Abstract
Insulin secretion and insulin sensitivity indexes are related by hyperbolic functions, allowing the calculation of the disposition index (DI) as the product of the acute insulin response (AIR) and the insulin sensitivity index (Si) from intravenous glucose tolerance test (IVGTT). Our objective was to develop an oral-DI based on the oral glucose tolerance test (OGTT) and to assess its association with glucose tolerance status. This research is structured in three studies. Study 1: OGTT were performed in 833 non-diabetic Chilean women (18–60 years) without family history of diabetes mellitus. Study 2: an independent group of n = 57 non-diabetic (18–46 years) without family history of diabetes mellitus carried out an OGTT and an abbreviated IVGTT. Study 3: a sample of 1674 Chilean adults (18–60 years) with different glycaemic status performed an OGTT. An adequate statistical fit for a rectangular hyperbola was found between the area under the curve of insulin-to-glucose ratio (AUCI/G-R) and the Matsuda ISI-COMP index (study 1). The oral-DI derived as AUCI/G-R × ISI-COMP was previously termed insulin-secretion-sensitivity index-2 (ISSI-2). ISSI-2 significantly correlated with DI from IVGTT (rho = 0.34; p = 0.009) (study 2). ISSI-2 shows important differences across groups of subjects with different glycaemic status (study 3). We have confirmed that ISSI-2 replicates the mathematical properties of DI, showing significant correlations with DI from the abbreviated MM-IVGTT. These results indicate that ISSI-2 constitutes a surrogate measure of insulin secretion relative to insulin sensitivity and emphasizes the pivotal role of impaired insulin secretion in the development of glucose homeostasis dysregulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is widely accepted that both impaired insulin secretion and systemic insulin sensitivity are found in classical type 2 diabetes mellitus (DM2) patients. In response to intravenous glucose stimulus, it has been described that insulin is released from pancreatic β-cells following a biphasic secretion pattern. The first phase consists of a brief spike lasting ∼10 min followed by a much longer second phase with increasing rate of secretion to peak at 30–40 min with a variable plateau of 2–3 h, as it has been observed both in rats and humans [14, 32]. In subjects with isolated impaired glucose tolerance (IGT), fasting glucose levels are still normal, but their first phase insulin response is already blunted. As a compensatory mechanism, persons with IGT also secrete more insulin during the second phase. It is important to consider that the compensatory ability of β-cells in insulin secretion is dependent on the degree of insulin resistance. However, it has been estimated that the pancreatic function is already decreased as much as 50 % in newly diagnosed DM2 cases, independently of the degree of insulin resistance [28]. During the course of DM2, there is also a progressive deterioration of β-cell function and mass, with a net loss in the ability of β-cells to compensate for insulin resistance. The loss of function/mass of β-cells has been attributed to a variety of factors such as glucotoxicity, lipotoxicity, action of pro-inflammatory cytokines, leptin and amyloid deposits [32]. Although there is a debate on which is the earliest and more important (quantitatively speaking) detectable defect in DM2, it is accepted that impaired insulin sensitivity precedes the onset of the disease and is necessarily accompanied by β-cell failure, with an earlier defect detectable in the first phase of insulin secretion [14].
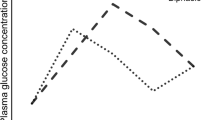
Using the minimal model (MM) intravenous glucose tolerance test (IVGTT), it is possible to compute the acute insulin response (AIR) and the insulin sensitivity index (Si) in a single day, as measures of early insulin secretion and systemic insulin sensitivity, respectively. It has been described that these indexes are hyperbolically related in such a way that their product (the disposition index (DI)) is constant for individuals with the same degree of glucose tolerance [33]. In this context, the shape of the graph that relates measures on insulin secretion with insulin action (rectangular hyperbolic function; insulin secretion = constant/insulin sensitivity) (Fig. 1) allows for the prediction of shifts in compensatory insulin secretion in response to or accompanied by changes in systemic insulin sensitivity/resistance. Therefore, the disposition index (DI = AIR × Si) is usually considered as a quantitative measure that describes the β-cell secretion normalized to the degree of systemic insulin sensitivity [10, 13, 25, 31].
