Abstract
Cerebral small vessel disease (cSVD) refers to the age-dependent pathological processes involving the brain small vessels and leading to vascular cognitive impairment, intracerebral hemorrhage, and acute lacunar ischemic stroke. Despite the significant public health burden of cSVD, disease-specific therapeutics remain unavailable due to the incomplete understanding of the underlying pathophysiological mechanisms. Recent advances in neuroimaging acquisition and processing capabilities as well as findings from cSVD animal models have revealed critical roles of several age-dependent processes in cSVD pathogenesis including arterial stiffness, vascular oxidative stress, low-grade systemic inflammation, gut dysbiosis, and increased salt intake. These factors interact to cause a state of endothelial cell dysfunction impairing cerebral blood flow regulation and breaking the blood brain barrier. Neuroinflammation follows resulting in neuronal injury and cSVD clinical manifestations. Impairment of the cerebral waste clearance through the glymphatic system is another potential process that has been recently highlighted contributing to the cognitive decline. This review details these mechanisms and attempts to explain their complex interactions. In addition, the relevant knowledge gaps in cSVD mechanistic understanding are identified and a systematic approach to future translational and early phase clinical research is proposed in order to reveal new cSVD mechanisms and develop disease-specific therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral small vessel disease (cSVD) encompasses the various age-dependent pathological processes of the brain small vessels leading to vascular cognitive impairment (VCI), intracerebral hemorrhage (ICH), and acute lacunar ischemic stroke [1]. Collectively, these diseases result in a major public health burden [2, 3]. The typical brain MRI findings of cSVD include white matter hyperintensity (WMH), lacunes, perivascular spaces (PVS), cerebral microbleeds, and recent small subcortical infarcts (Fig. 1) [4].
Neuroimaging features of cerebral small vessel disease according to STandards for ReportIng Vascular changes on nEuroimaging 1 (STRIVE-1) criteria. The brain MRI manifestations of cerebral small vessel disease include lacune, recent small subcortical infarct, white matter hyperintensity of presumed vascular origin, perivascular spaces, cerebral microbleed, and brain atrophy. CSF cerebrospinal fluid, DWI diffusion weighted imaging, FLAIR fluid-attenuated inversion recovery, MRI magnetic resonance imaging, SWI susceptibility weighted imaging
The spectrum of cSVD manifestations, however, continues to evolve over the years by including additional features such as cortical cerebral microinfarct or focusing on the quantitative measurements of brain structure or function and the summary scores of cSVD burden [5]. Despite the significant burden of cSVD, it remains without specific therapies due to the incomplete understanding of the underlying pathophysiological mechanisms. This review summarizes the current state of knowledge of cSVD mechanisms and attempts to explain their complex interactions. It also provides an approach to future research aiming to address the knowledge gaps of cSVD mechanisms and develop future therapeutics.
Classification
cSVD is classified into 6 types (Table 1) [1, 6,7,8]. This review focuses on the mechanisms of type I (arteriosclerosis) which is the most common form of cSVD [1]. Histopathologically, type I cSVD manifests by the loss of the vascular smooth muscle cells (VSMCs) in the medial vascular wall layer which are replaced by deposition of glass-like material (fibrohyalinosis) as well as microvascular necrosis (fibrinoid necrosis). These changes are observed primarily in the white matter (WM) arterioles [9]. Other histopathological changes include microatheromas (distal atherosclerosis), microaneurysms (dilated vessels), and microvascular rarefaction [1, 10, 11]. In addition, remodeling of the vascular wall with or without thickening occurs due to the rearrangement of the VSMCs organization, increase in their volume, or proliferation [9]. In association with the vascular changes, the WM exhibits areas of pallor (demyelination), cavitation with lacunar formation, small vessel rupture with red blood cell extravasation forming microbleeds, and PVS enlargement [4, 12].
The Cerebral Circulation and the Cerebral Small Vessels
The major intracranial arteries form a highly anastomotic vascular network on the brain surface (the pial arteries) from which the perforating arterioles originate (Fig. 2A, B) [13, 14]. When the perforating arterioles enter the brain, they are surrounded by the PVS that separates the vessel basement membrane from the astrocytic processes [13]. The PVS disappears when these vessels are smaller, and the astrocytic processes become in direct contact with the vessel basal lamina [13]. At this level, the endothelial cells (ECs) are encircled with a single layer of VSMCs that become discontinuous, and they are replaced with pericytes as the vessels transition to capillaries [13].
The cerebrovascular circulation and white matter perfusion. The internal carotid and basilar arteries combine at the base of the brain to form the circle of Willis from which the major intracranial arteries perfusing the brain originate (A). The lenticulostriate perforators perfuse the basal ganglia and its surrounding white matter and they originate from the middle cerebral artery at almost a vertical angle and are considered terminal vessels (B). The branches of the middle cerebral artery form the pial arteriolar network from which the pial perforators originate to perfuse the subcortical white matter in a centripetal vascularization pattern. Those vessels have extensive network of collaterals. The adjacent white matter to the ventricle (periventricular white matter) receives some of its perfusion from the choroidal arteries in a centrifugal vascularization pattern (B). ACA anterior cerebral artery, AICA anterior inferior cerebellar artery, BA basilar artery, ICA internal carotid artery, LV lateral ventricle, MCA middle cerebral artery, PCA posterior cerebral artery, SCA superior cerebellar artery, VA vertebral artery. The figure was created with Motifolio Toolkit (Motifolio, Inc, Ellicott City, MD)
The special histological and anatomical properties of the cerebral small vessels make them prone to the pathologies of cSVD. For example, the lenticulostriate perforators originate from the middle cerebral artery (MCA) or the circle of Willis at almost a vertical angle and there are little anastomoses among them making them susceptible to the effects of systemic hypertension. This explains the development of lacunes in the medial brain structures perfused by the lenticulostriate perforators [15]. Another important observation is the pressure differences within the cerebral circulation which are created by the cerebral artery branching patterns causing two arterial pressure systems which is referred to as an ambibaric brain model [16]. While the peripheral arteries on the brain surface follow a lower pressure system, the ones at the brain base which are in closer proximity to the larger vessels are rather under higher pressure [16]. This can also be predicted through mathematical modeling [17]. Knowledge of these different pressure systems may explain the deleterious effects of the elevated and low blood pressure (BP) in cSVD pathogenesis necessitating an individualized approach to BP control.
The WM vascularization has also roles in cSVD pathogenesis [18]. Historically, two vascularization models of the WM are described, centripetal and centrifugal models. In the centripetal one, the WM receives its blood supply from the dense pial arterial network in the periphery or the lenticulostriate perforators. In the centrifugal model, the WM receives its vascularization in addition from the choroidal arteries located in the lateral ventricles that perfuse that adjacent periventricular WM. This organization creates a WM watershed zone between the two vascularization systems which is susceptible to ischemia [18]. A more complex vascularization can also be constructed based on the interconnecting patterns of the perfusing vessels leading to 6 different brain areas [19]. The WM vascularization role in cSVD requires further investigation.
Mechanisms of Type I cSVD
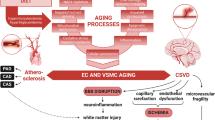
cSVD results from the interaction of several mechanisms involving cerebral microangiopathy, EC dysfunction, neuroinflammation, and BBB breakdown which will be reviewed in the next sections [20].
cSVD Risk Factors and Their Roles in cSVD Animal Models
cSVD exhibits a strong age dependency [21,22,23] since its prevalence increases with age reaching almost 100% in subjects who are older than 80 years [21]. In addition to age, other cardiovascular risk factors including hypertension, diabetes, hypercholesterolemia, and smoking have been all associated with cSVD [24]. Among these risk factors, hypertension has the strongest association [24]. Several measurements of BP including the systolic BP (SBP), diastolic BP (DBP), and the variability of BP were all significantly associated with cSVD [25, 26]. Hypertension is also associated with microstructural WM injury in healthy individuals [27, 28]. Among the environmental risk factors, increased salt intake has mostly been associated with cSVD [29, 30]. The genetic risk factors of cSVD are not entirely known. However, several genes causing the hereditary forms of cSVD and few loci associated with sporadic cSVD have been identified in the genome-wide association studies (GWAS) and their roles will be discussed in the following sections [31].
Exposure of animals to these risk factors, in particular hypertension, has been utilized to model many relevant features of cSVD. The spontaneously hypertensive rat stroke-prone (SHRSP) has been mainly used with or without additional dietary salt administration for this purpose [32]. The SHRSP is a genetic model for severe hypertension, and it develops several histopathological features of cSVD as it ages including BBB breakdown, microbleeds, vascular wall thickening, and enlargement of the PVS [33,34,35]. When additional salt is added to the diet and drinking water (Japanese modified diet), the appearance of cSVD lesions is accelerated [36]. The use of SHRSP has enhanced our understanding of several important mechanisms especially the role of EC dysfunction in cSVD [37]. However, the lack of demyelination in SHRSP studies has remained a critique for its utility as an optimal disease model [38]. To induce specific changes of the WM in SHRSP, unilateral carotid occlusion with Japanese modified diet was applied resulting in inflammatory changes and BBB breakdown with associated cognitive impairment which were thought to be secondary to hypoxia [39, 40]. Similarly, bilateral occlusion of the common carotid arteries (BCAO) in rats was used to induce a widespread demyelination and axonal damage that involves the optic nerves and tracts [41]. This model shows, however, acute demyelination with involvement of the optic tracts which may cloud the interpretation of the behavioral testing. Therefore, a bilateral carotid artery stenosis (BCAS) model was developed by attaching microcoils from outside the carotid arteries. In this model, WM changes and working memory deficit develop gradually 30 days following the procedure [42]. To overcome the sharp drop in CBF in the BCAS model, an ameroid constrictor device on bilateral common carotid arteries in rodents was used to more precisely replicate cerebral hypoperfusion. Results of this model show selective WM changes with significant spatial working memory impairment like the BCAS model at 28 days post-procedurally [43]. The main drawback of the hypoperfusion model is that the reduction of CBF does not appear to be an early mechanism of cSVD in human. Finally, focal injection of vasoconstrictors or fluorescent microspheres has been utilized as a model of WM lacunar strokes [44, 45]. These lacunar stroke models, however, lack the biological changes of the underlying vasculature that result in the human disease. Therefore, it is now widely accepted that there is no single model that replicates all cSVD findings due to the heterogeneity of the disease mechanisms and histopathological phenotypes in human and the inherent differences between the rodent and human brain anatomy and vasculature [32].
Large Artery Stiffness (AS) and cSVD
Large AS is the hallmark of vascular aging which alters the hemodynamics of the systemic circulation [46, 47]. The reduction of aortic elasticity in AS increases the pulse wave velocity (PWV) and decreases the aortic distension and collapse during systole and diastole, respectively (Fig. 3A, B) [48,49,50]. These changes result in an increase in SBP that far exceeds DBP with widening of the pulse pressure (PP) [46, 47, 49]. In response, the arterial wall remodels to reduce the harmful effect of the increased arterial pulsatility on the microcirculation [9]. Nevertheless, the increase in PP eventually reaches the cerebral microvasculature leading to its damage (Fig. 3A) [51].
