Abstract
Cholesterolemia is associated with pro-oxidative and proinflammatory effects. Glucomannan- or glucomannan plus spirulina-enriched surimis were included in cholesterol-enriched high-saturated diets to test the effects on lipemia; antioxidant status (glutathione status, and antioxidant enzymatic levels, expressions and activities); and inflammation biomarkers (endothelial nitric oxide synthase (eNOS), inducible nitric oxide synthase (iNOS), tumor necrosis factor alpha (TNF-α)) in Zucker fa/fa rats. Groups of eight rats each received diet containing squid-surimi (C), squid-surimi cholesterol-enriched diet (HC), glucomannan-squid-surimi cholesterol-enriched diet (HG), or glucomannan-spirulina-squid-surimi cholesterol-enriched diet (HGS) over a period of 7 weeks. HC diet induced severe hyperlipemia, hepatomegalia, increased inflammation markers, and impaired antioxidant status significantly (at least p < 0.05) vs. C diet. HG diet decreased lipemia and liver size and normalized antioxidant status to C group levels, but increased TNF-α with respect to HC diet (p < 0.05). In general terms, 3 g/kg of spirulina in diet maintained the positive results observed in the HG diet but, in addition, increased inflammation index [eNOS/(eNOS + iNOS)] and decreased plasma TNF-α (both p < 0.05). In conclusion, glucomannan plus a small amount of spirulina blocks negative effects promoted by hypercholesterolemic diets. Although more studies are needed, present results suggest the utility of including glucomannan and/or spirulina as functional ingredients into fish derivates to be consumed by people on metabolic syndrome risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A plethora of potential functional foods have been developed during the last few decades [3]. As ample evidence exists of the health benefits of fish and fish derivate consumption [15], our research team is engaged in assessing the health impact of potential new functional foods containing seaweeds [12] and fish derivates/ingredients [33]. Squid-surimi admits the addition of ingredients with known health benefits and/or removes potentially undesirable components for the development of functional foods [6, 33].
Westernized countries, where there is a high prevalence of degenerative chronic diseases, are also characterized by consumption of hyperenergetic, hypersaturated, cholesterol-rich diets believed to cause imbalances in reactive oxygen species (ROS) production [5]. Free radicals and ROS are considered to be crucial in the development of degenerative diseases such as obesity, cardiovascular diseases and type 2 diabetes [21]. In addition, hypercholesterolemia, increases free radical production and related inflammation mechanisms [30]. Oxidative stress is defined as an overbalance of the net levels of ROS and reactive nitrogen species (RNS) in comparison to the antioxidant capacity. Endogenous antioxidant defense capacity consists of enzymes that catalyze oxidant-modifying reactions and interacting molecules with antioxidant activity (Fig. 1). Endothelial nitric oxide synthase (eNOS) is habitually expressed in cells, synthesizing nitric oxide, which is involved in the regulation of the cardiovascular system and homeostatic mechanisms [31]. Inducible nitric oxide synthase (iNOS) activation is strongly promoted by pathophysiological situations in response to cytokines and leads to sustained higher nitric oxide levels, which have antioxidant and anti-inflammatory effects [32].
General endogenous antioxidant defense system diagram. Figure modified from Vázquez-Velasco et al. [33]
The role of plant bioactive components and dietary fiber as free radical scavengers is now an area of active research. Glucomannan, a dietary fiber extracted from Amorphophallus konjac, is known to possess satiating, laxative, and hypocholesterolemic properties [11], while Spirulina platensis, has been considered a microalga rich in minerals and antioxidant compounds such as carotenoids and phycocyanin [23].
Zucker fa/fa rats are very sensitive to hypercaloric and hyperlipemic diets, developing a chronic low-level inflammation state, closely linked to oxidative stress, which leads to cell damage [9].
To the best of our knowledge, there have been few studies to date on the effects of squid-surimi on antioxidant status and hypercholesterolemia, and much less on the effects of glucomannan-enriched surimi in the frame of hypercholesterolemic diets. A previous study demonstrated that glucomannan-enriched surimi induced antioxidant and proinflammatory effects, while glucomannan plus spirulina-enriched surimi kept the antioxidant effects but ameliorated the inflammatory ones [33]. The hypothesis of the present work is that glucomannan-enriched and glucomannan plus spirulina-enriched squid-surimis act as functional foods by reducing the oxidative and inflammatory status originated by hypercholesterolemia induction in fa/fa rats. The purpose of our study, then, is to determine the effects of large amounts of glucomannan-enriched squid-surimi consumption in a hyperenergetic, hypersaturated-fat diet, enriched with cholesterolemic agents, on liver fat and cholesterol; liver antioxidant (superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), catalase (CAT), oxidized and reduced glutathione); and inflammation (eNOS, iNOS, and tumor necrosis factor alpha (TNF-α) biomarkers.