Some insulin secretion and sensitivity indexes derived from plasma glucose/insulin measurements obtained during the oral glucose tolerance test (OGTT) have been widely used in the literature, showing all of them relatively high correlations with more elaborated measures related to glucose homeostasis obtained from different types of intravenous tests [5, 11, 23]. In contrast to the MM-IVGTT, the assessment of β-cell function after OGTT is not direct since it is necessary to take into account additional influences related glucose absorption and the incretin effect mediated by GLP-1 and GIP [16]. However, several protocols have been proposed in the literature to measure insulin secretion after standard OGTT such as the insulinogenic index at 30 min, the Stumvoll-1ST index, the Stumvoll-2ST index and the ratio of the area under the curve for insulin and glucose (AUCI/G-R) [1, 11, 15, 29].
In the last years, the typical hyperbolic relation between indexes of insulin secretion and insulin sensitivity obtained from MM-IVGTT has been also replicated by using surrogate indexes derived from OGTT [26]. This fact has led to the creation of novel OGTT-based measures mathematically analogous to the DI that have been collectively termed as “oral-DI”. One of these novel measures is the insulin secretion sensitivity index-2 (ISSI-2), calculated as the product AUCI/G-R × Matsuda index [18], has been shown modest significant correlations with the DI derived from the IVGTT [27]. It is important to note that both DI and oral-DI are relevant predictors of future DM2 [18, 31].
Given the limited applicability of IVGTT in epidemiological studies, it would be desirable to develop disposition indexes based on the oral glucose tolerance test rather than in IVGTT for population studies. Given the above, our objectives are (1) to develop an adequate oral disposition index (oral-DI) by evaluating the fit to a hyperbolic function between insulin secretion and insulin sensitivity indexes calculated from the OGTT, (2) to assess the relation between the selected oral-DI and the DI based on IVGTT, (3) to assess differences in oral-DI in Chilean subjects with different glucose tolerance status, as well as to evaluate the capacity of the oral-DI to discriminate normal and altered glycaemic status.
Subjects and methods
Study design and subjects
This cross-sectional research was structured in three studies described below. Table 1 shows the general characteristics of the participants. This research was approved by the Ethics committee of the School of Medicine, Pontificia Universidad Católica de Chile (Santiago, Chile).
Study 1: development of an oral-DI through the assessment of the hyperbolic relation between insulin secretion/sensitivity indexes derived from the OGTT
Standard OGTTs (75 g of oral glucose) were performed in 833 non-diabetic non-pregnant Chilean women without family history of diabetes mellitus (age range 18–60 years) and body mass index (BMI) >18.5 kg/m2 (see Table 1 for general characteristics). In this group, we tested couples of insulin secretion/sensitivity variables in order to find a pair that adequately fits the rectangular hyperbolic function. Glucose and insulin in plasma/serum samples were obtained at basal, 30, 60, 90 and 120 min at the clinical facilities of the Red Salud UC (Santiago, Chile) between the years 2009 and 2011 [3]. Venous blood samples were collected in BD tubes (Sodium fluoride and Na2-EDTA; for glucose analysis) and in serum separation gel tubes (for insulin analysis). Plasma/serum samples were analyzed in the accredited central laboratory Red Salud-UC by the colorimetric glucose-oxidase method for glucose analysis (expressed in mg/dL) and by an electrochemiluminescence immunoassay for insulin analysis (expressed in μU/mL).
Among the different combinations of insulin secretion/sensitivity indexes tested, the Stumvoll First Phase Secretion Index (Stumvoll-1PH) [29] and the ratio of the total area under the insulin curve to the total area under the glucose-curve (ratio of AUC insulin/glucose or AUCI/G-R) were used as surrogate variables of insulin secretion. On the other hand, the HOMA-S (the inverse of HOMA-IR × 100; https://www.dtu.ox.ac.uk/) and the Matsuda ISI-COMP index (http://mmatsuda.diabetes-smc.jp/english.html) were used as surrogates of insulin sensitivity indexes. Other measures of insulin secretion or sensitivity such as the Insulinogenic Index at 30 min or the OGIS [20] were also tested but are not shown herein given its poor performance in fitting hyperbolic functions.