Large artery stiffness, its mechanisms, and effects on the cerebrovascular circulation. The pulse wave originating from cardiac systole travels down the aorta and reflects backward against the arterial bifurcation points and the transition points of the compliant and resistant arterial systems to create the reflected wave (A). The reflected wave travels backward toward the heart. In arterial stiffness, the pulse wave and reflected wave combine at the end of systole to increase the systolic blood pressure and widen the pulse pressure which reaches the cerebral microcirculation causing its damage (A). The mechanisms and structural changes of the arterial wall and the changes of the aortic pressure waveform in the setting of arterial stiffness are displayed (B). DBP diastolic blood pressure, SBP systolic blood pressure. The figure was created with Motifolio Toolkit (Motifolio, Inc, Ellicott City, MD)
The harmful effect of the increased pulsatility on the cerebral microcirculation can be demonstrated in in vitro studies showing reduction of the expression of the tight junction (TJ) markers including occludin and claudin-5 in the setting of high shear stress and/or pulsatility [52]. Human studies provide evidence for the transmission of the systemic arterial pulsatility to the intracranial vessels as the increase in the MCA CBF pulsatility index is associated with higher systemic PWV [53]. In another study, the MCA pulsatility was the strongest physiological correlate of leukoaraiosis [54]. The MCA diameter was also associated with the WMH volume among subjects with elevated large AS [55]. The association of large AS with cSVD imaging markers is now well-established in epidemiological studies [56, 57]. However, the effect of large AS may not be homogenous across the brain. A recent study showed the PVWMH had greater association than the DWMH with vascular property measurements reflecting AS and vascular remodeling such as PP and hypertension response to exercise [58]. The regional differences in the WM susceptibility to the systemic hemodynamic alterations require further investigation.
The mechanisms of large AS involve structural changes of the vascular wall extracellular matrix (ECM) manifesting by reduction in the elastin/collagen ratio with subsequent increase of the intima-media thickness, hypertrophy of the VSMCs layer, and arterial wall calcifications (Fig. 3B) [47, 59]. The elastin/collagen ratio changes are mainly regulated by effects of matrix metalloproteases (MMPs) in addition to a smaller role of non-enzymatic protein glycation forming irreversible cross-links among collagen and elastin molecules.
Dysfunction of the ECs and VSMCs also contributes to AS. Circulating angiotensin II (Ang-II) causes hypertrophy and senescence of VSMCs and promotes collagen formation and vascular media remodeling. Ang-II activates NADPH oxidase (NOX) enzymes leading to superoxide production which impairs the production and decreases the bioavailability of nitric oxide (NO) leading to impairment of NO-dependent vasodilation of the arteries and increasing vascular tone [60]. Recently, the attention has shifted to the complex bidirectional communications between the cellular components and extracellular components/arterial hemodynamic changes as the cause of AS [61]. In these models, the dynamic effect of the sheer stress reaches the nuclear membrane of the VSMCs through the ECM-integrin-cytoskeletal axis complex to regulate the nucleocytoplasmic transfer of protein/RNA and gene expression leading to ECM stiffness [62].
Decreased Cerebral Perfusion: a Cause or an Effect of cSVD?
Ischemia and hypoperfusion have long been linked to cSVD pathogenesis since microangiopathy is a prominent histopathological feature [1]. Hypoperfusion is also an important mechanism for inducing WM injury in cSVD animal models [63]. However, clinical data supporting hypoperfusion in the mechanistic development of WMH have yielded conflicting results [64, 65]. In cross-sectional studies, larger WMH burden has been consistently associated with lower resting CBF. This has been demonstrated in a meta-analysis that included all the cross-sectional studies evaluating CBF and WMH [64]. It is important to note that the significance of this association decreased when removing studies that had no age-matched controls and subjects with dementia [64]. A follow-up updated meta-analysis that included the remaining studies from 2015 through 2020 confirmed the findings of the original meta-analysis by demonstrating the association of greater WMH burden with lower CBF in the cross-sectional studies [65]. CBF was also lower in WMH in comparison to the normally appearing WM [65]. In longitudinal studies, the baseline WMH volume was associated with future decline in CBF [66]. Taken together, these data suggest that WMH areas suffer from lower CBF. However, whether a decrease in CBF precedes the progression of WMH is less clear. In one large prospective study (N = 406), an association between baseline global CBF and WMH progression at 5-year follow-up was not found [67]. Another longitudinal study identified a decline in global CBF in association with PVWMH progression but not DWMH [68]. It is important to consider the limitations of the studies that measure global CBF since there are regional differences in CBF that may play roles in cSVD pathogenesis. Therefore, a small study (N = 52) analyzed the effect of CBF in the WMH penumbra which refers to the normally appearing WM surrounding WMH on brain MRI. The results indicated that low baseline CBF in the WMH penumbra was independently associated with the appearance of new PVWMH in these regions [69]. Larger-sample longitudinal studies are needed to identify the mechanistic link between CBF and cSVD.
Impairment of CBF Regulation: Can It Explain What Is Missing from Resting CBF Studies?
Regulation of CBF requires complex mechanisms to meet the continuous and rapidly changing energy demands of the neurons [70]. These mechanisms include the myogenic vascular control (autoregulation), metabolic regulation (cerebrovascular reactivity), and neurogenic mechanisms (neurovascular coupling) [70,71,72]. The neurovascular coupling (NVC) is controlled by the neurovascular unit (NVU) where the neural activity is tied to local CBF (Fig. 4A, B) [72]. Cerebrovascular reactivity (CVR) refers to the sensitivity of cerebral vasculature to changes in arterial CO2 and O2 levels (Fig. 4B) [71]. Cerebral autoregulation (CA) reflects the mechanisms that maintain constant CBF despite the changes of systemic BP which has a static component, referring to CBF changes in relation to systemic BP when the changes reach steady states, and dynamic component, referring to CBF changes in relation to fast BP changes [70].
The neurovascular unit and cerebral blood flow regulation. The neurovascular unit consists of endothelial cells that combine through the tight junctions, pericytes, cerebrovascular basement membrane, and the astrocyte end-feet (A). Cerebral blood flow regulation is maintained through mechanisms involving the neurovascular coupling, cerebral autoregulation, and cerebrovascular reactivity (B). The feedforward mechanism of neurovascular coupling is mediated by the glutamate release from neuronal activity resulting in neuronal release of NO and astrocyte release of prostaglandin leading to increase in local cerebral blood flow. The feedbackward mechanism is mediated by local increase in CO2 and decrease in pH following neuronal activity. Cerebrovascular reactivity is mediated by the release of neuronal NO through chemical stimulation. Cerebral autoregulation maintains cerebral blood flow in the setting of systemic blood pressure changes and is mediated by the myogenic control of the vessel lumen. CO2 carbon dioxide, EET epoxyeicosatrienoic acid, NO nitric oxide, O2 oxygen
As the link of CBF reduction and cSVD has not been clearly established, it is postulated that dynamic changes of CBF may lead to cSVD as in the case of adult onset cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoncephalopathy (CADASIL), a hereditary form of cSVD [73]. Measurement of CVR can be achieved through detecting amplitude changes in the blood-oxygenation-level-dependent (BOLD) signal of functional MRI per unit change in end-tidal CO2 (PETCO2) during breath-holding technique or inhaled 6% CO2 challenge [74, 75]. A systematic review involving five studies of CVR in cSVD revealed mixed findings as only two of the included studies identified an association of WMH or cSVD score with decreased CVR [76]. More recently, however, a clearer association is found. One study identified the association of reduced CVR with cSVD, higher venous pulsatility, and lower foramen magnum CSF stroke volume [77]. In another small prospective study, reduced CVR in the normally appearing WM preceded the progression of WMH [78]. Finally, vessel-clusters seen on SWI corresponding to the maximally dilated vessels in the WM approaching complete injury were associated with lower CVR in patients with cSVD [79].
Impairment of CA has been recognized in hypertension for a long time [70]. The question, however, is whether impairment of CA precedes the development of cSVD independent of hypertension. This was not the case in a small study investigating the relationship of WMH, dynamic CA, and CVR [80]. More recently, however, diffuse and sustained impairment of dynamic CA was found in patients with lacunar stroke compared to patients with territorial strokes who had localized impairment of the dynamic CA [81]. Another case-control study identified impairment of dynamic CA in cSVD patients while a larger burden of cSVD indicated worse CA [82]. The role of CBF regulation is undergoing further assessment in ongoing large prospective studies [12].
The Cerebral Capillary Networks
The cerebral capillary networks show inherent heterogeneity in their three-dimensional structures leading to heterogeneity of the resting cerebral capillary blood flow (CCBF). This heterogeneity is postulated to function as a reserve in allowing these networks’ flow to homogenize during the functional hyperemic response to the local neural activity [83]. Jespersen and Ostergaard showed through mathematical modeling that reduction of capillary transit time heterogeneity (CTTH) (i.e., flow homogenization within the capillary networks) improves the oxygen delivery to the brain tissue [84]. Their model predicted conditions where the tissue oxygen delivery is compromised due to the CCBF heterogeneity despite a higher state of CBF. We recently extended these results by demonstrating that perturbations of capillary segment diameter or length will always on average decrease the tissue oxygen level in a model of capillary networks with variability in geometric and three-dimensional structures [85]. The decrease in tissue oxygen level can reach a critical level that affects the neuronal activity [86]. A small cross-sectional study of patients with WMH provided evidence of capillary dysfunction by showing higher values of CTTH within the WMH with associated decrease in CBF [87]. Histopathological studies also show decrease in capillary density in patients with leukoaraiosis even outside the WMH areas [88, 89]. The mechanistic role of capillary dysfunction in cSVD requires further examination.
The Central Role of the NVU
The NVU components (ECs, mural cells, and astrocytic end-feet) form functionally the BBB and they harmonize to provide constant nutrient supply to the neurons, remove unwanted metabolites, enable neuroimmune trafficking, and maintain the homeostatic balance of the brain (Fig. 4A) [90]. The roles of the NVU components in cSVD will be discussed in the next sections.
The Endothelial Cells (ECs)
The ECs control the local CBF through secreting vasodilatory mediators (NO and prostacyclin) or vasoconstrictors (endothelin) in response to chemical or mechanical stimulators which constitute the endothelial-dependent vasodilatory or vasoconstrictor response [13]. The ECs also participate in forming the BBB through their TJs [13]. “ECs dysfunction” term primarily refers to the impairment of endothelium synthesis, release, and/or activity of endothelium-derived NO [91, 92]. “ECs activation” term refers to the expression of adhesion molecules such as VCAM-1 and ICAM-1 on the ECs that is induced by proinflammatory cytokines [93]. The reduction in NO synthesis in EC dysfunction, however, can also lead to VCAM-1 expression and EC activation [92]. Therefore, dysfunctional ECs exhibit a wide-range impairment of their functions with a switch toward reduced vasodilation, proinflammatory state, and prothrombotic properties [94]. EC dysfunction is considered now by many as the starting point of cSVD [12]. The dysfunctional ECs can lead to cSVD through multiple mechanisms including impairment of CBF regulation [95], breakdown of the BBB with subsequent fibrinogen leakage into the brain and neuroinflammation [96], and diminishing the flow and waste clearance in the PVS (Fig. 5) [97]. Furthermore, a direct link between the dysfunctional ECs and oligodendrocyte precursor cells has been identified in both SHRSP and human with WMH through heat shock protein 90α secretion by the dysfunctional ECs which inhibits oligodendrocyte precursor cell maturation leading to demyelination [37].
