Abstract
Intracranial atherosclerotic disease (ICAD) is a major cause of ischemic stroke and transient ischemic attack (TIA) worldwide. The culprit of ICAD is frequently a high-grade intracranial atherosclerotic stenosis (ICAS) pertaining to the infarct territory, and by then, the ICAS is described as symptomatic. A high-grade ICAS may progressively limit cerebral perfusion downstream, demanding collateral compensation. Collateral circulation refers to the pre-existing and dynamic emergence of vascular channels that maintain and compensate for a failing principal vascular route. Collaterals through the Circle of Willis and leptomeningeal circulation are of utmost importance in this regard. In this article, we first discussed the epidemiology, stroke mechanisms, contemporary therapeutics, and prognosis of symptomatic ICAD. Then, we reviewed the collateral routes in ICAS, factors associated with recruitment and development of the collaterals and diagnostic imaging modalities in assessing the origin and function of collateral circulation. We discussed the associations between collateral circulation and clinical outcomes after acute reperfusion treatment in ICAD-related ischemic strokes with or without large vessel occlusion (LVO). We also conducted a systematic review and meta-analysis on the associations of collateral circulation with the risk of recurrent stroke and the functional outcome in symptomatic ICAS patients on medical treatment as secondary stroke prevention. Finally, we summarized current evidence in these aspects and proposed the future directions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intracranial atherosclerotic disease (ICAD) is a major cause of ischemic stroke and transient ischemic attack (TIA) worldwide [1]. ICAD frequently involves terminal segment of internal carotid artery (ICA), middle cerebral artery (MCA) and anterior cerebral artery (ACA) in the anterior circulation, and intracranial vertebral artery (VA), basilar artery (BA), and posterior cerebral artery (PCA) in the posterior circulation [1]. ICAD is often used interchangeably with intracranial atherosclerotic stenosis (ICAS) in the literature when the culprit atherosclerotic plaque causes an obstructive narrowing in the vessel lumen [2]. Yet, the term ICAD could be more accurate in reference to atherosclerosis in an intracranial artery, as low-to-moderate grade or non-stenotic plaques (because of positive vascular remodeling) could also cause thromboembolic strokes and be symptomatic.
Collateral circulation refers to the pre-existing and dynamic emergence of vascular channels that compensate for a failing principal vascular route. As flow-limiting ICAS may progressively attenuate perfusion downstream, collaterals through the Circle of Willis and leptomeningeal circulation are crucial to maintain cerebral perfusion [3]. In this review, we shall discuss collateralization in flow-limiting ICAS only, as collateral recruitment is minimal, if any, when the ICAD lesions are hemodynamically insignificant [4]. We shall review the collateral routes in ICAS, factors associated with the recruitment and development of collaterals, and imaging modalities and methods to assess the origin and function of collateral circulation.
Collateral circulation may help prevent or delay the onset of stroke. Individuals may remain largely “asymptomatic” even when one or more major cerebral arteries are incidentally found severely stenotic or occluded in neurovascular imaging examination [5,6,7]. More robust leptomeningeal collateral flow has been observed in patients with asymptomatic unilateral MCA stenosis than those with symptomatic MCA stenosis in a small-scale study [8]. However, clinical correlation of the collateralization in asymptomatic ICAS was scarce, probably related to the relatively low stroke risk in these symptom-free carriers than those with symptomatic ICAS. In this article, we shall discuss the relevance of collateral circulation in the context of symptomatic ICAS. We shall also discuss the associations between collateral circulation and clinical outcomes after intravenous or intra-arterial reperfusion therapy in ICAD-related ischemic strokes with or without large vessel occlusion (LVO). We then systematically review published evidence on the association of collateral circulation with the risk of recurrent stroke and the functional outcome of symptomatic ICAS. Finally, we summarized current evidence in these aspects and proposed some research directions in ICAD and collateral circulation.