Study 2: testing the relation between oral-DI and DI derived from IVGTT
An independent group of n = 57 non-diabetic nulliparous, non-pregnant women without parental family history of diabetes (age range 18–46 years) (see Table 1), carried out an OGTT and IVGTT separated by approximately 1 week. The inclusion criteria of this group included normal levels of plasma lipids, no hypertension, no obesity, as well as normal hematologic parameters and no alterations in plasma hepatic enzymes. We assessed the correlation between oral and endovenous disposition indexes derived from OGTT and IVGTT. The OGTT in study 2 was carried out analogously to the one of study 1, except that basal measurements were carried out in study 2 at times −15 and −5 min instead of a single basal measurement. Additionally, a short protocol for the MM-IVGTT was performed in this group using an intravenous dose of 0.3 g of glucose per kilogram of body weight as 50 % water solution, administered during 60 s. During the abbreviated MM-IVGTT, measurements of serum glucose and insulin were carried out at −15 and −5 min and at times 2, 3, 4, 5, 6, 8 and 10 min to calculate the AIR index [19]. After AIR, additional blood samples over a period of 50 min were drawn to complete a total of 15 blood samples, in which circulating insulin and glucose were measured to estimate the calculated sensitivity index (CSi) [30].
Study 3: evaluating differences in oral-DI among Chilean subjects with different glucose tolerance status
A cross-sectional sample of n = 1674 Chilean adults (both sexes; age 18–60 years) (see Table 1) were included in this study. Following general cut-off points of the American Diabetes Association [2], participants were classified into 5 study groups: (1) normal glucose tolerance (NGT; n = 1393) defined as fasting plasma glucose <100 mg/dL (<5.6 mmol/L) and 2-h glucose levels <140 mg/dL (<7.8 mmol/L); (2) isolated impaired fasting glucose (I-IFG; n = 86) with fasting plasma glucose in the range 100–125 mg/dL (5.6–6.9 mmol/L) and 2-h glucose levels <140 mg/dL (<7.8 mmol/L); (3) isolated impaired glucose tolerance (I-IGT; n = 136) with fasting plasma glucose <100 mg/dL (<5.6 mmol/L) and 2-h glucose levels in the range of 140–200 mg/dL (7.8–11.1 mmol/L); (4) Combined IFG + IGT (n = 27); and (5) diabetes mellitus (DM; n = 32; 32.1 % with family history of diabetes in parents) with 2-h glucose levels >200 mg/dL (>11.1 mmol/L). The NGT group of study 3 is composed of the n = 833 women from the study 1 with the additional inclusion of 560 participants (both sexes) (see Table 1). OGTT in all study participants of study 3 was carried out in the same way as in study 1. It is important to note that participants of the NGT group are notably younger and shows lower BMI than the other groups within study 3 (Table 1).
Statistical methods
In the studies 1 and 2, the evaluation of a hyperbolic relation (Insulin-secretion = k/insulin sensitivity) between insulin secretion and sensitivity indexes was statistically assessed after logarithmic transformation and test of the negative slope (m = −1) by Deming regression. This regression method estimates a straight line by minimizing the sum of distance between the measured values and the regression line at an angle specified by the variance ratio of variables in both axes. In the particular case of a variance ratio of 1, Deming regression minimizes the perpendicular distances to the regression line, and it became orthogonal regression. In more general terms, this method is equivalent to the perpendicular least squares properly weighted regression used by Renatkaran et al. [26]. This type of regression is widely used in clinical chemistry as a tool for comparing analytical methods. However, it can also be used for our purpose of estimating the adequate fit to a hyperbolic function if the slope after logarithmic transformation is approximately equal to −1.