The pathophysiological processes involving the neurovascular unit and microglia in cerebral small vessel disease. Oxidative stress impairs eNOS function and uncouples it resulting in lower NO levels. Dysfunctional ECs also express VCAM and ICAM-1 facilitating leukocyte adhesion. They also show impairment of their tight junctions leading to fibrinogen infiltration. Dysfunctional ECs secrete HSP-90α affecting ODC precursors and resulting in demyelination. These processes as well as hypoxia activate astrocytes to secrete MMP-2, pericytes which secrete MMP-3, and microglia which produce MMP-9. MMP-3 activates inflammatory MMP-9 leading to neuroinflammation, ECM degradation, BM destruction, demyelination, and neuronal injury. BM basement membrane, ECM extracellular matrix, ECs endothelial cells, eNOS endothelial nitric oxide synthase, HSP-90α heat shock protein 90α, ICAM-1 intercellular adhesion molecule-1, MMP matrix metalloprotease, NO nitric oxide, ODC oligodendrocyte, ROS reactive oxygen species, TJ tight junction, VCAM vascular cell adhesion molecule. The figure was created with Motifolio Toolkit (Motifolio, Inc, Ellicott City, MD)
There is currently abundant evidence supporting the existence of EC dysfunction in cSVD. Hypertensive animal models of cSVD, such as SHRSP, show EC dysfunction in the brain manifesting by reduction in NO levels or impairment of eNOS even before the onset of hypertension [37, 98]. The brain of SHRSP also shows breakdown of the BBB with reduction of claudin-5 level, a component of the EC TJs [35, 99]. Hypoperfusion models of cSVD show signs of EC activation including overexpression of ICAM-1 and VCAM-1 leading to glial cell activation [100]. In human, data supporting dysfunction of the ECs and NVU can be inferred by studying the BBB function. Using dynamic contrast enhanced imaging, diffuse BBB breakdown was identified in the WM in patients with lacunar stroke and WMH [101, 102]. BBB leakage can be seen in areas of WMH, the normal-appearing WM, and the grey matter suggesting that this finding is universal in the brain and potentially predates the development of WMH [102, 103]. In another study, BBB leakage increased in proximity of the WMH lesions suggesting its role in WMH expansion [104]. Another body of evidence for EC dysfunction in patients with cSVD comes from studies examining cSVD association with several circulating markers of EC dysfunction and activation [105]. In a cohort of asymptomatic patients, serum ICAM-1 level was associated with PVWMH and DWMH independent of cardiovascular risk factors [106]. Higher levels of circulating MMP-9 were found in stroke-free subjects with WMH vs those without WMH [107]. Longitudinally, ICAM-1 levels were associated with the progression of WMH at 3- and 6-year follow-up in a cohort of subjects free of stroke and dementia [108]. These circulating biomarkers’ studies do not provide direct evidence of cerebrovascular EC dysfunction versus supporting a state of systemic EC dysfunction in the setting of cSVD.
The mechanisms of EC dysfunction in cSVD are not fully understood. Age and cardiovascular risk factors, especially hypertension, are associated with systemic vascular oxidative stress with production of reactive oxygen species (ROS) [109, 110]. ROS may directly interact with NO reducing its bioavailability (Fig. 5). Additionally, by oxidizing the eNOS cofactor Tetrahydrobiopterin (BH4), ROS may uncouple eNOS resulting in the paradoxical production of the ROS superoxide anion instead of NO. ROS also impair the responsiveness of the endothelial-dependent vasodilators through multiple interacting pathways [109]. The sources of ROS in the vascular wall are several including NOX, the mitochondrial respiratory chain, xanthine oxidase, and the uncoupled eNOS [111]. The perivascular macrophages (PVMs) have gained recent attention as the main source of superoxide in the brain in hypertension [112]. In a model of Ang-II induced hypertension, the entry of Ang-II through the broken BBB to the brain results in the production of ROS through NOX2 secondary to the activation of type I Ang-II receptor (AT1R) in the PVM. Mechanistically, depletion of PVM or AT1R counteracts the harmful cerebrovascular effect of Ang-II [112]. In another work, PVM was necessary for the full expression of BBB disruption while EC AT1R initiated the BBB disruption seen in hypertension [113]. This was evident as the depletion of brain PVMs or AT1R deletions in PVMs attenuated, but did not abolish, the BBB breakdown in the cerebrovascular arterioles. However, AT1R deletion in cerebral ECs prevented BBB disruption completely [113].
Dysregulation of systemic hemodynamics and systemic inflammation are additional contributors to EC dysfunction [114]. As preceded, the increase in arterial pulsatility in the setting of large AS can be transmitted to the cerebral circulation leading to EC injury [52, 114]. Age is also associated with chronic low-grade inflammation “inflammaging” resulting in vascular oxidative stress and impairment of EC-dependent vascular tone with associated increase in vascular permeability and procoagulant activity [114,115,116,117]. Excessive dietary salt intake, an environmental cSVD risk factor, can cause cerebrovascular EC dysfunction through the expansion of TH17 in the small intestine with marked increase in plasma interleukin-17 (IL-17) which in turn promotes the inhibition of phosphorylation of eNOS and reduce NO production in the cerebral ECs [118]. The cognitive impairment induced by excessive salt intake is mediated by hyperphosphorylation of tau and can be prevented by restoring endothelial NO production providing a mechanistic link between dietary salt, EC dysfunction, and tau pathology [119].
The Cerebrovascular Basement Membrane (BM)
The BMs are layers of complex ECM proteins providing support of the ECs and contributing to the formation of the BBB [120]. The vascular BM along the interstitial matrix and the perineuronal network of the brain parenchyma form the brain’s ECM [120].
Impairment of the BM and ECM is found in models of cSVD and VCI [121, 122]. BCAO in normotensive rats or unilateral carotid artery occlusion in SHRSP on Japanese modified diet shows disruption of the BM and TJs due to the action of MMPs [123, 124]. The hypoxia induced by carotid occlusion leads to expression of hypoxia inducible factor-1α (HIF-1α) with subsequent activation of MMP-2 in the astrocytes resulting in BM disruption and demyelination. As the hypoxia persists, the inducible MMP (MMP-3 and MMP-9) production is stimulated by cytokine (TNF-α) release leading to irreversible BBB disruption and neuroinflammation (Fig. 5) [121, 122].
Like EC dysfunction, impairment of the BM function in forming the BBB can be inferred in human with cSVD by utilizing dynamic contrast-enhanced brain MRI [102, 103]. Monogenic mutations affecting type IV collagen genes, an important component of the BM, provide additional evidence for the BM role in human cSVD [31]. More than half of the individuals with mutations in COL4A1 have cSVD lesions. Large-scale genetic population studies have also identified variants in COL4A2 gene in association with lacunar ischemic stroke and deep ICH while a meta-analysis of healthy and stroke participants identified a common variant in COL4A2 in association with WMH burden [31].
The Pericytes
The crosstalk of pericyte with the ECs and astrocytes maintains the BBB, angiogenesis, CCBF, and material clearance from the brain. The pericytes also regulate inflammation and immune cell activation in the brain [125].
The roles of pericyte in BBB breakdown and cSVD are emerging. In the BCAS model, pericyte count and coverage of the small vessels decrease reaching a nadir 3 days following carotid surgery with an increase in BBB permeability that precedes WM injury or behavioral deficits [126]. Mechanistically, ablation of pericytes in mice by diphtheria toxin administration to an inducible pericyte-specific Cre line results in acute BBB breakdown, severe loss of CBF, and rapid neuronal loss [127]. Furthermore, pericyte-deficient mice exhibit disruption of the WM microcirculation with accumulation of fibrinogen deposits and associated decrease in blood-flow triggering a loss of myelin, axons, and oligodendrocytes [128]. The negative cerebrovascular effects of APOE4 causing BBB breakdown are mediated through activation of a proinflammatory CypA-nuclear factor-ĸB-matrix-metalloproteinase-9 pathway in pericyte providing additional evidence for the pericyte role in maintaining the BBB. These vascular deficits precede the neuronal dysfunction and can initiate neurodegenerative changes [129]. The production of MMP-3 by pericytes is also important for MMP-9 activation and neuroinflammation [121, 122].
The role of pericytes in human with cSVD can be demonstrated in CADASIL where patients develop cSVD at an early age [130]. The extracellular fragment of the responsible gene, NOTCH3, accumulates at the cytoplasmic membrane of the VSMCs and pericytes leading to the disease [131]. Evidence for the role of NOTCH3 in sporadic cSVD is still lacking except for a single GWAS in healthy subjects that found an association between common variants on EFEMP1 and higher WMH load [31].
The Astrocytes
The astrocytes support the neuronal function through their roles in potassium buffering and osmotic and glutamatergic homeostasis. The astrocyte end-feet also participate in the BBB formation and NVC through the release of COX-1 metabolites [132,133,134].
Evidence of astrocytic functional impairment in cSVD models already exists. Ang-II induced impairment in NVC in mice is mediated by the increase in calcium in astrocytes which subsequently promotes vasoconstrictor over vasodilatory response to neuronal activity [135]. The increase in the glial fibrillary acid protein (GFAP) expression, a marker for reactive astrocytes [136], is consistently found in SHRSP [98, 99]. Astrocytes and microglia produce MMPs which play important roles in models of cSVD. MMP-2 and MMP-9 expression is increased in hyperhomocysteinemia-induced VCI models [137]. Similar observations are made in cerebral hypoperfusion models which show astrocyte activation through HIF-1 expression leading to MMP-2 activation and subsequent ECM alteration [121]. Inflammatory mediators secreted by activated microglia such as IL-1α or TNF-α are additional sources for astrocyte activation which are seen in neuroinflammatory disorders and yet to be determined in cSVD [138].