ICAD: Epidemiology, Stroke Mechanisms, Therapeutic Methods, and Prognosis
Compared to Caucasians, ICAD has an ethnic predilection in East Asians, Africans, and Hispanics, attributed to genetic and environmental disparities [9, 10]. Earlier post-mortem studies revealed a higher prevalence of ICAS in Asians died of various causes, for instance, > 30% in those died at 60–90 years old in a Chinese study (n = 114) [11], but below 10% in counterparts from a large Caucasian study (n = 3942) [12]. The prevalence of asymptomatic ICAS varied in different stroke-free populations, for instance, 7–13% in rural and urban Chinese aged over 40 years who had no history of stroke/TIA [13, 14], 13% in Chinese (mean age of 55.8 years) with one or more cardiovascular risk factors [15], and 13% in predominantly Caucasians (mean age 71.4 years) referred for carotid Doppler examination [16]. However, the heterogeneity of age and vascular risk profile in these cohorts may limit the interpretation, as these factors may govern the development and evolution of ICAD.
Overall, ICAS caused 30–50% of all ischemic strokes and TIAs in East Asian populations and 5–10% in Caucasians in studies from 1990 and 2000s [17, 18]. However, among 1368 minor stroke and TIA patients in the population-based Oxford Vascular Study from 2011 to 2018, the prevalence of symptomatic ICAS (50–99% stenosis) increased from 4.9% of patients < 70 years old to 19.6% of those ≥ 90 years old (p for trend < 0.001), where ICAS was diagnosed by transcranial Doppler (TCD), MR angiography (MRA), or CT angiography (CTA) [19]. Therefore, ICAS prevalence in Caucasian stroke patients could have been underestimated, or alternatively, the prevalence has been increasing in the past decade given the aging population. Interestingly, in Asians, the proportions of stroke/TIA due to ICAS might have significantly declined in the last 20 years, attributed mostly to more intensive vascular risk factor management, as well as the escalating cardioembolic strokes related to atrial fibrillation in the aging population [20]. For instance, in a hospital-based stroke registry in Hong Kong, the proportion of stroke/TIA with “large-artery atherosclerotic etiology” (mostly ICAS) dropped from 23.3% in 2004–2006 to 8.8% in 2016–2018, in contrast to the surge of cardioembolic stroke/TIA from 20.4 to 29.3% in the same period [20]. Yet, the ethnic disparity in ICAD prevalence among age- and vascular risk factor–matched Asians and Caucasians remains evident [10]. More studies are warranted to reveal the underlying mechanisms for more effective primary and secondary prevention.
ICAD can cause ischemic stroke or TIA by diverse stroke mechanisms, which include (1) parent artery atherosclerosis occluding the orifice of a perforator, resulting in a lacunar syndrome; (2) artery-to-artery thromboembolism from an unstable plaque; and (3) hypoperfusion distal to a high-grade stenotic plaque [21, 22]. Of note, these mechanisms may interplay and contribute to a stroke. For example, thromboemboli from an ICAD plaque could be stranded distally in the hypoperfused watershed regions, resulting in rosary-like borderzone infarcts. In a cohort of 153 patients with a symptomatic ICAS (50–99% stenosis), this “mixed mechanism” accounted for 37% of ischemic strokes [22].
Current secondary stroke prevention strategy for symptomatic ICAS (50–99% stenosis) are predominantly based on WASID (warfarin–aspirin symptomatic intracranial disease) [23] and SAMMPRIS (stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis) [24] trials, consisting of antiplatelet treatment and stringent vascular risk factor control (lifestyle modifications such as aerobic exercise and dietary restrictions, high-intensity statin, blood pressure, and glycemic control) [25]. For patients with symptomatic 70–99% ICAS, or minor stroke/high-risk TIA patients with symptomatic 50–99% ICAS, short-term dual antiplatelets with aspirin and clopidogrel (3 weeks to 90 days) are recommended to reduce subsequent stroke risk based on the medical arm of SAMMPRIS [24] and the dual-antiplatelet arm of CHANCE (clopidogrel in high-risk patients with acute non-disabling cerebrovascular events) trial [26]. Angioplasty ± stenting is not recommended for ICAS < 70% even if symptomatic. For symptomatic ICAS > 70%, given the high periprocedural complication rate in SAMMPRIS (14.7%) [24] and VISSIT (Vitesse stent ischemic therapy trial) (25%) [27], angioplasty ± stenting is not recommended as initial treatment [25]. Yet, as a much lower rate of periprocedural complication was observed in WEAVE (Wingspan stent system post-market surveillance cohort) (2.6%) and other registry studies (approximately 6%) [28, 29], the efficacy of angioplasty ± stenting for refractory strokes due to a 70–99% ICAS despite optimal medical treatment warrants further randomized study.