We have not carried out replicated OGTTs of the same subjects to estimate intra-subject variances or coefficients of variation of insulin secretion/sensitivity indexes to include in the Deming regression. Then, we have calculated the coefficient of variation (CV) of the HOMA-S index, which was measured twice in the same subjects: 81.6 ± 44.5 (mean ± standard deviation in the day of OGTT) and 77.4 ± 31.3 (day of IVGTT) (both the day of OGTT and IVGTT, insulin was measured at −15 and −5 min). These measures yielded a standard deviation of reproducibility of 4.07 μU/mL with a coefficient of variation of CV = 5.1 %. As an operational strategy, the CV of HOMA-S was used as a measure of error for all insulin secretion/sensitivity indexes in order to estimate slope and 95 % confidence intervals in Deming regression. It is worth mentioning that Renatkaran et al. [26] report coefficients of variation of similar magnitude for different indexes derived from OGTT. Assuming a constant CV in these insulin secretion/sensitivity indexes means that we assume that errors are proportional to mean values, instead of being constant across all measured values.
In the study 2 and 3, associations among numerical variables were additionally analysed using non-parametric Spearman correlation coefficients. Differences in oral-DI and secretion/sensitivity indexes across study groups in study 3 were assessed though the Kruskall-Wallis tests and multiple regression techniques. The discriminatory capacity of insulin secretion/sensitivity indexes for classification of subjects with different glycaemic status categories was also evaluated by the receiver operating characteristic (ROC) curve. All statistical analyses were carried out with the STATA 12.0 software (http://www.stata.com) and MedCalc 14 (http://www.medcalc.org).
Results
Study 1
Table 1 shows the general characteristics and glycaemic traits of participants in this study. In the group of normoglycaemic women (n = 833 subjects), we used different combinations of surrogates for insulin secretion derived from OGTT (Stumvoll-1PH and AUCI/G-R) on one hand, and insulin sensitivity (HOMA-S and Matsuda ISI-COMP index) on the other hand, to evaluate hyperbolic relations between insulin secretion versus insulin sensitivity indexes. We found that the pair of variables represented by AUCI/G-R (surrogate of insulin secretion) and the Matsuda ISI-COMP (surrogate of insulin sensitivity) showed the most satisfactory statistical fit for a rectangular hyperbola (Figs. 2a-d and 3a-d). Statistically, this is formally tested by assessing the slope of m = −1 through Deming regression using log-transformed data. The oral-DI derived from this combination of insulin secretion/sensitivity indexes (AUCI/G-R × Matsuda ISI-COMP) is the same as the previously described ISSI-2 [27].
Study 2
Table 1 shows the general characteristics and glycaemic traits of participants in this study. In this group of n = 57 non-diabetic women who carried out both OGTT and IVGTT, AUCI/G-R significantly correlated with AIR (rho = 0.37; p = 0.004) (Fig. 4a), while Matsuda Index significantly correlated with CSI (rho = 0.54; p < 0.0001) (Fig. 4b). The hyperbolic relation was satisfactory for the combination of AUCI/G-R and Matsuda ISI-COMP derived from OGTT (Fig. 4c). In this group, the ISSI-2 index calculated from OGTT (ISSI-2 = AUCI/G-R × Matsuda ISI-COMP) correlated significantly with the DI calculated from IVGTT (DI = AIR × CSi) (rho = 0.34; p = 0.009) (Fig. 4d). Transformations of both axes with standardization of DI and the use of logarithm of the standardized ISSI-2, as proposed by Renatkaran et al. (2009), yielded a Pearson correlation coefficient of 0.32 (p = 0.01). Using linear regression techniques, the association between ISSI-2 and CSi remained statistically significant after adjusting by the covariates age and BMI.
Study 3
Table 1 shows the general characteristics and glycaemic traits of participants in this study. In all study groups (NGT, I-IFG, I-IGT, IFG + IGT and diabetes mellitus), there were an approximate hyperbolic relation between AUCI/G-R and Matsuda ISI-COMP, indicating that ISSI-2 is an adequate oral-DI (Fig. 5a-e). Curves were closer to the origin as the severity of the glucose dysregulation is increased (Fig. 5f). Additionally, the ISSI-2 showed important and significant differences among study groups (Kruskall-Wallis p < 0.0001; Table 1). Statistical analysis with multiple regression indicated that there are significant differences in ISSI-2 across groups of glycaemic status even after adjustment by gender, age and family history of diabetes.