The Microglia and Neuroinflammation
Microglia constitute 5–10% of total brain cells and are the only true CNS parenchymal macrophages [139]. Microglia activation is a prominent feature of the neuroinflammatory process [139]. Evidence of neuroinflammation and microglia activation is consistently seen across cSVD experimental models. Iba-1, a marker of microglia, is increased in the brain of SHRSP early in life compared to age-matched normotensive Wistar-Kyoto (WKY) rat [98, 99]. Pathway mapping of the differentially expressed genes in the brain of SHRSP compared to age-matched WKY identified significant differences in the responsible pathways for glia and microglia activation [140]. In the chronic hypertension model of deoxycorticosterone acetate (DOCA)–salt-treated Wistar rats following unilateral nephrectomy, the resting perivascular microglia directly switched to the pro-inflammatory M1 state [141]. Microglia activation is also present in the models of BCAO in rats [142, 143] as well as a lacunar infarct model induced by stereotactic injection of ouabain into the striatum in the adult rats [144]. Mechanistically, pharmacological depletion of microglia using PLX5622, a highly selective CSF1R tyrosine kinase inhibitor, in Cx3Cr1 gfp/wtxThy1 yfp/0 reporter mice protects against the cognitive impairment induced by prolonged Ang-II infusion [145].
In post-mortem human studies, markers of BBB breakdown, activated microglia, and astroglia are seen in the brain of patients with cSVD [146]. In an autopsy study from the Cognitive Function and Ageing Study (CFAS), heterogeneity in the inflammatory response profile was identified within the brain in patients with WM lesions. In particular, the expression of microglia and astrocyte markers increased from the subcortical to the subventricular WM. Furthermore, WM inflammation showed a weak relationship to microvascular disease pathology and no relationship to Alzheimer’s neuropathology [147]. Recent advances in neuroimaging techniques have allowed in vivo measurement of neuroinflammation in patients with cSVD. By using 11C-PK-11195 PET imaging for microglia activation, 11C-PK-11195 binding was associated with cSVD particularly in regions typical of hypertensive arteriopathy [148]. In another study combining sporadic cSVD, CADASIL patients, and normal controls, microglial activation identified by 11C-PK11195 PET and increased BBB permeability were found in sporadic cSVD in the normal appearing WM while CADASIL patients did not have increase in BBB permeability [149].
Systemic Inflammation, Gut Dysbiosis, and cSVD
Age is closely associated with a state of chronic, low-grade, systemic, and asymptomatic inflammation that is known as “inflammaging” [115, 117]. The cellular and molecular mechanisms of inflammaging are complex, and they involve cellular senescence, immunosenescence, mitochondrial dysfunction, defective autophagy, metaflammation, and gut microbiota dysbiosis [117]. While it has not been established how inflammaging may cause cSVD, the increase in cytokine levels such as TNF-α, IL-6, or IL-1β and vascular oxidative stress may cause EC dysfunction [114,115,116,117]. Indeed, the association of systemic inflammatory markers (e.g., CRP, IL-6, fibrinogen) and cSVD is well-documented [150]. In a systematic review, longitudinal studies showed an association of elevated levels of systemic inflammatory markers at baseline and future cSVD severity and progression [150]. Furthermore, a robust association of systemic markers of vascular inflammation (e.g., homocysteine, von Willebrand factor) and cSVD was also seen [150].
The gastrointestinal microbiota is a particular source of inflammaging that has gained special attention as a cause of vascular inflammation and EC dysfunction [115, 151]. Mechanistically, lipopolysaccharides, a component of the gram-negative gut bacteria, can directly influence the ECs by initiating interrelated redox and kinase-dependent signally pathways through endothelial TLR4 activation [151]. When the neutrophils of both human and mouse are exposed to fecal bacterial extracts from a patient with cSVD, they activate with an increase in their capacity to provide IL-17A demonstrating the effect of gut dysbiosis to induce systemic inflammation in cSVD [152]. Evidence of gut dysbiosis is also found in experimental models of cSVD with mechanistic effect on hypertension and BBB function [153, 154]. When normotensive WKY at young age were gavaged with SHRSP microbiota, their SBPs were increased as they aged. While SHR gavaged with WKY microbiota tended to have lower BP than SHR gavaged with SHRSP microbiota [153]. SHRSP fostered on WKY dams had also lower SBP and improved BBB function compared to SHRSP fostered on SHRSP dams [154].
The association of dysbiosis of the oral or the gut microbiota with cSVD markers has been identified in several retrospective studies of different cohorts including stroke patients and patients without dementia or stroke history [155,156,157]. However, larger prospective studies are needed to determine the role of gut dysbiosis in cSVD.
The Glymphatic System: a New Target for cSVD
The glymphatic system utilizes a network of perivascular channels formed by the astroglial cells for the brain waste clearance [97]. The roles of the glymphatic system are being revealed in various neurological diseases [158]. Enlarged PVS have long been recognized as cSVD feature [4]. However, questions remain whether an associated impairment of the glymphatic transport exists in association with PVS. Experimentally, this was the case as solute transport in the PVS and PVS-to-tissue transfer were slower in SHRSP compared to normal rats [159]. Recent reports show progressive enlargement of PVS in SHRSP with age enhancing this hypothesis further [33, 34]. SHR also shows similar findings to SHRSP of the enlarged PVS association with impaired glymphatic clearance [160]. However, given the presence of hydrocephalus in SHR, these results should be interpreted with caution [161]. Chronic cerebral hypoperfusion model induced by BCAS in mice also shows evidence for glymphatic dysfunction with loss of AQP4 polarization [162].
Novel MRI techniques are being developed to measure the glymphatic clearance in human both invasively by using intrathecal tracer injections [163] and non-invasively by utilizing diffusion tensor image analysis along the perivascular space (DTI-ALPS) [164]. Indeed, the DTI-ALPS index was associated with cSVD markers and cognitive function in small studies of sporadic cSVD and cerebral amyloid angiopathy [165, 166]. In another study, the association of the DTI-ALPS index with cognitive function was independent of cSVD four classical markers including WMH, lacunes, microbleeds and PVS [167]. The mechanistic role of glymphatic system impairment in cSVD requires further investigation.
Conclusions and Future Recommendations
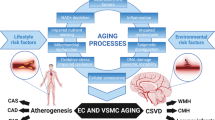
As preceded, cSVD mechanisms are complex and involve several overlapping systems including the brain, the systemic circulation, and other body organs (Fig. 6).
Overview of cSVD mechanisms. Age leads to large artery stiffness, systemic low-grade inflammation, vascular oxidative stress, and gut dysbiosis which combine to cause cerebrovascular EC dysfunction. Because of these events, CBF regulation becomes impaired with BBB breakdown leading to neuroinflammation and neuronal injury. Age also impairs waste clearance through the glymphatic system resulting in tau and amyloid-β accumulation. These pathological events eventually result in cerebral small vessel disease and vascular cognitive impairment. BBB blood brain barrier, CBF cerebral blood flow, cSVD cerebral small vessel disease, ECs endothelial cells, MRI magnetic resonance imaging, VCI vascular cognitive impairment
In the setting of advanced age, vascular oxidative stress, chronic systemic inflammation, and AS-related alterations of the systemic hemodynamics interact to cause a state of cerebrovascular EC dysfunction. The dysfunctional ECs induce the subsequent neurovascular dysfunction and neuroinflammation (Fig. 6). The dysfunctional ECs exacerbate superoxide production through the uncoupled eNOS, impair CBF regulation, and lose their BBB-protective capabilities. Hypoxia and passage of fibrinogen into the brain activate the microglia and astrocytes leading to MMP secretion and neuroinflammation. These events worsen the BBB breakdown with potential recruitment of systemic inflammatory cells, ECM damage, and neuronal injury. The astrocytic dysfunction and neuronal injury may further impair the NVC. It remains unknown whether the initial neurovascular dysfunction is sufficient alone or whether it requires the secondary neuroinflammation to induce VCI [9]. Aging also impairs the glymphatic waste clearance system leading to phosphorylated tau accumulation with additional neuronal injury (Fig. 6).
The management of covert cSVD focuses on cardiovascular risk factor modification and they are currently supported by weak quality evidence (Table 2) [168].
Prior attempts to develop disease-specific treatments have not been successful such as NO donors and cGMP-modifying agents, while others remain under investigation such as minocycline for modifying neuroinflammation and BBB breakdown [12, 169, 170]. The translational failure in bringing cSVD therapies to bedside is due to several factors, many of which are linked to the complexity of cSVD mechanisms and phenotypes. Therefore, important research questions need to be addressed to improve the mechanistic understanding of cSVD and develop new therapies (Fig. 7). First, knowledge gaps need to be addressed as identified in this review. Given the significance of EC dysfunction and BBB breakdown in cSVD, understanding of the early oxidative stress process that induces EC dysfunction and how the dysfunctional ECs lead to neuronal injury needs to be prioritized [91]. In doing so, the heterogeneity of the EC molecular composition across the brain vessels should be considered [171]. Neuroinflammation is another consistent finding in cSVD. Most of the prior cSVD research has focused on its negative consequences while recent discoveries have noted the positive roles of the inflammatory response in neuroregeneration [138, 172]. Therefore, the positive and negative roles of neuroinflammation in cSVD should be deciphered. The mechanistic contribution of the glymphatic system to cSVD is another knowledge gap, especially, whether the glymphatic system explains the overlaps of aging, cSVD, and AD [173,174,175].
A proposed paradigm for testing cerebral small vessel disease mechanisms and therapeutics. When testing new mechanistic interventions for cerebral small vessel disease (cSVD), important considerations should include the specific mechanisms for inducing cSVD in the selected model and the pre-clinical cognitive and histological phenotypes that would serve as the pre-clinical study endpoints. Testing the intervention in more than one model reflecting the different mechanisms for inducing cSVD will be favorable. In early-phase clinical studies, enriching the study sample with a homogenous group of patients is important. This can be achieved by utilizing artificial intelligence that can integrate the clinical, neuroimaging, and genomic characteristics of the sample. The identified mechanisms in exploratory early clinical studies should be confirmed by a reverse translational approach in relevant animal models. AD Alzheimer’s disease, AI artificial intelligence, cSVD cerebral small vessel disease, ECs endothelial cells
Following identifying the relevant knowledge gaps, testing mechanistic and therapeutic interventions requires a systematic approach to pre-clinical models of cSVD (Fig. 7). Utilizing more than one translational model reflecting the different mechanisms for inducing cSVD is needed. The cognitive and histological endpoints of cSVD in pre-clinical studies also need to carefully consider the underlying neurochemical profile of the utilized model [176]. Testing of therapeutic and mechanistic interventions in subsequent early-phase human studies should utilize advanced neuroimaging, genomics, and artificial intelligence (AI) techniques to enrich the study sample with a homogenous population (Fig. 7). An important step toward this aim has been recently made by a group of international experts [177]. Endpoints and treatment goals of cSVD clinical studies also need to closely align with the specific mechanism that is targeted. An example is utilizing large AS as a treatment target in cSVD studies [178, 179]. Beyond utilizing AI for cSVD segmentation [180], it can also infer the underlying mechanisms by combining the genetic, imaging, and clinical characteristics of the studied sample [181, 182]. New mechanistic pathways identified in early exploratory human studies should also be tested in animal models through a reverse translational approach to confirm their findings.
In conclusion, a systematic approach to translational and clinical cSVD research combined with advancements in neuroimaging, genomics, and AI will move the field closer to developing specific therapeutics to improve the lives of patients with cSVD.
References
Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. https://doi.org/10.1016/S1474-4422(10)70104-6.