However, despite uniform “best” medical treatment, the recurrent stroke risk still varied in ICAD subgroups. For instance, in the medical arm of SAMMPRIS, patients with anterior-circulation borderzone infarcts, presumably with hypoperfusion, were associated with a particularly high risk of recurrence in the same territory at 1 year (21.1%) [30]. In another cohort of medically treated patients with 50–99% ICAS (n = 122), a mixed stroke mechanism with artery-to-artery embolism and hypoperfusion conferred a significantly higher stroke relapse rate in 1 year than those with a stroke mechanism other than hypoperfusion or artery-to-artery embolism (24.4% versus 3.2%; hazard ratio [HR] 8.50; 95% confidence interval [CI] 2.51–28.82; P = 0.013) [22]. Understandably, medical treatment alone would not mitigate distal hypoperfusion if plaque regression or positive remodeling does not occur. In fact, without endovascular recanalization, aggressive blood pressure control in the absence of sufficient collateral compensation may aggravate hypoperfusion further [22, 31]. Therefore, understanding precisely the stroke mechanism could be a key to more effective secondary prevention.
In flow-limiting ICAS, functional collateral circulation maintains cerebral perfusion [4] and reduces the risk of hypoperfusion stroke [32]. Moreover, effective collaterals may facilitate washout and clearance of small emboli in the distal vascular bed, enhance exposure to thrombolytics, and restore cerebral perfusion. In contrast, impaired emboli clearance from poor collateralization may be an important link in the vicious cycle of flow-limiting ICAS, hypoperfusion, artery-to-artery embolism, and watershed infarctions [33, 34]. The collateral routes, assessment methods, and the protective effects of collateral circulation in symptomatic ICAS will be further discussed below.
Collateral Circulation in ICAS: Associated Factors and Assessment Methods
Cerebral collateral circulation consists mostly of primary network involving the Circle of Willis and secondary network through leptomeningeal anastomoses that connect terminal cortical branches of major cerebral arteries along the surface of the brain. These collaterals are dormant under normal conditions when blood flow from all major cerebral arteries is not impeded, but are recruited when one major artery is either progressively or acutely occluded [7]. In the presence of an occlusive or flow-limiting lesion in extracranial arteries or intracranial arterial segments proximal to these networks, flow diversion through arterial segments in the Circle of Willis can provide rapid collateral perfusion to the contralateral anterior circulation (via anterior communicating artery and A1 ACA) or the ipsilateral anterior or posterior circulation (through forward or backward flow via posterior communicating artery). Leptomeningeal anastomoses are of utmost importance when collateral compensation is insufficient through the Circle of Willis, or when the flow-limiting ICAS is distal to the Circle of Willis, for example, an M1 MCA stenosis. In this situation, collateral flow through the leptomeningeal vessels plays a more prominent role in maintaining distal cerebral perfusion than collateral flow via the Circle of Willis [35]. The leptomeningeal anastomoses are small arterioles (~ 50–400 μm) that connect the distal territories between ACA and MCA and between MCA and PCA. In the presence of severe stenosis or occlusion of these cerebral arteries, the leptomeningeal anastomoses provide retrograde flow to the ischemic area, for instance, from ACA or PCA to MCA territory [7]. In the posterior fossa, anastomoses between the distal branches of superior cerebellar artery (SCA), anterior inferior cerebellar artery (AICA), and posterior inferior cerebellar artery (PICA) also exist [7].