The ROC analysis showed good discriminatory capacity of ISSI-2 when comparing the NGT group versus other groups characterized by impaired glucose tolerance or diabetes mellitus (Fig. 6a-d). When comparing discriminatory ability of several indexes, ISSI-2 shows a much greater area under the ROC curve that Matsuda ISI-COMP and AUCI/G-R, having the latter no appreciable capacity in this sense (Fig. 6a-d).
Discussion
Insulin secretion and insulin sensitivity indexes derived from the intravenous glucose tolerance tests (IVGTT) have been mathematically related by hyperbolic functions. This type of relation allows the calculation of the glucose disposition index (DI) as the product of the acute insulin response (AIR) and the insulin sensitivity index (Fig. 1). The usefulness of DI is exemplified in pregnancy, which is a physiological state characterized by insulin resistance: in this situation, insulin response in pregnant women is apparently similar to volunteers, but when corrected by insulin sensitivity through the DI, it results in a defective insulin secretion/clearance relative to insulin sensitivity [10] (Fig. 1, top centre). As an example using fictitious numbers, the top right part of Fig. 1 shows that, at similar levels of insulin sensitivity, a person with normal β-cell secretion (light-dotted line; hyperbolic) has a higher DI compared to a person with impaired β-cell secretion (darker dotted line; closer to the origin) (DI = 1000 versus DI = 900) (Fig. 1). However, if insulin sensitivity decreases due any cause (puberty, pregnancy, increased adiposity), this difference becomes more evident (DI = 1000 versus DI = 500) (Fig. 1; top right). Although it is accepted that both impaired insulin secretion and insulin resistance are found in classical type 2 diabetes mellitus (DM2) patients, our results showing important differences in the average ISSI-2 across subjects with different degree of glucose tolerance (from NGT to diabetes mellitus) remark the importance of the assessment of beta cell function and relative insulin secretion in the development of glucose dysregulation [22].
The MM-IVGTT is a clinical test that simultaneously allows the calculation of insulin secretion and sensitivity indexes in a single day. The original MM-IVGTT used a total of 27 serial blood samples during 180 min after initial intravenous glucose administration, allowing the assessment of both AIR (ten first minutes) of the MM-IVGTT and the insulin sensitivity index (SI) [6–10, 24]. The minimal model evolved from its original description to more elaborated protocols with the additional infusion of a bolus of 0.03 UI/Kg of insulin at t = 20 min. [4, 6, 25]. In the last years, several abbreviated MM-IVGTT have been developed in order to reduce the time, cost and complexity associated with the complete MM-IVGTT. In our study, we have used the short IVGTT proposed by Tura et al. (2010). This abbreviated protocol generates a complete estimation of AIR (first 10 min after glucose infusion) and a measure of insulin sensitivity called “calculated sensitivity index” (CSi) that is based in plasma insulin/glucose measurements carried out during 50 min. Both Si and CSi show very high correlations with insulin sensitivity measures derived from the hyperinsulinemic euglycemic clamp [30].
In spite of the reduced cost of using abbreviated protocols for MM-IVGTT instead of full elaborated protocols, it is still difficult to apply IVGTT in large-scale human studies. Given that the oral-DI is a predictor of type 2 diabetes [31] and taking into account the limited applicability of IVGTT in epidemiological studies, it would be desirable to develop adequate disposition indexes based on the oral glucose tolerance test rather than in IVGTT in the Chilean population. In our study, we have confirmed that the ISSI-2 (ISSI-2 = AUCI/G-R × Matsuda ISI-COMP), previously described by Renatkaran et al. [18], displays an adequate adjustment to a rectangular hyperbolic function. We have also confirmed that the ISS-2 shows a modest although significant correlation with the DI computed as the product of AIR × CSi derived from an abbreviated MM-IVGTT in agreement with the results of Renatkaran et al. (2009). Moreover, ROC analysis indicated that AUCI/G-R, as a measure of insulin secretion, has no capacity for classify participants with different glycaemic status. However, when insulin secretion is corrected by insulin sensitivity through ISSI-2, then this index shows a great ability to correctly classify subjects with glucose dysregulation compared to normal glucose tolerance. Then, the results of our study support the use of ISSI-2 as a surrogate measure of DI, which is generally interpreted as a measure of insulin secretion/clearance relative to the level of insulin resistance. However, it is also worth noting that some authors have suggested that other nonlinear inverse functions may also explain the relation between insulin secretion/sensitivity indexes [11, 21]. Finally, it has been suggested that the meaning of the DI resides in its information on the integrated response between insulin secretion and the metabolic clearance of insulin sensitivity [12]. Regardless of its interpretation, the hyperbolic relation between AIR and Si is a remarkable, consistent and reproducible observation in the scientific literature [17].