Shi Y, Wardlaw JM. Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc Neurol. 2016;1(3):83–92. https://doi.org/10.1136/svn-2016-000035.
Dichgans M, Leys D. Vascular cognitive impairment. Circ Res. 2017;120(3):573–91. https://doi.org/10.1161/CIRCRESAHA.116.308426.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–38. https://doi.org/10.1016/S1474-4422(13)70124-8.
Duering M, Biessels GJ, Brodtmann A, Chen C, Cordonnier C, de Leeuw FE, et al. Neuroimaging standards for research into small vessel disease-advances since 2013. Lancet Neurol. 2023; https://doi.org/10.1016/S1474-4422(23)00131-X.
Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA. Venous collagenosis and arteriolar tortuosity in leukoaraiosis. J Neurolog Sci. 2002;203-204:159–63. https://doi.org/10.1016/s0022-510x(02)00283-6.
Revesz T, Holton JL, Lashley T, Plant G, Rostagno A, Ghiso J, et al. Sporadic and familial cerebral amyloid angiopathies. Brain Pathol. 2002;12(3):343–57. https://doi.org/10.1111/j.1750-3639.2002.tb00449.x.
Sondergaard CB, Nielsen JE, Hansen CK, Christensen H. Hereditary cerebral small vessel disease and stroke. Clin Neurol Neurosurg. 2017;155:45–57. https://doi.org/10.1016/j.clineuro.2017.02.015.
Santisteban MM, Iadecola C, Carnevale D. Hypertension, neurovascular dysfunction, and cognitive impairment. Hypertension. 2022; https://doi.org/10.1161/HYPERTENSIONAHA.122.18085.
Craggs LJ, Yamamoto Y, Deramecourt V, Kalaria RN. Microvascular pathology and morphometrics of sporadic and hereditary small vessel diseases of the brain. Brain Pathol. 2014;24(5):495–509. https://doi.org/10.1111/bpa.12177.
Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T. Towards defining the neuropathological substrates of vascular dementia. J Neurolog Sci. 2004;226(1-2):75–80. https://doi.org/10.1016/j.jns.2004.09.019.
Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18(7):684–96. https://doi.org/10.1016/S1474-4422(19)30079-1.
Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension. Circ Res. 2019;124(7):1025–44. https://doi.org/10.1161/CIRCRESAHA.118.313260.
Chandra A, Li WA, Stone CR, Geng X, Ding Y. The cerebral circulation and cerebrovascular disease I: anatomy. Brain Circ. 2017;3(2):45–56. https://doi.org/10.4103/bc.bc_10_17.
Li Q, Yang Y, Reis C, Tao T, Li W, Li X, et al. Cerebral small vessel disease. Cell Transplant. 2018;27(12):1711–22. https://doi.org/10.1177/0963689718795148.
Hachinski V, Ostergaard L. The ambibaric brain: pathophysiological and clinical implications. Stroke. 2021;52(6):e259–e62. https://doi.org/10.1161/STROKEAHA.120.033492.
Blanco PJ, Muller LO, Spence JD. Blood pressure gradients in cerebral arteries: a clue to pathogenesis of cerebral small vessel disease. Stroke Vasc Neurol. 2017;2(3):108–17. https://doi.org/10.1136/svn-2017-000087.
Smirnov M, Destrieux C, Maldonado IL. Cerebral white matter vasculature: still uncharted? Brain : a J Neurol. 2021;144(12):3561–75. https://doi.org/10.1093/brain/awab273.
Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR Am J Neuroradiol. 1990;11(3):431–9.
Ihara M, Yamamoto Y. Emerging evidence for pathogenesis of sporadic cerebral small vessel disease. Stroke. 2016;47(2):554–60. https://doi.org/10.1161/STROKEAHA.115.009627.
de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70(1):9–14. https://doi.org/10.1136/jnnp.70.1.9.
Hilal S, Mok V, Youn YC, Wong A, Ikram MK, Chen CL. Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry. 2017;88(8):669–74. https://doi.org/10.1136/jnnp-2016-315324.
Simoni M, Li L, Paul NL, Gruter BE, Schulz UG, Kuker W, et al. Age- and sex-specific rates of leukoaraiosis in TIA and stroke patients: population-based study. Neurology. 2012;79(12):1215–22. https://doi.org/10.1212/WNL.0b013e31826b951e.
Wang Z, Chen Q, Chen J, Yang N, Zheng K. Risk factors of cerebral small vessel disease: a systematic review and meta-analysis. Medicine (Baltimore). 2021;100(51):e28229. https://doi.org/10.1097/MD.0000000000028229.
Wilkinson I, Webb AJS. Consistency of associations of systolic and diastolic blood pressure with white matter hyperintensities: a meta-analysis. Int J stroke : official J Int Stroke Soc. 2022;17(3):291–8. https://doi.org/10.1177/17474930211043364.
Zhang B, Huo Y, Yang Z, Lv H, Wang Y, Feng J, et al. Day to day blood pressure variability associated with cerebral arterial dilation and white matter hyperintensity. Hypertension. 2022;79(7):1455–65. https://doi.org/10.1161/HYPERTENSIONAHA.122.19269.
Hannawi Y, Yanek LR, Kral BG, Vaidya D, Becker LC, Becker DM, et al. Hypertension is associated with white matter disruption in apparently healthy middle-aged individuals. AJNR Am J Neuroradiol. 2018;39(12):2243–8. https://doi.org/10.3174/ajnr.A5871.
Hannawi Y, Yanek LR, Kral BG, Becker LC, Vaidya D, Haughey NJ, et al. White matter injury is associated with reduced manual dexterity and elevated serum ceramides in subjects with cerebral small vessel disease. Cerebrovasc Dis. 2021a;50(1):100–7. https://doi.org/10.1159/000511937.
Makin SDJ, Mubki GF, Doubal FN, Shuler K, Staals J, Dennis MS, et al. Small vessel disease and dietary salt intake: cross-sectional study and systematic review. J Stroke Cerebrovasc Dis : the official J Nat Stroke Assoc. 2017;26(12):3020–8. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.08.004.
Heye AK, Thrippleton MJ, Chappell FM, Hernandez Mdel C, Armitage PA, Makin SD, et al. Blood pressure and sodium: association with MRI markers in cerebral small vessel disease. J Cereb Blood Flow Metab. 2016;36(1):264–74. https://doi.org/10.1038/jcbfm.2015.64.
Marini S, Anderson CD, Rosand J. Genetics of cerebral small vessel disease. Stroke. 2020;51(1):12–20. https://doi.org/10.1161/STROKEAHA.119.024151.
Bailey EL, McCulloch J, Sudlow C, Wardlaw JM. Potential animal models of lacunar stroke: a systematic review. Stroke. 2009;40(6):e451–8. https://doi.org/10.1161/STROKEAHA.108.528430.
Monte B, Constantinou S, Koundal S, Lee H, Dai F, Gursky Z, et al. Characterization of perivascular space pathology in a rat model of cerebral small vessel disease by in vivo magnetic resonance imaging. J Cerebral Blood Flow Metabol : Official J Int Soc Cerebral Blood Flow Metabol. 2022:271678X221105668. https://doi.org/10.1177/0271678X221105668.
Hannawi Y, Caceres E, Ewees MG, Powell KA, Bratasz A, Schwab JM, et al. Characterizing the neuroimaging and histopathological correlates of cerebral small vessel disease in spontaneously hypertensive stroke-prone rats. Front Neurol. 2021b;12:740298. https://doi.org/10.3389/fneur.2021.740298.
Schreiber S, Bueche CZ, Garz C, Braun H. Blood brain barrier breakdown as the starting point of cerebral small vessel disease? - new insights from a rat model. Exp Transl Stroke Med. 2013;5(1):4. https://doi.org/10.1186/2040-7378-5-4.
Bailey EL, Smith C, Sudlow CL, Wardlaw JM. Is the spontaneously hypertensive stroke prone rat a pertinent model of sub cortical ischemic stroke? A systematic review. Int J Stroke : official J Int Stroke Soc. 2011a;6(5):434–44. https://doi.org/10.1111/j.1747-4949.2011.00659.x.
Rajani RM, Quick S, Ruigrok SR, Graham D, Harris SE, Verhaaren BFJ, et al. Reversal of endothelial dysfunction reduces white matter vulnerability in cerebral small vessel disease in rats. Sci Transl Med. 2018;10(448) https://doi.org/10.1126/scitranslmed.aam9507.
Brittain JF, McCabe C, Khatun H, Kaushal N, Bridges LR, Holmes WM, et al. An MRI-histological study of white matter in stroke-free SHRSP. J Cereb Blood Flow Metab. 2013;33(5):760–3. https://doi.org/10.1038/jcbfm.2013.14.
Jalal FY, Yang Y, Thompson JF, Roitbak T, Rosenberg GA. Hypoxia-induced neuroinflammatory white-matter injury reduced by minocycline in SHR/SP. J Cerebral Blood Flow and Metabol : official J Int Soc Cerebral Blood Flow Metabol. 2015;35(7):1145–53. https://doi.org/10.1038/jcbfm.2015.21.
Weaver J, Jalal FY, Yang Y, Thompson J, Rosenberg GA, Liu KJ. Tissue oxygen is reduced in white matter of spontaneously hypertensive-stroke prone rats: a longitudinal study with electron paramagnetic resonance. J Cerebral Blood Flow Metabol: Official J Int Soc Cerebral Blood Flow Metabol. 2014;34(5):890–6. https://doi.org/10.1038/jcbfm.2014.35.
Wakita H, Tomimoto H, Akiguchi I, Matsuo A, Lin JX, Ihara M, et al. Axonal damage and demyelination in the white matter after chronic cerebral hypoperfusion in the rat. Brain Res. 2002;924(1):63–70. https://doi.org/10.1016/s0006-8993(01)03223-1.
Shibata M, Yamasaki N, Miyakawa T, Kalaria RN, Fujita Y, Ohtani R, et al. Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke. 2007;38(10):2826–32. https://doi.org/10.1161/STROKEAHA.107.490151.
Kitamura A, Fujita Y, Oishi N, Kalaria RN, Washida K, Maki T, et al. Selective white matter abnormalities in a novel rat model of vascular dementia. Neurobiol Aging. 2012;33:e25–35. https://doi.org/10.1016/j.neurobiolaging.2011.10.033.
Silasi G, She J, Boyd JD, Xue S, Murphy TH. A mouse model of small-vessel disease that produces brain-wide-identified microocclusions and regionally selective neuronal injury. J Cerebral Blood Flow Metabol: Official J Int Soc Cerebral Blood Flow Metabol. 2015;35(5):734–8. https://doi.org/10.1038/jcbfm.2015.8.
Hinman JD, Rasband MN, Carmichael ST. Remodeling of the axon initial segment after focal cortical and white matter stroke. Stroke. 2013;44(1):182–9. https://doi.org/10.1161/STROKEAHA.112.668749.