Material and methods
Diet preparation and experimental design
All experiments were performed in compliance with Directive 86/609/EEC of 24 November 1986 (modified by Directive 2003/65/CE of 22 July 2003) for the protection of scientific research animals. The present study was approved by the Spanish Science and Technology Advisory Committee (project AGL 2008-04892-C03-02 and Consolider Ingenio 2010, CSD 2007–00016) and by an ethics committee of the Universidad Complutense of Madrid (Spain). A total of 24 male growing Zucker fa/fa rats with an initial body weight of approximately 120 g were obtained from Harlan Laboratories Models (Harlan, SL, Barcelona, Spain). The animals were housed individually in metabolic cells in a temperature-controlled room (22.3 ± 1.9 °C) with a 12-h light/12-h dark cycle. The rats were fed commercial rat pellets (Panlab, Barcelona, Spain) during a 1-week adaptation period to environmental conditions and then distributed into three groups of eight animals each, according to their average body weight.
Four experimental semi-synthetic diets were prepared in a room under appropriate environmental conditions (4 °C and low enlightenment) to reduce changes in their antioxidant properties blending AIM-93 M diets and surimi. Vitamin and mineral contents of AIN-93 M diets were designed to cover requirements for rats once the whole diet was prepared (Table 1). Control diet (C) was composed of a homogeneous mixture of 70 % rodent diet (AIN-93 M #102634 purified rodent diet; Dyets, Inc., Bethlehem, PA, USA) and 30 % freeze-dried restructured squid-surimi (with 15 % microcrystalline cellulose). Hypercholesterolemic control diet (HC) was composed of a homogeneous mixture of 70 % rodent diet (AIN-93 M #102636 purified rodent diet; Dyets, Inc., Bethlehem, PA, USA) and 30 % freeze-dried restructured squid-surimi (with 15 % microcrystalline cellulose); Hypercholesterolemic glucomannan diet (HG) consisted of a mixture of AIN-93 M #102637 feed (70 %) and freeze-dried, restructured glucomannan-enriched squid-surimi (30 %, 15 % of glucomannan into surimi) and hypercholesterolemic glucomannan plus spirulina diet (HGS) consisted in a mixture of AIN-93 M #102637 feed (70 %) and freeze-dried, restructured glucomannan plus spirulina-enriched squid-surimi (30, 15 % glucomannan into surimi and 3 g/kg diet of spirulina). All hypercholesterolemic diets contained 2 % cholesterol (95–98 % purity) and 0.4 % cholic acid (98 % purity). Water and food were provided ad libitum over the 7-week experimental period. At the end of the experiment, in order to avoid inter-assay variations that could affect the comparison of data from the different groups, fasting rats were taken, one at a time from each of the six groups, anesthetized and euthanized by extracting blood from the descending aorta.
Liver fat and cholesterol determinations
Lipids from homogeneous samples of hepatic tissue from the major lobe of liver were extracted with chloroform/methanol (2:1, v/v). Extract was dissolved in isopropanol and tested for total cholesterol, using the enzymatic colorimetric method (kit references #1001090) of Spinreact (Sant Esteve de Bas, Girona, Spain). Turbidity in samples, when present, was eliminated by centrifuging at 2200 g at 4 °C for 5 min after the enzymatic-colorimetric reaction and before spectrophotometric reading.
Glutathione determination
Total, reduced (GSH) and oxidized glutathione (GSSG) levels were determined in liver following the Hissin and Hill [13] method. Liver tissue was homogenized with phosphate-EDTA (0.1 M sodium phosphate and 0.005 M EDTA) buffer (pH = 8) at 100 mg/mL concentration, adding 10 μL/mL tissue of HClO4 (60 %). Then, tissue homogenates were spun at 10,000 RPM (6000g) for 10 min at 4 °C and supernatant were at 4 °C until GSH and GSSG determination. Fluorescence was measured in a FLX 800 fluorimeter (Bio-Tek Instruments, Winooski, Vermont, USA) at λexc = 350 nm and λem = 420 nm.
The redox index (RI), a parameter that indicates the antioxidant status of the tissue, was expressed as follows: RI = GSH/(GSH + GSSG).
Western blotting. Antioxidant enzyme and inflammation biomarker levels
Equal amounts of protein lysates obtained from rat liver homogenates were separated in 10 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The gels were blotted onto a PVDF Amersham Hybond-P membrane (GE Healthcare, Buckinghamshire, UK) and incubated with the appropriate antibodies (S2147, C0979, A2228 and A9917 from Sigma-Aldrich, St. Louis, MI, USA; Ab60275, Ab5589 and Ab21775 from Abcam, Cambridge, UK; sc-32886, sc-1350, sc-2490 and sc-2004 from Santa Cruz Biotechnology, Dallas, TX, USA). Blots were developed by enhanced chemo luminescence using an Amersham ECL Plus Western Blotting Detection Reagents (GE Healthcare, Buckinghamshire, UK) according to the manufacturer’s instructions. β-actin was used as a loading control.