Our study has some limitations: First, insulin secretion and sensitivity indexes show intrinsic interdependence because the same data are partially involved in shared mathematical expressions used to derive such indexes. Second, autocorrelation may arise since some measures are derived in a single test in the same day, such as AUCI/G-R and ISI-COMP from OGTT (from OGTT) as well as AIR and CSi (from abbreviated MM-IVGTT). In contrast, more elaborated MM-IVGTT protocols include the infusion of insulin at minute 20, introduces some degree of independence in the estimation of AIR and Si [21]. Third, it is worth noting that subjects involved in studies 1 and 3 were selected in this research based on a health-related context rather than as a part of a designed research study, which may introduce bias regarding the particular previous health conditions related with each participant. Fourth, we do not have estimates of coefficient of variation of the insulin secretion/sensitivity indexes involved in ISSI-2, which can be obtained in repeated measures of oral/endovenous glucose tests [26]. However, we believe that the application of the coefficient of variation from HOMA-S calculated directly in our study provides an adequate measure to estimate trustable slopes in Deming regression. In this context, the coefficient of variation reported by Renatkaran el al. [26] for HOMA-IR (the inverse of HOMA-S) is of similar magnitude compared to coefficients of variation of other insulin secretion/sensitivity indexes. Fifth, an unavoidable limitation of our study resides in the fact that circulating glucose/insulin levels during the OGTT are affected by other factors apart from β-cell function, such as the action of incretin hormones and hepatic extraction. In this context, a relevant part of variability in glucose/insulin levels is also related to day-to-day variability in gastrointestinal function such as gastric emptying and glucose absorption. On the other hand, insulin sensitivity measured either with IVGTT or OGTT represents an aggregate of skeletal muscle, hepatic and adipose insulin sensitivity that may vary in different conditions. Sixth, the cross-sectional nature of our study does not allow the evaluation of the predictive capacity of oral-DI (ISSI-2) in predicting future impaired glucose regulation.
In summary, we have confirmed that ISSI-2, an oral-DI derived from the OGTT, replicates the mathematical properties of the DI from IVGTT, showing significant correlations with the DI calculated from the abbreviated MM-IVGTT. These results indicate that ISSI-2 constitutes a surrogate measure of insulin secretion relative to insulin sensitivity that is useful in epidemiologic studies of diabetes and glycaemic-related traits. The important differences found in ISSI-2 in adults with varying degree of glucose tolerance emphasize the pivotal role of impaired insulin secretion in the development of glucose homeostasis dysregulation.