Shirwany NA, Zou MH. Arterial stiffness: a brief review. Acta Pharmacol Sin. 2010;31(10):1267–76. https://doi.org/10.1038/aps.2010.123.
Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, et al. Interaction between hypertension and arterial stiffness. Hypertension. 2018;72(4):796–805. https://doi.org/10.1161/HYPERTENSIONAHA.118.11212.
Dart AM, Kingwell BA. Pulse pressure--a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37(4):975–84. https://doi.org/10.1016/s0735-1097(01)01108-1.
London GM. Large arteries haemodynamics: conduit versus cushioning function. Blood Press Suppl. 1997;2:48–51.
Tang KS, Medeiros ED, Shah AD. Wide pulse pressure: a clinical review. J Clin Hypertens (Greenwich). 2020;22(11):1960–7. https://doi.org/10.1111/jch.14051.
Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain : a J Neurol. 2011;134(Pt 11):3398–407. https://doi.org/10.1093/brain/awr253.
Garcia-Polite F, Martorell J, Del Rey-Puech P, Melgar-Lesmes P, O’Brien CC, Roquer J, et al. Pulsatility and high shear stress deteriorate barrier phenotype in brain microvascular endothelium. J Cerebral Blood Flow Metabol: Official J Int Soc Cerebral Blood Flow Metabol. 2017;37(7):2614–25. https://doi.org/10.1177/0271678X16672482.
Kwater A, Gasowski J, Gryglewska B, Wizner B, Grodzicki T. Is blood flow in the middle cerebral artery determined by systemic arterial stiffness? Blood Press. 2009;18(3):130–4. https://doi.org/10.1080/08037050902975114.
Webb AJ, Simoni M, Mazzucco S, Kuker W, Schulz U, Rothwell PM. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke. 2012;43(10):2631–6. https://doi.org/10.1161/STROKEAHA.112.655837.
Caughey MC, Qiao Y, Meyer ML, Palta P, Matsushita K, Tanaka H, et al. Relationship between central artery stiffness, brain arterial dilation, and white matter hyperintensities in older adults: the ARIC study-brief report. Arterioscler Thromb Vasc Biol. 2021;41(6):2109–16. https://doi.org/10.1161/ATVBAHA.120.315692.
de Havenon A, Wong KH, Elkhetali A, McNally JS, Majersik JJ, Rost NS. Carotid artery stiffness accurately predicts white matter hyperintensity volume 20 years later: a secondary analysis of the atherosclerosis risk in the community study. AJNR Am J Neuroradiol. 2019;40(8):1369–73. https://doi.org/10.3174/ajnr.A6115.
Saji N, Kimura K, Shimizu H, Kita Y. Silent brain infarct is independently associated with arterial stiffness indicated by cardio-ankle vascular index (CAVI). Hypertension Res : official J Japanese Soc Hypertens. 2012;35(7):756–60. https://doi.org/10.1038/hr.2012.20.
Hannawi Y, Vaidya D, Yanek LR, Johansen MC, Kral BG, Becker LC, et al. Association of vascular properties with the brain white matter hyperintensity in middle-aged population. J Am Heart Assoc. 2022a;11(11):e024606. https://doi.org/10.1161/JAHA.121.024606.
Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932–43. https://doi.org/10.1161/01.ATV.0000160548.78317.29.
Lyle AN, Raaz U. Killing me unsoftly: causes and mechanisms of arterial stiffness. Arterioscler Thromb Vasc Biol. 2017;37(2):e1–e11. https://doi.org/10.1161/ATVBAHA.116.308563.
Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev. 2017;97(4):1555–617. https://doi.org/10.1152/physrev.00003.2017.
Lacolley P, Regnault V, Laurent S. Mechanisms of arterial stiffening: from mechanotransduction to epigenetics. Arterioscler Thromb Vasc Biol. 2020;40(5):1055–62. https://doi.org/10.1161/ATVBAHA.119.313129.
Hainsworth AH, Allan SM, Boltze J, Cunningham C, Farris C, Head E, et al. Translational models for vascular cognitive impairment: a review including larger species. BMC Med. 2017;15(1):16. https://doi.org/10.1186/s12916-017-0793-9.
Shi Y, Thrippleton MJ, Makin SD, Marshall I, Geerlings MI, de Craen AJM, et al. Cerebral blood flow in small vessel disease: a systematic review and meta-analysis. J Cerebral Blood Flow Metabol: official J Int Soc Cerebral Blood Flow Metabol. 2016;36(10):1653–67. https://doi.org/10.1177/0271678X16662891.
Stewart CR, Stringer MS, Shi Y, Thrippleton MJ, Wardlaw JM. Associations between white matter hyperintensity burden, cerebral blood flow and transit time in small vessel disease: an updated meta-analysis. Front Neurol. 2021;12:647848. https://doi.org/10.3389/fneur.2021.647848.
van der Veen PH, Muller M, Vincken KL, Hendrikse J, Mali WP, van der Graaf Y, et al. Longitudinal relationship between cerebral small-vessel disease and cerebral blood flow: the second manifestations of arterial disease-magnetic resonance study. Stroke. 2015;46(5):1233–8. https://doi.org/10.1161/STROKEAHA.114.008030.
Nylander R, Fahlstrom M, Rostrup E, Kullberg J, Damangir S, Ahlstrom H, et al. Quantitative and qualitative MRI evaluation of cerebral small vessel disease in an elderly population: a longitudinal study. Acta Radiol. 2018;59(5):612–8. https://doi.org/10.1177/0284185117727567.
ten Dam VH, van den Heuvel DM, de Craen AJ, Bollen EL, Murray HM, Westendorp RG, et al. Decline in total cerebral blood flow is linked with increase in periventricular but not deep white matter hyperintensities. Radiology. 2007;243(1):198–203. https://doi.org/10.1148/radiol.2431052111.
Promjunyakul NO, Dodge HH, Lahna D, Boespflug EL, Kaye JA, Rooney WD, et al. Baseline NAWM structural integrity and CBF predict periventricular WMH expansion over time. Neurology. 2018;90(24):e2119–e26. https://doi.org/10.1212/WNL.0000000000005684.
Claassen J, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101(4):1487–559. https://doi.org/10.1152/physrev.00022.2020.
Ozturk ED, Tan CO. Human cerebrovascular function in health and disease: insights from integrative approaches. J Physiol Anthropol. 2018;37(1):4. https://doi.org/10.1186/s40101-018-0164-z.
Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17–42. https://doi.org/10.1016/j.neuron.2017.07.030.
Huneau C, Houot M, Joutel A, Beranger B, Giroux C, Benali H, et al. Altered dynamics of neurovascular coupling in CADASIL. Ann Clin Transl Neurol. 2018;5(7):788–802. https://doi.org/10.1002/acn3.574.
Sleight E, Stringer MS, Marshall I, Wardlaw JM, Thrippleton MJ. Cerebrovascular reactivity measurement using magnetic resonance imaging: a systematic review. Front Physiol. 2021;12:643468. https://doi.org/10.3389/fphys.2021.643468.
Thrippleton MJ, Shi Y, Blair G, Hamilton I, Waiter G, Schwarzbauer C, et al. Cerebrovascular reactivity measurement in cerebral small vessel disease: rationale and reproducibility of a protocol for MRI acquisition and image processing. Int J Stroke : official J Int Stroke Soc. 2018;13(2):195–206. https://doi.org/10.1177/1747493017730740.
Blair GW, Doubal FN, Thrippleton MJ, Marshall I, Wardlaw JM. Magnetic resonance imaging for assessment of cerebrovascular reactivity in cerebral small vessel disease: a systematic review. J Cerebral Blood Flow Metabol: official J Int Soc Cerebral Blood Flow Metabol. 2016;36(5):833–41. https://doi.org/10.1177/0271678X16631756.
Blair GW, Thrippleton MJ, Shi Y, Hamilton I, Stringer M, Chappell F, et al. Intracranial hemodynamic relationships in patients with cerebral small vessel disease. Neurology. 2020;94(21):e2258–e69. https://doi.org/10.1212/WNL.0000000000009483.
Sam K, Crawley AP, Conklin J, Poublanc J, Sobczyk O, Mandell DM, et al. Development of white matter hyperintensity is preceded by reduced cerebrovascular reactivity. Ann Neurol. 2016;80(2):277–85. https://doi.org/10.1002/ana.24712.
Rudilosso S, Chui E, Stringer MS, Thrippleton M, Chappell F, Blair G, et al. Prevalence and significance of the vessel-cluster sign on susceptibility-weighted imaging in patients with severe small vessel disease. Neurology. 2022; https://doi.org/10.1212/WNL.0000000000200614.
Birns J, Jarosz J, Markus HS, Kalra L. Cerebrovascular reactivity and dynamic autoregulation in ischaemic subcortical white matter disease. J Neurol Neurosurg Psychiatry. 2009;80(10):1093–8. https://doi.org/10.1136/jnnp.2009.174607.
Guo ZN, Xing Y, Wang S, Ma H, Liu J, Yang Y. Characteristics of dynamic cerebral autoregulation in cerebral small vessel disease: Diffuse and sustained. Sci Rep. 2015;5:15269. https://doi.org/10.1038/srep15269.
Liu Z, Ma H, Guo ZN, Wang L, Qu Y, Fan L, et al. Impaired dynamic cerebral autoregulation is associated with the severity of neuroimaging features of cerebral small vessel disease. CNS Neurosci Ther. 2022;28(2):298–306. https://doi.org/10.1111/cns.13778.
Hartmann DA, Coelho-Santos V, Shih AY. Pericyte control of blood flow across microvascular zones in the central nervous system. Annu Rev Physiol. 2022;84:331–54. https://doi.org/10.1146/annurev-physiol-061121-040127.
Jespersen SN, Ostergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cerebral Blood Flow Metabol Official J Int Soc Cerebral Blood Flow Metabol. 2012;32(2):264–77. https://doi.org/10.1038/jcbfm.2011.153.
Terman D, Chen L, Hannawi Y. Mathematical modeling of cerebral capillary blood flow heterogeneity and its effect on brain tissue oxygen levels. J Theor Biol. 2021;527:110817. https://doi.org/10.1016/j.jtbi.2021.110817.
Chen L, Hannawi Y, Terman D. Modeling the effect of cerebral capillary blood flow on neuronal firing. J Theor Biol. 2022;537:111018. https://doi.org/10.1016/j.jtbi.2022.111018.
Dalby RB, Eskildsen SF, Videbech P, Frandsen J, Mouridsen K, Sorensen L, et al. Oxygenation differs among white matter hyperintensities, intersected fiber tracts and unaffected white matter. Brain Commun. 2019;1(1):fcz033. https://doi.org/10.1093/braincomms/fcz033.
Brown WR, Moody DM, Thore CR, Challa VR, Anstrom JA. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. J Neurol Sci. 2007;257(1-2):62–6. https://doi.org/10.1016/j.jns.2007.01.015.
Moody DM, Thore CR, Anstrom JA, Challa VR, Langefeld CD, Brown WR. Quantification of afferent vessels shows reduced brain vascular density in subjects with leukoaraiosis. Radiology. 2004;233(3):883–90. https://doi.org/10.1148/radiol.2333020981.