Extraction and analysis of RNA and quantification by reverse transcription-polymerase chain reaction. Enzymes’ gene expression
Total RNA was isolated from 100 mg of liver using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA samples were then treated with DNase I RNase-free, DNase treatment and removal reagents (Thermo Fisher Scientific, Waltham, MA, USA) to remove any contamination with genomic DNA. The yield and quality of the RNA were assessed by measuring absorbance at 260, 270, 280, and 310 nm and by electrophoresis on agarose gels (1.3 %). Total RNA of each sample (1.5 mg) was reverse-transcribed to first-strand complementary DNA (cDNA) using a revert aid H minus first-strand cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA).
Relative CAT, Cu, Zn-SOD, Mn-SOD, GPx, GR, and cytochrome P450 7A1 (CYP7A1) messenger RNA (mRNA) levels were quantified using real-time PCR with a LightCyclerTM Real-Time PCR Detection System (Roche diagnostics, Indianapolis, IN, USA), using a SYBR® Green (Biotools, Madrid, Spain) for the normalization of the results.
The PCR parameters were as follows: preincubation at 95 °C for 5 s followed by 40 cycles of denaturation at 95 °C for 5 s, with an annealing temperature dependent of each couple primer (55 °C for Mn-SOD; 56 °C for Cu, Zn-SOD and 60 °C for CAT, GPx, GR and CYP7A1), extension 72 °C for 30 s. Melting curve 95–65–95 °C. Cooling 40 °C.
Primers sequences:
-
Cu, Zn-SOD: sense 5ʹ-GCCGTGTGCGTGCTGAA-3ʹ
-
antisense ʹ-TGACGATGCCGTGCTGCATG-3ʹ
-
Mn-SOD: sense 5ʹ-GACAAACCTGAGCCCTAAGGG-3ʹ
-
antisense 5ʹ-CTTCTTGCAAACTATG-3ʹ
-
CAT: sense 5ʹ-ATCAGGGATGCCATGTTGTT-3ʹ
-
antisense 5ʹ-GGGTCCTTCAGGTGAGTTTG-3ʹ
-
GPx: sense 5ʹ-GCAATCAGTTCGGACACCAG-3ʹ
-
antisense 5ʹ-AAAGTTCCAGGCAATGTCGT-3ʹ
-
GR: sense 5ʹ-TCACTGCTCCGCACATCC-3ʹ
-
antisense 5ʹ-CTCAACACCGCCAGCGTTCTCC-3ʹ
-
CYP7A1: sense 5ʹ-CACCATTCCTGCAACCTT-3ʹ
-
antisense 5ʹ-GTACCGGCAGGTCATTCA-3ʹ
All sample mRNA levels were normalized to their values of β-actin and the results expressed as fold changes of threshold cycle (Ct) value relative to controls using the 2−ΔΔCt method [18].
Enzyme assays
GPx activity was determined as Se-dependent GPx and total GPx activities. Se-dependent GPx activity was assessed following the Paglia and Valentine method [24], while total GPx activity was assessed by the Lawrence and Burk method [16]. CAT activity was determined according to Aebi [1]. Total SOD activity was determined as indicated by Marklund [20]. Enzyme activities were standardized to liver protein concentrations that were determined according to Bradford [7].
Plasma assays
Cholesterol and triglycerides were determined by the enzymatic colorimetric method (kit references #1001090 and #1001312, respectively) of Spinreact (Sant Esteve de Bas, Girona, Spain). TNF-α was measured using the #DE4774 rat ELISA kit from Diagenics (Milton Keynes, Buckinghamshire, UK).
Statistical analyses
Statistical analyses were performed using the SPSS version 22.0 statistical analysis package (SPSS, Inc., Chicago, IL, USA). Results were expressed as means and standard deviations. One-way ANOVA followed by Bonferroni test was used to assess the effect of the diets. When variances were assumed not to be equal, the T2 of Tamhane post hoc test was applied. Contingence tables to assess differences in prevalence of severe hypercholesterolemia between groups were performed by chi-square test. Results were accepted as significant when p < 0.05.
Results
Body and liver weight, hepatosomatic index
As shown in Table 2, all fa/fa rats became obese. HC diet intake reduced body weight (p < 0.05), but increased liver weight and hepatosomatic index in comparison with C rats (both p < 0.001).
Liver fat and cholesterol
HG diet significantly reduced liver weight and hepatosomatic index vs. HC diet (both p < 0.001), but no extra effect of spirulina addition was observed (HGS vs. HG, p > 0.05).