Less commonly, new vessels may emerge through angiogenesis (new capillaries induced by ischemia and hypoxia) or arteriogenesis (new arterioles or remodeling of preexisting arterio-arteriolar anastomoses induced mostly by physical forces such as shear stress) adjacent to the ischemic core to provide collateral flow in high-grade ICAS, when primary and secondary collateral pathways are ineffective [7, 36, 37]. The increase in microvessel density in the periphery of ischemic core has been used to quantify angiogenesis after induced ischemia in animal models [38]. In patients with ICAS, new vessel formation (NVF) along steno-occlusive MCA is not uncommon in digital subtraction angiography (DSA) [37], which has also been observed in high-resolution MR imaging, i.e., multiple deep tiny flow voids along steno-occlusive MCA [39]. Preliminary investigations indicated associations of NVF in DSA with poor primary and secondary collaterals and worse functional outcome, in patients with isolated, atherosclerotic stenosis, or occlusion of MCA [37]. This supports NVF as a marker of exhausted primary and secondary collaterals. However, the nature, mechanisms, and clinical implications of NVF visible in neurovascular imaging in ICAS patients are far from being fully appreciated, which are not discussed in details in this review article. In the discussions below over the assessment methods, associated factors, and prognostic values of collateral circulation in symptomatic ICAS patients, we are mostly referring to the primary and secondary collaterals.
A high-pressure gradient across ICAS and a reduced antegrade flow could drive collateral recruitment from preexisting anastomoses or development of new collateral routes [34, 40]. The extent and capacity of the collateral circulation may change over time [3]; yet, the dynamic evolution of collateral circulation in ICAD has not been clearly delineated as serial neurovascular imaging tests (like CTA or DSA) are needed in such studies. Ultimately, the summation of retrograde collateral flow and residual antegrade flow determines the distal perfusion and dictates if hemodynamic ischemia would happen distal to an ICAS. For hemodynamically significant ICAS, retrograde collateral flow would be more crucial to maintain downstream perfusion than in cases where the ICAD lesions are hemodynamically insignificant [4]. Without sufficient compensation from leptomeningeal collaterals, borderzone ischemia and infarction may develop in ICAS with poor antegrade flow, independent of thromboembolic risk.
In terms of predictors of collateralization, in a cohort of 206 patients with acute ischemic stroke due to M1 MCA occlusion with or without intracranial ICA occlusion, metabolic syndrome [41], hyperuricemia, and older age were independently associated with poor leptomeningeal collaterals assessed in CTA [42]. In a larger dataset (n = 857) of patients with acute proximal MCA occlusion from the Acute STroke Registry and Analysis of Lausanne (ASTRAL), older age and creatinine levels were associated with poor collaterals, but dyslipidemia and no previous statin use were associated with good collaterals, graded by the extent of vessel filling in the territory distal to the occluded artery in CTA [43]. Another large cohort (n = 1988) incorporating data from the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) trial and MR CLEAN Registry indicated older age, male sex, and higher blood glucose level as independent predictors of poor collaterals, in stroke patients with acute intracranial LVO in the anterior circulation. Interestingly, in these thrombectomy studies where cardioembolism rather than ICAD was the predominant stroke mechanism, cardiovascular risk factors were not associated with the collateral status in the analyses [44].
The heterogeneous etiology of acute LVO (i.e., chiefly ICAD atherothrombosis vs cardioembolic occlusion) may explain the mixed findings of these studies. In case of ICAS where hypoperfusion is slowly progressive, time-dependent development or recruitment of collaterals may set in. Therefore, the more robust collateral flow observed in ICAD-related acute LVO might reflect the long-standing ischemia prior to the abrupt occlusion [35]. This phenomenon has been observed in a recent meta-analysis, which showed a higher chance of good pre-treatment collaterals in those with large artery atherosclerotic (versus cardioembolic) strokes, among patients receiving intravenous or intra-arterial reperfusion treatment (risk ratio [RR] 1.24; 95%CI 1.04–1.50; p = 0.020), although there were significant between-study heterogeneities [45]. By far, factors associated with the collateral status in ICAS have been less frequently investigated. Post hoc analysis of WASID reported a neutral relationship between systolic, diastolic, or mean blood pressures and the collateral status in either moderate or severe ICAS [35]. Larger-scale cross-sectional or longitudinal studies are warranted to study associated factors of collateralization in ICAS.