Schaeffer S, Iadecola C. Revisiting the neurovascular unit. Nat Neurosci. 2021;24(9):1198–209. https://doi.org/10.1038/s41593-021-00904-7.
Quick S, Moss J, Rajani RM, Williams A. A vessel for change: endothelial dysfunction in cerebral small vessel disease. Trends Neurosci. 2021;44(4):289–305. https://doi.org/10.1016/j.tins.2020.11.003.
Liao JK. Linking endothelial dysfunction with endothelial cell activation. J Clin Invest. 2013;123(2):540–1. https://doi.org/10.1172/JCI66843.
Hunt BJ, Jurd KM. Endothelial cell activation. A central pathophysiological process. BMJ. 1998;316(7141):1328–9. https://doi.org/10.1136/bmj.316.7141.1328.
Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9(10):1057–69. https://doi.org/10.7150/ijbs.7502.
Lansdell TA, Chambers LC, Dorrance AM. Endothelial cells and the cerebral circulation. Compr Physiol. 2022;12(3):3449–508. https://doi.org/10.1002/cphy.c210015.
Petersen MA, Ryu JK, Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci. 2018;19(5):283–301. https://doi.org/10.1038/nrn.2018.13.
Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res. 2015;40(12):2583–99. https://doi.org/10.1007/s11064-015-1581-6.
Hannawi Y, Ewees MG, Moore JT, Zweier JL. Characterizing CD38 expression and enzymatic activity in the brain of spontaneously hypertensive stroke-prone rats. Front Pharmacol. 2022b;13:881708. https://doi.org/10.3389/fphar.2022.881708.
Bailey EL, Wardlaw JM, Graham D, Dominiczak AF, Sudlow CL, Smith C. Cerebral small vessel endothelial structural changes predate hypertension in stroke-prone spontaneously hypertensive rats: a blinded, controlled immunohistochemical study of 5- to 21-week-old rats. Neuropathol Appl Neurobiol. 2011b;37(7):711–26. https://doi.org/10.1111/j.1365-2990.2011.01170.x.
Huang Y, Zhang W, Lin L, Feng J, Chen F, Wei W, et al. Is endothelial dysfunction of cerebral small vessel responsible for white matter lesions after chronic cerebral hypoperfusion in rats? J Neurol Sci. 2010;299(1-2):72–80. https://doi.org/10.1016/j.jns.2010.08.035.
Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Munoz Maniega S, et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65(2):194–202. https://doi.org/10.1002/ana.21549.
Zhang CE, Wong SM, van de Haar HJ, Staals J, Jansen JF, Jeukens CR, et al. Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology. 2017;88(5):426–32. https://doi.org/10.1212/WNL.0000000000003556.
Dobrynina LA, Shamtieva KV, Kremneva EI, Zabitova MR, Akhmetzyanov BM, Gnedovskaya EV, et al. Daily blood pressure profile and blood-brain barrier permeability in patients with cerebral small vessel disease. Sci Rep. 2022;12(1):7723. https://doi.org/10.1038/s41598-022-11172-1.
Wong SM, Jansen JFA, Zhang CE, Hoff EI, Staals J, van Oostenbrugge RJ, et al. Blood-brain barrier impairment and hypoperfusion are linked in cerebral small vessel disease. Neurology. 2019;92(15):e1669–e77. https://doi.org/10.1212/WNL.0000000000007263.
Poggesi A, Pasi M, Pescini F, Pantoni L, Inzitari D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: a review. J Cereb Blood Flow Metab. 2016;36(1):72–94. https://doi.org/10.1038/jcbfm.2015.116.
Han JH, Wong KS, Wang YY, Fu JH, Ding D, Hong Z. Plasma level of sICAM-1 is associated with the extent of white matter lesion among asymptomatic elderly subjects. Clin Neurol Neurosurg. 2009;111(10):847–51. https://doi.org/10.1016/j.clineuro.2009.08.018.
Kim Y, Kim YK, Kim NK, Kim SH, Kim OJ, Oh SH. Circulating matrix metalloproteinase-9 level is associated with cerebral white matter hyperintensities in non-stroke individuals. Eur Neurol. 2014;72(3-4):234–40. https://doi.org/10.1159/000362876.
Markus HS, Hunt B, Palmer K, Enzinger C, Schmidt H, Schmidt R. Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: longitudinal results of the Austrian Stroke Prevention Study. Stroke. 2005;36(7):1410–4. https://doi.org/10.1161/01.STR.0000169924.60783.d4.
Schulz E, Gori T, Munzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res official J Japanese Soc Hypertens. 2011;34(6):665–73. https://doi.org/10.1038/hr.2011.39.
Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond). 2011;120(9):357–75. https://doi.org/10.1042/CS20100476.
Sena CM, Leandro A, Azul L, Seica R, Perry G. Vascular oxidative stress: impact and therapeutic approaches. Front Physiol. 2018;9:1668. https://doi.org/10.3389/fphys.2018.01668.
Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. 2016;126(12):4674–89. https://doi.org/10.1172/JCI86950.
Santisteban MM, Ahn SJ, Lane D, Faraco G, Garcia-Bonilla L, Racchumi G, et al. Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension. Hypertension. 2020;76(3):795–807. https://doi.org/10.1161/HYPERTENSIONAHA.120.15581.
Peng Z, Shu B, Zhang Y, Wang M. Endothelial response to pathophysiological stress. Arterioscler Thromb Vasc Biol. 2019;39(11):e233–e43. https://doi.org/10.1161/ATVBAHA.119.312580.
Sanada F, Taniyama Y, Muratsu J, Otsu R, Shimizu H, Rakugi H, et al. Source of chronic inflammation in aging. Front Cardiovasc Med. 2018;5:12. https://doi.org/10.3389/fcvm.2018.00012.
Guarner V, Rubio-Ruiz ME. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdiscip Top Gerontol. 2015;40:99–106. https://doi.org/10.1159/000364934.
Li T, Huang Y, Cai W, Chen X, Men X, Lu T, et al. Age-related cerebral small vessel disease and inflammaging. Cell death Dis. 2020;11(10):932. https://doi.org/10.1038/s41419-020-03137-x.
Faraco G, Brea D, Garcia-Bonilla L, Wang G, Racchumi G, Chang H, et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat Neurosci. 2018;21(2):240–9. https://doi.org/10.1038/s41593-017-0059-z.
Faraco G, Hochrainer K, Segarra SG, Schaeffer S, Santisteban MM, Menon A, et al. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature. 2019;574(7780):686–90. https://doi.org/10.1038/s41586-019-1688-z.
Thomsen MS, Routhe LJ, Moos T. The vascular basement membrane in the healthy and pathological brain. J Cerebral Blood Flow Metabol: Official J Int Soc Cerebral Blood Flow Metabol. 2017;37(10):3300–17. https://doi.org/10.1177/0271678X17722436.
Rosenberg GA. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin Sci (Lond). 2017;131(6):425–37. https://doi.org/10.1042/CS20160604.
Rosenberg GA. Matrix metalloproteinase-mediated neuroinflammation in vascular cognitive impairment of the Binswanger type. Cell Mol Neurobiol. 2016;36(2):195–202. https://doi.org/10.1007/s10571-015-0277-4.
Jalal FY, Yang Y, Thompson J, Lopez AC, Rosenberg GA. Myelin loss associated with neuroinflammation in hypertensive rats. Stroke. 2012;43(4):1115–22. https://doi.org/10.1161/STROKEAHA.111.643080.
Sood R, Yang Y, Taheri S, Candelario-Jalil E, Estrada EY, Walker EJ, et al. Increased apparent diffusion coefficients on MRI linked with matrix metalloproteinases and edema in white matter after bilateral carotid artery occlusion in rats. J Cereb Blood Flow Metab. 2009;29(2):308–16. https://doi.org/10.1038/jcbfm.2008.121.
Uemura MT, Maki T, Ihara M, Lee VMY, Trojanowski JQ. Brain microvascular pericytes in vascular cognitive impairment and dementia. Front Aging Neurosci. 2020;12:80. https://doi.org/10.3389/fnagi.2020.00080.
Liu Q, Radwanski R, Babadjouni R, Patel A, Hodis DM, Baumbacher P, et al. Experimental chronic cerebral hypoperfusion results in decreased pericyte coverage and increased blood-brain barrier permeability in the corpus callosum. J Cerebral Blood Flow Metabol : Official J Int Soc Cerebral Blood Flow Metabol. 2019;39(2):240–50. https://doi.org/10.1177/0271678X17743670.
Nikolakopoulou AM, Montagne A, Kisler K, Dai Z, Wang Y, Huuskonen MT, et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci. 2019;22(7):1089–98. https://doi.org/10.1038/s41593-019-0434-z.
Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med. 2018;24(3):326–37. https://doi.org/10.1038/nm.4482.
Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485(7399):512–6. https://doi.org/10.1038/nature11087.
Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8(7):643–53. https://doi.org/10.1016/S1474-4422(09)70127-9.
Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, et al. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000;105(5):597–605. https://doi.org/10.1172/JCI8047.
Price BR, Norris CM, Sompol P, Wilcock DM. An emerging role of astrocytes in vascular contributions to cognitive impairment and dementia. J Neurochem. 2018;144(5):644–50. https://doi.org/10.1111/jnc.14273.
Wiese S, Karus M, Faissner A. Astrocytes as a source for extracellular matrix molecules and cytokines. Front Pharmacol. 2012;3:120. https://doi.org/10.3389/fphar.2012.00120.
Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9(2):260–7. https://doi.org/10.1038/nn1623.
Boily M, Li L, Vallerand D, Girouard H. Angiotensin II disrupts neurovascular coupling by potentiating calcium increases in astrocytic endfeet. J Am Heart Assoc. 2021;10(17):e020608. https://doi.org/10.1161/JAHA.120.020608.
Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. https://doi.org/10.1007/s00401-009-0619-8.
Sudduth TL, Powell DK, Smith CD, Greenstein A, Wilcock DM. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J Cerebral Blood Flow Metabol Official J Int Soc Cerebral Blood Flow Metabol. 2013;33(5):708–15. https://doi.org/10.1038/jcbfm.2013.1.
Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9(1):42. https://doi.org/10.1186/s40035-020-00221-2.
Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18(4):225–42. https://doi.org/10.1038/nri.2017.125.
Bailey EL, McBride MW, Beattie W, McClure JD, Graham D, Dominiczak AF, et al. Differential gene expression in multiple neurological, inflammatory and connective tissue pathways in a spontaneous model of human small vessel stroke. Neuropathol Appl Neurobiol. 2014;40(7):855–72. https://doi.org/10.1111/nan.12116.
Koizumi T, Taguchi K, Mizuta I, Toba H, Ohigashi M, Onishi O, et al. Transiently proliferating perivascular microglia harbor M1 type and precede cerebrovascular changes in a chronic hypertension model. J Neuroinflamm. 2019;16(1):79. https://doi.org/10.1186/s12974-019-1467-7.