Liver fat and liver cholesterol significantly increased in HC vs. C rats (p < 0.001). Both HG and HGS diets significantly reduced liver fat but only HGS diet was able to decrease significantly liver cholesterol with respect to HC diet (at least p < 0.05).
Plasma lipids and CYP7A1 expression
HC diet considerably modified the cholesterolemia and hypertriglyceridemia with respect to C diet (Table 2). Glucomannan-supplemented diets significantly reduced (p < 0.001) plasma cholesterol and triglycerides in comparison to HC diet (Table 2). In fact, the prevalence of severe hypercholesterolemia (plasma cholesterol ≥200 mg/dL [27]) differed significantly (p = 0.002) between rat groups: all HC rats were severely hypercholesterolemic, but the same applied to only 37.5 % and 12.5 % from groups HG and HGS, respectively. Furthermore, some of the animals in the HG and HGS groups became normocholesterolemic (<100 mg/dL [27]). Glucomannan consumption reduced triglycerides by more than 80 %; all triglycerides values in HG and HGS groups were below 150 mg/dL. Liver weight appears to correlate significantly with both plasma cholesterol and triglycerides (p < 0.01).
Low cytochrome P450 expression has been reported in fa/fa rats [14]. The HC diet reduced this theoretically low CYP7A1 expression even more. Both HG and HGS diets significantly increased CYP7A1 expression (p < 0.05). However, the addition of spirulina (HGS vs. HG) has no extra effects (p > 0.05) on CYP7A1 expression.
Total, reduced, and oxidized glutathione levels
Dietary cholesterol significantly reduced GSH levels (p < 0.05) and the redox index (p < 0.001) in HC vs. C rats (Table 2). GSH levels were greater in HG rats than in their HC counterparts, but GSSG levels were unaffected (Table 2). The addition of Spirulina (HGS vs. HG) has no extra effects (p > 0.05) on GSH or GSSG levels. The redox index was higher in HG and HGS vs. HC liver extracts (p < 0.05).
Antioxidant enzyme activities, levels, and expressions
Table 3 and Fig. 2 show information on liver antioxidant enzyme activities, levels, and expressions. SOD activity increased significantly (p < 0.05) while total GPx, Se-GPx, and non-Se-GPx activities tended to increase, and CAT activity to decrease in HC vs. C rats.
Effects of cholesterol enriched diets containing glucomannan-squid surimi added or not with spirulina on major enzymatic antioxidants, determined by western blotting. 2.1. Effects on superoxide dismutase (SOD) level. 2.2. Effects on catalase (CAT) level. 2.3. Effects on glutathione peroxidase (GPx) level. 2.4. Effects on glutathione reductase (GR) level. Bars (mean ± standard deviation of eight rats per group) bearing unlike superscript letters were significantly different (a > b > c, post hoc Bonferroni test or T2 of Tamhane, p < 0.05). A representative band of each enzyme result is set together to a β-actin band, used as a loading control. C, fa/fa rats fed modified AIM-93 diet containing control-surimi; HC, fa/fa rats fed cholesterol-enriched modified AIM-93 diet containing control-surimi; HG, fa/fa rats fed cholesterol-enriched modified AIM-93 diet containing glucomannan-surimi; HGS, fa/fa rats fed cholesterol-enriched modified AIM-93 diet containing glucomannan-surimi plus spirulina
HC diet significantly increased GPx levels (14 %) and MnSOD expression (38-fold), and reduced CAT (65 %) and GPx (58 %) expressions. As noted, HC rats displayed cholesterol levels six times higher than C rats.
HG vs. HC diet reduced SOD and total GPx activities (Table 3). In fact, the MnSOD expression was reduced considerably. The addition of spirulina to HG diet induced important changes. GPx and MnSOD expression decreased. Levels of GR and GPx also diminished.
Inflammation biomarkers
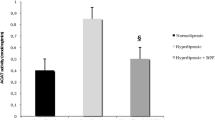
Table 2 and Fig. 3 show data on inflammation biomarkers. C rats presented very high plasma TNF-α values. HC diet increased liver eNOS and iNOS levels (p < 0.05) but not the eNOS/(eNOS + iNOS) index in comparison to C diet. eNOS and iNOS expressions were not significantly different (p > 0.05) in HG animals with respect to their HC counterparts. HGS diet reduced significantly iNOS expression and the inflammation ratio with respect to the other three experimental diets (at least p < 0.05).