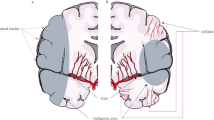
Several imaging modalities may assess the vascular anatomy and grade primary and secondary collaterals (Fig. 1), including conventional DSA, CTA, MRA, fluid-attenuated inversion recovery MR imaging (FLAIR) and TCD. By far, DSA is the gold standard in assessing cerebral collateral circulation, and the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) grading system is widely recognized for grading leptomeningeal collaterals [46]. In brief, the ASITN/SIR collateral grading system classifies leptomeningeal collaterals as no (grade 0), slow and incomplete (grade 1), rapid but incomplete (grade 2), slow but complete (grade 3), and rapid and complete (grade 4) in the ischemic territory [46]. Single- or multi-phase CTA is also commonly used to assess leptomeningeal collaterals in ICAS by grading the contrast filling in the vascular territory distal to the stenotic/occluded cerebral artery, or by comparing the pial vessels in the ipsilesional versus the contralesional side [47,48,49,50,51]. With the rapid development in neurovascular imaging, more methods are developed to assess the function or the effects of collaterals on cerebral perfusion, such as CT perfusion, dynamic susceptibility contrast and arterial spin labeling MR perfusion, and quantitative MR angiography [51].
Cerebral collaterals in ICAD shown in DSA and CTA images. A–D DSA images of different phases showing leptomeningeal collateral flow from left ACA filling the left MCA territory (grade 3 by the ASITN/SIR collateral flow grading system), in a 67-year-old female patient with acute occlusion of the left MCA. E, F Maximum intensity projections of single-phase CTA showing good leptomeningeal collaterals (more prominent pials in ACA and PCA territories than the contralateral side; double arrows), in a 71-year-old female patient with 70% stenosis of the left M1 MCA (arrow). G, H Maximum intensity projections of single-phase CTA showing poor leptomeningeal collaterals (equal pials in ACA and PCA territories with the contralateral side), in a 60-year-old male patient with 70% stenosis of the right M1 MCA (arrow). ICAD, intracranial atherosclerotic disease; DSA, digital subtraction angiography; CTA, computed tomography angiography; ACA, anterior cerebral artery; MCA, middle cerebral artery; ASITN/SIR, the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology; PCA, posterior cerebral artery
Other articles under this special issue “Collaterals and the Ephemeral Ischemic Penumbra” will discuss with details over the anatomy of cerebral collateral circulation, vascular biology in collateral development, diagnostic imaging modalities and scales for assessing collateral circulation, and therapeutic augmentation of the collateral flow [52].
Collateral Circulation and Outcomes After Reperfusion Therapy in Acute Stroke due to ICAD
In acute ischemic stroke, reperfusion therapies with intravenous thrombolysis and/or endovascular treatment are first-line treatment in eligible patients presented within 4.5 h for intravenous thrombolysis and up to 24 h for mechanical thrombectomy [53]. A systematic review and meta-analysis showed the prognostic significance of good collaterals in acute ischemic stroke treated with intravenous thrombolysis, in terms of a lower risk of symptomatic intracranial hemorrhage (RR 0.38; 95%CI 0.16–0.90; p = 0.03), a higher incidence of early neurological improvement (RR 4.21; 95%CI 1.57–11.28; p = 0.004), and a higher chance of achieving functional independence (modified Rankin Scale (mRS) 0–2 or 0–1 as defined in different primary studies) at 3–6 months (RR 2.45; 95%CI 1.94–3.09; p < 0.001) [54]. Another two systematic reviews and meta-analyses revealed that good pre-treatment collaterals in acute LVO patients receiving endovascular treatment ± prior intravenous thrombolysis was associated with slightly higher rates of successful recanalization (RR 1.23; 95%CI 1.06–1.42; p = 0.006) and reperfusion (RR 1.28; 95%CI 1.17–1.40; p < 0.001) [55], and more importantly, a significantly lower risk of symptomatic intracranial hemorrhage (RR 0.59; 95%CI 0.43–0.81; p = 0.001), an almost doubled chance of achieving functional independence (mRS 0–2) at 3 months (RR 1.98; 95%CI 1.64–2.38; p < 0.001), and a halved 3-month mortality (RR 0.49; 95%CI 0.38–0.63; p < 0.001) [56]. HERMES, a meta-analysis of individual patient data by the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials collaboration, demonstrated that acute LVO patients with good collaterals are more likely to benefit from endovascular treatment than conventional medical treatment alone, compared to those with poor collaterals [57].