Zhang LY, Pan J, Mamtilahun M, Zhu Y, Wang L, Venkatesh A, et al. Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics. 2020;10(1):74–90. https://doi.org/10.7150/thno.35841.
Farkas E, Donka G, de Vos RA, Mihaly A, Bari F, Luiten PG. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol. 2004;108(1):57–64. https://doi.org/10.1007/s00401-004-0864-9.
Dabrowska S, Andrzejewska A, Kozlowska H, Strzemecki D, Janowski M, Lukomska B. Neuroinflammation evoked by brain injury in a rat model of lacunar infarct. Exp Neurol. 2021;336:113531. https://doi.org/10.1016/j.expneurol.2020.113531.
Kerkhofs D, van Hagen BT, Milanova IV, Schell KJ, van Essen H, Wijnands E, et al. Pharmacological depletion of microglia and perivascular macrophages prevents vascular cognitive impairment in Ang II-induced hypertension. Theranostics. 2020;10(21):9512–27. https://doi.org/10.7150/thno.44394.
Tomimoto H, Akiguchi I, Suenaga T, Nishimura M, Wakita H, Nakamura S, et al. Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer’s disease patients. Stroke. 1996;27(11):2069–74. https://doi.org/10.1161/01.str.27.11.2069.
Waller R, Narramore R, Simpson JE, Heath PR, Verma N, Tinsley M, et al. Heterogeneity of cellular inflammatory responses in ageing white matter and relationship to Alzheimer’s and small vessel disease pathologies. Brain Pathol. 2021;31(3):e12928. https://doi.org/10.1111/bpa.12928.
Low A, Mak E, Malpetti M, Passamonti L, Nicastro N, Stefaniak JD, et al. In vivo neuroinflammation and cerebral small vessel disease in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2020; https://doi.org/10.1136/jnnp-2020-323894.
Walsh J, Tozer DJ, Sari H, Hong YT, Drazyk A, Williams G, et al. Microglial activation and blood-brain barrier permeability in cerebral small vessel disease. Brain : a J Neurol. 2021;144(5):1361–71. https://doi.org/10.1093/brain/awab003.
Low A, Mak E, Rowe JB, Markus HS, O'Brien JT. Inflammation and cerebral small vessel disease: a systematic review. Ageing Res Rev. 2019;53:100916. https://doi.org/10.1016/j.arr.2019.100916.
Grylls A, Seidler K, Neil J. Link between microbiota and hypertension: focus on LPS/TLR4 pathway in endothelial dysfunction and vascular inflammation, and therapeutic implication of probiotics. Biomed Pharmacother. 2021;137:111334. https://doi.org/10.1016/j.biopha.2021.111334.
Cai W, Chen X, Men X, Ruan H, Hu M, Liu S, et al. Gut microbiota from patients with arteriosclerotic CSVD induces higher IL-17A production in neutrophils via activating RORgammat. Sci Adv. 2021;7(4) https://doi.org/10.1126/sciadv.abe4827.
Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genom. 2017;49(2):96–104. https://doi.org/10.1152/physiolgenomics.00081.2016.
Nelson JW, Phillips SC, Ganesh BP, Petrosino JF, Durgan DJ, Bryan RM. The gut microbiome contributes to blood-brain barrier disruption in spontaneously hypertensive stroke prone rats. FASEB J : Official Public Federation Am Soc Exp Biol. 2021;35(2):e21201. https://doi.org/10.1096/fj.202001117R.
Hosoki S, Saito S, Tonomura S, Ishiyama H, Yoshimoto T, Ikeda S, et al. Oral carriage of Streptococcus mutans harboring the cnm gene relates to an increased incidence of cerebral microbleeds. Stroke. 2020;51(12):3632–9. https://doi.org/10.1161/STROKEAHA.120.029607.
Saji N, Murotani K, Hisada T, Tsuduki T, Sugimoto T, Kimura A, et al. The relationship between the gut microbiome and mild cognitive impairment in patients without dementia: a cross-sectional study conducted in Japan. Sci Rep. 2019;9(1):19227. https://doi.org/10.1038/s41598-019-55851-y.
Saji N, Murotani K, Hisada T, Tsuduki T, Sugimoto T, Kimura A, et al. The association between cerebral small vessel disease and the gut microbiome: a cross-sectional analysis. J Stroke Cerebrovasc Dis the official J Nat Stroke Assoc. 2021;30(3):105568. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105568.
Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J. The glymphatic system and waste clearance with brain aging: a review. Gerontology. 2019;65(2):106–19. https://doi.org/10.1159/000490349.
Koundal S, Elkin R, Nadeem S, Xue Y, Constantinou S, Sanggaard S, et al. Optimal mass transport with Lagrangian workflow reveals advective and diffusion driven solute transport in the glymphatic system. Sci Rep. 2020;10(1):1990. https://doi.org/10.1038/s41598-020-59045-9.
Xue Y, Liu N, Zhang M, Ren X, Tang J, Fu J. Concomitant enlargement of perivascular spaces and decrease in glymphatic transport in an animal model of cerebral small vessel disease. Brain Res Bull. 2020;161:78–83. https://doi.org/10.1016/j.brainresbull.2020.04.008.
Mortensen KN, Sanggaard S, Mestre H, Lee H, Kostrikov S, Xavier ALR, et al. Impaired glymphatic transport in spontaneously hypertensive rats. J Neurosci official J Soc Neurosci. 2019;39(32):6365–77. https://doi.org/10.1523/JNEUROSCI.1974-18.2019.
Cao J, Yao D, Li R, Guo X, Hao J, Xie M, et al. Digoxin ameliorates glymphatic transport and cognitive impairment in a mouse model of chronic cerebral hypoperfusion. Neurosci Bull. 2022;38(2):181–99. https://doi.org/10.1007/s12264-021-00772-y.
Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain : a J Neurol. 2017;140(10):2691–705. https://doi.org/10.1093/brain/awx191.
Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol. 2017;35(4):172–8. https://doi.org/10.1007/s11604-017-0617-z.
Xu J, Su Y, Fu J, Wang X, Nguchu BA, Qiu B, et al. Glymphatic dysfunction correlates with severity of small vessel disease and cognitive impairment in cerebral amyloid angiopathy. Eur J Neurol. 2022; https://doi.org/10.1111/ene.15450.
Zhang W, Zhou Y, Wang J, Gong X, Chen Z, Zhang X, et al. Glymphatic clearance function in patients with cerebral small vessel disease. NeuroImage. 2021a;238:118257. https://doi.org/10.1016/j.neuroimage.2021.118257.
Tang J, Zhang M, Liu N, Xue Y, Ren X, Huang Q, et al. The association between glymphatic system dysfunction and cognitive impairment in cerebral small vessel disease. Front Aging Neurosci. 2022;14:916633. https://doi.org/10.3389/fnagi.2022.916633.
Wardlaw JM, Debette S, Jokinen H, De Leeuw FE, Pantoni L, Chabriat H, et al. ESO Guideline on covert cerebral small vessel disease. Eur Stroke J. 2021;6(2):CXI–CLXII. https://doi.org/10.1177/23969873211012132.
Bath PM, Wardlaw JM. Pharmacological treatment and prevention of cerebral small vessel disease: a review of potential interventions. Int J Stroke : official J Int Stroke Soc. 2015;10(4):469–78. https://doi.org/10.1111/ijs.12466.
Markus HS. Erik de Leeuw F. Cerebral small vessel disease: recent advances and future directions. Int J Stroke : official J Int Stroke Soc. 2023;18(1):4–14. https://doi.org/10.1177/17474930221144911.
Matsuoka RL, Buck LD, Vajrala KP, Quick RE, Card OA. Historical and current perspectives on blood endothelial cell heterogeneity in the brain. Cellul Mol Life Sci: CMLS. 2022;79(7):372. https://doi.org/10.1007/s00018-022-04403-1.
Sas AR, Carbajal KS, Jerome AD, Menon R, Yoon C, Kalinski AL, et al. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat Immunol. 2020;21(12):1496–505. https://doi.org/10.1038/s41590-020-00813-0.
Benveniste H, Elkin R, Heerdt PM, Koundal S, Xue Y, Lee H, et al. The glymphatic system and its role in cerebral homeostasis. J Appl Physiol. 1985;129(6):1330–40. https://doi.org/10.1152/japplphysiol.00852.2019.
Xu J, Su Y, Fu J, Shen Y, Dong Q, Cheng X. Glymphatic pathway in sporadic cerebral small vessel diseases: from bench to bedside. Ageing Res Rev. 2023;86:101885. https://doi.org/10.1016/j.arr.2023.101885.
Skoog I, Gustafson D. Update on hypertension and Alzheimer’s disease. Neurol Res. 2006;28(6):605–11. https://doi.org/10.1179/016164106X130506.
Ueno KI, Togashi H, Mori K, Matsumoto M, Ohashi S, Hoshino A, et al. Behavioural and pharmacological relevance of stroke-prone spontaneously hypertensive rats as an animal model of a developmental disorder. Behav Pharmacol. 2002;13(1):1–13. https://doi.org/10.1097/00008877-200202000-00001.
Markus HS, van Der Flier WM, Smith EE, Bath P, Biessels GJ, Briceno E, et al. Framework for Clinical Trials in Cerebral Small Vessel Disease (FINESSE): a review. JAMA Neurol. 2022; https://doi.org/10.1001/jamaneurol.2022.2262.
Laurent S, Boutouyrie P. Arterial stiffness and hypertension in the elderly. Front Cardiovasc Med. 2020;7:544302. https://doi.org/10.3389/fcvm.2020.544302.
Vatner SF, Zhang J, Vyzas C, Mishra K, Graham RM, Vatner DE. Vascular stiffness in aging and disease. Front Physiol. 2021;12:762437. https://doi.org/10.3389/fphys.2021.762437.
Zhang Z, Powell K, Yin C, Cao S, Gonzalez D, Hannawi Y, et al. Brain Atlas guided attention U-Net for white matter hyperintensity segmentation. AMIA Jt Summits Transl Sci Proc. 2021b;2021:663–71.
Rodriguez S, Hug C, Todorov P, Moret N, Boswell SA, Evans K, et al. Machine learning identifies candidates for drug repurposing in Alzheimer’s disease. Nat Commun. 2021;12(1):1033. https://doi.org/10.1038/s41467-021-21330-0.
Phuah CL, Chen Y, Strain JF, Yechoor N, Laurido-Soto OJ, Ances BM, et al. Association of data-driven white matter hyperintensity spatial signatures with distinct cerebral small vessel disease etiologies. Neurology. 2022; https://doi.org/10.1212/WNL.0000000000201186.
Funding
This work was supported through pilot awards from the neurological research institute at The Ohio State University and The William T. Tozer Hemorrhagic Stroke Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hannawi, Y. Cerebral Small Vessel Disease: a Review of the Pathophysiological Mechanisms. Transl. Stroke Res. (2023). https://doi.org/10.1007/s12975-023-01195-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12975-023-01195-9