Effects of cholesterol enriched diets containing glucomannan-squid surimi added or not with spirulina on inflammation biomarkers, determined by western blotting. 3.1. Effects on endothelial nitric oxide synthase (eNOS) level. 3.2. Effects on inducible nitric oxide synthase (iNOS) level. 3.3. Effects on tumor necrosis factor alpha (TNF-α) level. Bars (mean ± standard deviation of eight rats per group) bearing unlike superscript letters were significantly different (a > b > c, post hoc Bonferroni test or T2 of Tamhane, p < 0.05). A representative band of each enzyme result is set together to a β-actin band, used as a loading control. C, fa/fa rats fed modified AIM-93 diet containing control-surimi; HC, fa/fa rats fed cholesterol-enriched modified AIM-93 diet containing control-surimi; HG, fa/fa rats fed cholesterol-enriched modified AIM-93 diet containing glucomannan-surimi; HGS, fa/fa rats fed cholesterol-enriched modified AIM-93 diet containing glucomannan-surimi plus spirulina
HG reduced liver TNF-α (p < 0.05) while HGS reduced liver iNOS and TNF-α levels (all p < 0.05) with respect to the HC diet. In comparison with HG diet, HGS diet improved iNOS and the inflammation index but increased liver TNF-α levels (p < 0.05). Plasma TNF-α values were higher in HG than in HC rats (p < 0.05). However, HGS diet significantly reduced plasma TNF-α vs. all other tested diets (at least p < 0.05).
Discussion
Present results show for the first time that the consumption of squid surimi added with glucomannan highly arrested the negative effect of cholesterol feeding on cholesterolemia and antioxidant status in fa/fa rats, although induced negative proinflammatory effects. The inclusion of small amounts of spirulina in the glucomannan-squid surimi blocked those negative effects but enhanced the hypolipemic and antioxidant effects of glucomannan-squid surimi.
In order to control the results of the present study in the fa/fa rats fed cholesterol-enriched diets, the inclusion of a fa/fa group fed control diet in the study was obliged. Nonetheless, it has to be pointed out that these results were also included in other parallel study testing the effect of glucomannan and and/or glucomannan plus spirulina in the frame of non-added cholesterol diets where squid surimis were tested [33].
Body and liver weight, fat, and cholesterol
According to a previous study from Aguirre et al. [2], all fa/fa rats became obese. Hepatomegalia and steatosis have been previously observed in fa/fa rats suffering from metabolic syndrome [8]. Results reveal that dietary cholesterol reduced body weight but increased liver weight and hepatosomatic index and this organ fat and cholesterol contents, suggesting that a high cholesterol dietary enrichment aggravates liver enlargement in this animal model already fed with a high saturated fat diet. Results agree with previous reports in Wistar rats fed semisynthetic cholesterol-enriched diets [4, 28].
Glucomannan addition to restructured squid surimi diet added with hypercholesterolemic agent reduced liver weight and fat and the hepatosomatic index in comparison to the HC diet. Thus, these results can be attributed to the dietary inclusion of konjac fiber. However, spirulina addition decreased liver cholesterol, suggesting that some ingredients of spirulina (e.g., omega-3 fatty acids, polyphenols) exert this extra effect. We are far from knowing the precise mechanism involved.
Plasma lipids and CYP7A1 expression
The dyslipemic profile of the control group was similar to the one reported by Aguirre et al. [2]. This effect could be ascribed, at least partially, to the ability of glucomannan to create a matrix which partially blocks fat, cholesterol and bile acid absorption [11].
CYP7A1 is a hemo enzyme of the cytochrome P450 protein family which helps to remove cholesterol from the liver via bile acid, generating free radicals [29]. Glucomannan addition increased CYP7A1 expression, helping to improve cholesterol excretion from the liver. Spirulina inclusion did not display extra effects on CYP7A1 expression, although in previous studies on hypercholesterolemic rats fed algae, this expression were found to increase [28].
Total, reduced, and oxidized glutathione levels
The present results clearly suggest a decline of the glutathione status, given by the redox index, in comparison to that found in Wistar rats fed cholesterol enriched diets [28]. However, the redox index was higher when glucomannan was added to cholesterol enriched diet. Similar tendencies were observes in fa/fa rats fed non-added cholesterol diets containing squid surimis [33]. Fiber is known to act as an antioxidant [17]; thus suggesting the ability of glucomannan to scavenge free radicals.
Antioxidant enzyme activities, levels, and expressions
A comparison of these activities with previous results in Wistar rats shows that C and HC rats in the present study displayed similar values of SOD but four times higher Se-GPx values and six to eight times lower CAT values in rats fed with and without added cholesterol than in Wistar rats [4, 28], suggesting antioxidant status impairment in fa/fa rats, as previously discussed for GSH and GSSG.