References
Abdul-Ghani MA, Tripathy D, DeFronzo R (2006) Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 29:1130–1139
American Diabetes Association (2015) Classification and diagnosis of diabetes. Diabetes Care 38(Suppl 1):S8–S16
Arancibia C, Galgani J, Valderas JP, Morales M, Santos JL, Pollak F (2014) Evaluation of serum insulin levels after an oral glucose load for the diagnosis of insulin resistance. Rev Med Chil 142:1106–1112
Ascaso JF, Pardo S, Real JT et al (2003) Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care 26:3320–3325
Bastard JP, Vandernotte JM, Faraj M et al (2007) Relationship between the hyperinsulinemic-euglycaemic clamp and a new simple index assessing insulin sensitivity in overweight and obese postmenopausal women. Diabetes Metab 33:261–268
Bergman RN (2005) Minimal model: perspective from 2005. Horm Res 64(suppl 3):8–15
Bergman RN, Ider YZ, Bowden CR, Cobelli C (1979) Quantitative estimation of insulin sensitivity. Am J Physiol 236:E667–E677
Bergman RN, Phillips LS, Cobelli C (1981) Physiologic evaluation of factors controlling glucose tolerance in man. J Clin Invest 68:1456–1467
Bergman RN, Prager R, Volund A, Olefsky JM (1987) Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest 79:790–800
Bergman RN, Ader M, Huecking K, Van Citters G (2002) Accurate assessment of β-cell function: the hyperbolic correction. Diabetes 51(Suppl 1):S212–S220
Cobelli C, Toffolo GM, Man CD et al (2007) The insulin secretory pathway is complex and regulated by multiple factors including glucose, protein, fat, and incretins. Am J Physiol Endocrinol Metab 293:E1–E15
DeFronzo RA, Tripathy D, Abdul-Ghani M, Musi N, Gastaldelli A (2014) The disposition index does not reflect β-cell function in IGT subjects treated with pioglitazone. J Clin Endocrinol Metab 99(10):3774–3781
Denti P, Toffolo GM, Cobelli C (2012) The disposition index: from individual to population approach. Am J Physiol Endocrinol Metab 303:E576–E586
Gerich JE (2002) Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes 51(Suppl 1):8117–8121
Herzberg-Schafer SA, Staiger H, Heni M et al (2010) Evaluation of fasting state-/oral glucose tolerance test-derived measures of insulin release for the detection of genetically impaired b-cell function. PLoS One 5(12):e14194
Holst JJ, Gromada J (2004) Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab 287:E199–E206
Kahn SE, Prigeon RL, McCulloch DK et al (1993) Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42:1663–1672
Lorenzo C, Wagenknecht LE, Rewers MJ, Karter AJ, Bergman RN, Hanley AJ, Haffner SM (2010) Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 33:2098–2103
Marcelli-Tourvieille S, Hubert T, Pattou F, Vantyghem MC (2006) Acute insulin response (AIR): review of protocols and clinical interest in islet transplantation. Diabetes Metab 32:295–303
Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ (2001) A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 24:539–548
Mari A, Ahren B, Pacini G (2005) Assessment of insulin secretion in relation to insulin resistance. Curr Opin Clin Nutr Metab Care 8:529–533
Mari A, Tura A, Natali A, Laville M, Laakso M, Gabriel R, Beck-Nielsen H, Ferrannini E (2010) RISC investigators impaired beta cell glucose sensitivity rather than inadequate compensation for insulin resistance is the dominant defect in glucose intolerance. Diabetologia 53:749–756
Matsuda M, DeFronzo R (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470
Pacini C, Bergman RN (1986) MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsitivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Prog Biomed 23:113–122
Plum L, Ahmed L, Febres G et al (2011) Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity 19:2149–2157
Retnakaran R, Shen S, Hanley AJ et al (2008) Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity 16:1901–1907
Retnakaran R, Qi Y, Goran M, Hamilton J (2009) Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med 26:1198–1203
Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC (2006) Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes Care 29:717–718
Stumvoll M, Mitrakou A, Jenssen T et al (2000) Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 23:295–301
Tura A, Sbrignadello S, Succurro E et al (2010) An empirical index of insulin sensitivity from short IVGTT: validation against the minimal model and glucose clamp indices in patients with different clinical characteristics. Diabetologia 53:144–152
Utzschneider KM, Prigeon RL, Faulenbach MV et al (2009) Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 32:335–341
Wajchenberg BL (2007) Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 28:187–218
Wallace TM, Levy JC, Matthews DR (2004) Use and abuse of HOMA modeling. Diabetes Care 27:1487–1493
Acknowledgments
This study is supported by Chilean grant FONDECYT 1150416 and (Fondo Nacional de Desarrollo Científico y Tecnológico)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This research was approved by the Ethics committee of the School of Medicine, Pontificia Universidad Católica de Chile (Santiago, Chile).
Rights and permissions
About this article
Cite this article
Santos, J.L., Yévenes, I., Cataldo, L.R. et al. Development and assessment of the disposition index based on the oral glucose tolerance test in subjects with different glycaemic status. J Physiol Biochem 72, 121–131 (2016). https://doi.org/10.1007/s13105-015-0458-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-015-0458-0