The tremendous increase in MnSOD expression together with the 73 % increase in SOD activity, was not observed in a parallel study performed in non-cholesterol fed-fa/fa rats [33], suggesting that cholesterol feeding and/or cholesterolemia regulation fueled a process linked to O2 − production and elimination in HC rats. The CYP7A1 enzyme implies O2 − production [29], which would explain the observed increase in SOD. However, the SOD increase was coupled with an increase of total GPx (40 %, 15.26 vs. 19.14 mmol NADPH/min/mg protein) but not of CAT. In fact, the CAT/GPx activity ratio was reduced almost to the half in HC rats vs. C rats. We are far from understanding the mechanism involved. A decrease in CAT, together with the decrease in NADPH needed for a normal GSSG/GSH pathway, has been reported in diabetes [34]. Preliminary data suggest that HC rats presented higher insulinemia than C rats (18.57 vs. 10.81 μ UI/mL; p = 0.017); that would explain, at least in part, data on the redox index, that in turn appears as a consequence of the tendency of GPx activity to increase (Tables 2 and 3, Fig. 1). Nonetheless, there would have to be some modulations in the expressions and levels of GPx and GR to avoid excess conversion of GSH to GSSG. In fact, the ratio of GPx/GR expressions was lower in HC vs. C animals (0.31 vs. 1, respectively).
HG vs. HC diet reduced SOD activity (Table 3) suggesting that less ROS (O2 −) is produced. In fact, the MnSOD expression was reduced considerably (Table 3). The increase in GPx expression appears to be linked to H2O2 elimination. Here, again, no clear explanation is available, but the increase in CYP7A1 expression and the lower cholesterol levels of HG with respect to HC animals, could at least partially explain those results. The redox index improvement in HG vs. HC rats appears to be related to the decrease in GPx activity (Table 3). Preliminary data on these fa/fa rats suggest that insulinemia was lower (21 %) in HG than in HC positively affecting GSSG/GSH pathway as reported by Winiarska et al. [34].
The addition of spirulina to HG diet induced important changes. GPx and MnSOD expression decreased. Levels of GR and GPx also diminished, suggesting that the GSH ↔ GSSG pathway was less active. Spirulina contains bioactive compounds of recognized antioxidant activity [23], explaining the need for less antioxidant enzymes. It also seems possible that spirulina or its ingredients modulate the gene expression of these antioxidant enzymes. It can be speculated again that the relationship of the GSH/GSSG pathway and insulinemia, as HGS rats presented much lower insulinemia than HC rats (5.75 vs. 18.57 μU/mL; p < 0.001).
Inflammation biomarkers
HC diet ingestion increased both eNOS and iNOS expressions keeping stable the inflammation index with respect to the C diet. The consumption of glucomannan in squid surimi did not modify thus parameters suggesting that this fermentable fiber did not affect NOS system in our experimental conditions. However, glucomannan plus spirulina largely decreased the iNOS expression. Spirulina contains C-phycocyanin that present anti-inflammatory effects [19]. The anti-inflammatory effects of glucomannan plus spirulina were also observed in fa/fa rats fed squid surimis in the frame of non-added cholesterol diets [33].
C rats presented very high plasma TNF-α values, approximately 3.4-times higher than reported by Plaza-Díaz et al. [25] in obese fa/fa rats, suggesting considerable inflammation caused by the high dietary consumption of saturated fat in this sensitive model. These data suggest that cholesterol feeding further increased the liver inflammation present in fa/fa rats, although there seems to have been a compensatory effect from activation of nitric oxide production. Mells et al. [22] reported increased TNF-α on a high-fat, high-cholesterol fed metabolic syndrome murine model, suggesting a clear relationship between cholesterol feeding and liver inflammation.
Plasma TNF-α values were augmented by cholesterol feeding in the glucomannan group. However, HGS diet significantly reduced plasma TNF-α, suggesting anti-inflammatory effects of spirulina. We are far from having an understanding of the mechanisms involved in differences between liver and plasma levels. Raju and Bird [26] proposed that increased levels of plasma TNF-α are in part due to its rapid release from other tissues to the vascular bed, including that released from the fatty liver. Thus, the increase in plasma could be due to higher liver TNF-α release induced by an inflammatory reaction. Glucomannan has been posited as a cause of jaundice and increased transaminase counts after the exclusion of other causes of liver injury such as alcohol consumption [10]. Spirulina contains omega-3 polyunsaturated fatty acids and C-phycocyanin. The former have anti-inflammatory effects [19], and the structure of the latter is very similar to biliverdin, a free radical scavenger and NADPH oxidase inhibitor [35], which would at least partially explain the present results.
Some potential limitations of the present paper are as follows: (a) only male growth fa/fa rats were used, (b) the study was performed with only one level of glucomannan or glucomannan plus spirulina added to squid surimi, and( c) only one dose of hypercholesterolemic agent was tested. Future studies should assess potential benefits of different levels of glucomannan/and glucomannan plus spirulina-squid surimis and in an ampler age range of fa/fa rats and their possible extrapolation to obese and dyslipidemic humans.
Conclusions
Adding cholesterol to a high-energy, high-saturated-fat, squid-surimi diet increased cholesterolemia in fa/fa rats, leading to antioxidant status impairment. Glucomannan addition to a cholesterol-enriched squid-surimi diet produced strongly hypolipemic effects, improving antioxidant status but inducing proinflammatory effects. The inclusion of spirulina maintained the hypolipemic effects, enhanced the antioxidant benefits, and partially blocked the negative proinflammatory effects induced by glucomannan. More studies are needed to understand the effects induced by HG and HGS diets in the glutathione and NOS systems, and hence to ascertain the utility of including different doses of these ingredients in the diet to determine the optimal dosage for functional food design and to avoid potential liver damage.
Abbreviations
- CAT:
-
Catalase
- eNOS:
-
Endothelial nitric oxide synthase
- GPx:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- iNOS:
-
Inducible nitric oxide synthase
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TNF-α:
-
Tumor necrosis factor alpha
References
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126
Aguirre L, Hijona E, Macarulla MT, Gracia A, Larrechi I, Bujanda L, Hijona L, Portillo MP (2013) Several statins increase body and liver fat accumulation in a model of metabolic syndrome. J Physiol Pharmacol 64:281–288
Arai S, Osawa T, Ohigashi H, Yoshikawa M, Kaminogawa S, Watanabe M, Ogawa T, Okubo K, Watanabe S, Nishino H, Shinohara K, Esashi T, Hirahara T (2001) A mainstay of functional food science in Japan: history, present status, and future outlook. Biosci Biotech Bioch 65:1–13
Bocanegra A, Benedí J, Sánchez-Muniz FJ (2006) Differential effects of konbu and nori seaweed dietary supplementation on liver glutathione status in normo- and hypercholesterolaemic growing rats. Br J Nutr 95:696–702
Bondia-Pons I, Ryan L, Martínez JA (2012) Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem 68:701–711
Borderías A, Sánchez-Alonso I, Pérez-Mateos M (2005) New applications of fibres in foods: addition to fishery products. Trends Food Sci Tech 16:458–465
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brockman D, Chen X, Gallaher D (2012) Hydroxypropyl methylcellulose, a viscous soluble fiber, reduces insulin resistance and decreases fatty liver in Zucker diabetic fatty rats. Nutr Metab 9:100
Codoñer-Franch P, Valls-Bellés V, Arilla-Codoñer Á, Alonso-Iglesias E (2011) Oxidant mechanisms in childhood obesity: the link between inflammation and oxidative stress. Transl Res 158:369–384
Fernández-Villaverde A, Benlloch S, Berenguer M, Miguel-Rayón J, Pina R, Berenguer J (2004) Acute hepatitis of cholestatic type possibly associated with the use of glucomannan (Amorphophalus konjac). J Hepatol 41:1061–1067
González-Canga A, Fernández-Martínez N, Sahagún AM, García-Vieitez JJ, Diez-Liébana MJ, Calle-Pardo AP, Castro-Robles LJ, Sierra-Vega M (2004) Glucomanano: propiedades y aplicaciones terapéuticas. Nutr Hosp 19:45–50
González-Torres L, Churruca I, Schultz Moreira AR, Bastida S, Benedí J, Portillo MP, Sánchez-Muniz FJ (2012) Effects of restructured pork containing Himanthalia elongata on adipose tissue lipogenic and lipolytic enzyme expression of normo- and hypercholesterolemic rats. J Nutrigenet Nutrigenomics 5:158–167
Hissin P, Hill R (1976) Fluorimetric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226
Irizar A, Barnett CR, Flatt PR, Ioannides C (1995) Defective expression of cytochrome P450 proteins in the liver of the genetically obese Zucker rat. Eur J Pharmacol 293:385–393
Kris-Etherton P, Harris W, Appel LJ (2003) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler Thromb Vasc Biol 23:20–30
Lawrence R, Burk R (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958
Lecumberri E, Mateos R, Ramos S, Alia M, Rupérez P, Goya L, Izquierdo-Pulido M, Bravo L (2006) Characterization of cocoa fiber and its effect on the antioxidant capacity of serum in rats. Nutr Hosp 21:622–628
Livak K, Schmittgen T (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Lorente-Cebrián S, Costa AG, Navas-Carretero S, Zabala M, Laiglesia LM, Martínez JA, Moreno-Aliaga MJ (2015) An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J Physiol Biochem 71:341–349
Marklund SL (1985) Pyrogallol oxidation. In: Greenwald R (ed) Handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 243–247
Martínez JA (2006) Mitochondrial oxidative stress and inflammation: an slalom to obesity and insulin resistance. J Physiol Biochem 62:303–306
Mells JE, Fu PP, Kumar P, Smith T, Karpen SJ, Anania FA (2015) Saturated fat and cholesterol are critical to inducing murine metabolic syndrome with robust nonalcoholic steatohepatitis. J Nutr Biochem 26:285–292
Nagaoka S, Shimizu K, Kaneko H, Shibayama F, Morikawa K, Kanamaru Y, Otsuka A, Hirahashi T, Kato T (2005) A novel protein C-phycocyanin plays a crucial role in the hypocholesterolemic action of Spirulina platensis concentrate in rats. J Nutr 135:2425–2430
Paglia D, Valentine W (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Plaza-Diaz J, Gomez-Llorente C, Abadia-Molina F, Saez-Lara MJ, Campaña-Martin L, Muñoz-Quezada S, Romero F, Gil A, Fontana L (2014) Effects of Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillusrhamnosus CNCM I-4036 on hepatic steatosis in Zucker rats. PLoS One 9:e98401
Raju J, Bird RP (2006) Alleviation of hepatic steatosis accompanied by modulation of plasma and liver TNF-a levels by Trigonella foenum graecum (fenugreek) seeds in Zucker obese (fa/fa) rats. Int J Obes 30:1298–1307
Sánchez-Muniz FJ, Bastida S (2008) Do not use the Friedewald formula to calculate LDL-cholesterol in hypercholesterolaemic rats. Eur J Lipid Sci Technol 110:295–301
Schultz-Moreira A, Benedí J, González-Torres L, Olivero-David R, Bastida S, Sánchez-Reus MI, González-Muñoz MJ, Sánchez-Muniz FJ (2011) Effects of diet enriched with restructured meats, containing Himanthalia elongata, on hypercholesterolaemic induction, CYP7A1 expression and antioxidant enzyme activity and expression in growing rats. Food Chem 129:1623–1630
Shinkyo R, Guengerich F (2011) Cytochrome P450 7A1 cholesterol 7alpha-hydroxylation: individual reaction steps in the catalytic cycle and rate-limiting ferric iron reduction. J Biol Chem 286:4632–4643
Stokes KY, Cooper D, Tailor A, Granger DN (2002) Hypercholesterolemia promotes inflammation and microvascular dysfunction: role of nitric oxide and superoxide. Free Rad Biol Med 33:1026–1036
Stuehr D (1999) Mammalian nitric oxide synthases. Biochim Biophys Acta 1411:217–230
Tousoulis D, Kampoli A, Tentolouris C, Papageorgiou N, Stefanadis C (2012) The role of nitric oxide on endothelial function. Curr Vasc Pharmacol 10:4–18
Vázquez-Velasco M, González-Torres L, López-Gasco P, Bastida S, Benedí J, Sánchez-Reus MI, González-Muñoz MJ, Sánchez-Muniz FJ (2014) Liver oxidation and inflammation in fa/fa rats fed glucomannan/spirulina-surimi. Food Chem 159:215–221
Winiarska K, Focht D, Sierakowski B, Lewandowski K, Orlowska M, Usarek M (2014) NADPH oxidase inhibitor, apocynin, improves renal glutathione status in Zucker diabetic fatty rats: a comparison with melatonin. Chem Biol Interact 218:12–19
Zheng J, Inoguchi T, Sasaki S, Maeda Y, McCarty MF, Fujii M, Ikeda N, Kobayashi K, Sonoda N, Takayanagi R (2013) Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am J Physiol Regul Integr Comp Physiol 304:110–120
Acknowledgments
The present study was supported by the Spanish projects AGL-2011-29644-C02-02, AGL-2008-04892-C03-02 and Consolider-Ingenio 2010 project # CSD2007-00016. We gratefully acknowledge the foreign fellowship for graduate studies granted by the Consejo Nacional de Ciencia y Tecnología (CONACYT) of México to Laura González-Torres.
Author’s contributions
All authors have significantly contributed to the paper and agree with the present version of the manuscript. FJ S-M is the corresponding author and guarantor of the paper, M V-V has contributed to the study design, data discussion, and writing of the paper, L G-T and P L-G have contributed to the data acquisition and analysis and writing of the paper. S B, J B, and MJ G-M have contributed to data discussion and have made a critical review of the paper.
Compliance with ethical standards
The present study was approved by the Spanish Science and Technology Advisory Committee (project AGL-2008-04892-C03-02) and by an ethics committee of the Universidad Complutense de Madrid (Spain). All experiments were performed in compliance with Directive 86/609/EEC of November 24, 1986 for the protection of scientific research animals.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Miguel Vázquez-Velasco and Laura González-Torres are the first authors of this study.
Rights and permissions
About this article
Cite this article
Vázquez-Velasco, M., González-Torres, L., López-Gasco, P. et al. Effects of glucomannan/spirulina-surimi on liver oxidation and inflammation in Zucker rats fed atherogenic diets. J Physiol Biochem 71, 611–622 (2015). https://doi.org/10.1007/s13105-015-0425-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-015-0425-9