However, these meta-analyses collectively reviewed all patients eligible for reperfusion therapy, and the prognostication of pre-treatment collaterals in ischemic strokes of certain etiologies (e.g., ICAD) remains largely unknown. As mentioned above, better pre-treatment collaterals have been observed in those with large artery atherosclerotic (versus cardioembolic) strokes, among patients receiving intravenous or intra-arterial reperfusion treatment [45]. Yet, other major confounders may come into play when correlating outcome with collaterals in each stroke subtype (ICAD vs cardioembolism), for example, clot location and composition, choice of endovascular device (aspiration vs stent-retriever), refractoriness and propensity to re-occlusion, duration of endovascular treatment, and so on [58, 59]. Therefore, the associations between collaterals and outcomes after acute reperfusion therapy in ischemic strokes in general may not fully apply in ICAD-related strokes ± LVO.
A single-center study of patients with acute MCA occlusion revealed a good 3-month functional outcome after intravenous thrombolysis and/or endovascular therapy in 87 cardioembolic strokes with good pre-treatment leptomeningeal collaterals in multi-phase CTA (odds ratio [OR] 3.223; 95%CI 1.212–8.570; p = 0.019), but not in 30 large artery atherosclerotic strokes (OR 1.011; 95%CI 0.276–3.700; p = 0.987) [60]. Although the finding needs confirmation in larger-scale studies, the observation may reflect the exhaustion of collateral recruitment in progressive large artery atherosclerosis prior to the stroke, as well as the fact that leptomeningeal collaterals would not protect the subcortical regions vulnerable in ICAD. On the contrary, there remained consistently a positive impact of good collaterals on the outcome of cardioembolic strokes where collaterals were in general worse.
Overall, good pre-treatment collateral circulation has been identified as a protective factor for better functional outcomes after acute reperfusion therapies in existing literature, when acute strokes of various etiologies were analyzed as a whole. Nevertheless, more studies are needed to verify these findings in acute strokes ± LVO with different etiologies, e.g., ICAD, given the potential differences in pathophysiology and temporal patterns of collateral recruitment, and in its prognostic values, in strokes of different etiologies [61].
Collaterals and Prognosis of Medically Treated Symptomatic ICAS: a Systematic Review and Meta-analysis
Medical treatment with antiplatelet(s) and stringent risk factor control is currently the best practice for secondary stroke prevention in symptomatic ICAS [25]. Good collateral circulation may serve as a protective factor against recurrent stroke in medically treated symptomatic ICAS patients and predict a favorable functional outcome. Yet, there has been no summary of the evidence. We hence conducted a systematic review and meta-analysis on the associations between collateral status and prognosis (risk of recurrent stroke and chance of achieving a favorable functional outcome) of medically treated symptomatic ICAS patients, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [62] and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) [63] statements.
Methods: Search Strategy and Study Screening
We searched Medline and Embase via PubMed and OVID on 3 January 2022, for adult, human studies reporting the association between collaterals and any outcome measure in symptomatic ICAS patients, with a full-text article published in English from 1 January 2001 to 31 December 2021. The search terms included stroke, TIA, ICAD, ICAS, and collateral, with a more detailed search strategy provided in Supplementary Tables 1 and 2. We also manually searched references in pertinent review articles for potentially relevant articles.
We screened the records retrieved, for cohort studies (including post hoc analysis of randomized studies) reporting associations of baseline collaterals with the risk of recurrent stroke, or with a favorable functional outcome, in symptomatic ICAS (> 50% stenosis or occlusion) patients receiving medical treatment for secondary stroke prevention. Definitions of the two outcomes could vary among the primary studies. We excluded studies with a considerable proportion of patients receiving acute reperfusion therapy (intravenous thrombolysis, endovascular treatment, or bridging therapy), with non-atherosclerotic stroke, or receiving angioplasty/stenting therapy, if data were not separately presented in those with symptomatic ICAS receiving medical treatment only for secondary stroke prevention.
Methods: Data Collection and Risk of Bias Assessment
We collected country/region of the study, inclusion criteria of symptomatic ICAS patients (e.g., time from stroke onset to enrollment, definition of symptomatic ICAS), sample size, mean/median age, male percentage, NIH Stroke Scale (NIHSS) at baseline, imaging modality and methods to assess the collateral circulation at baseline, duration of follow-up and treatments for secondary prevention, from the included primary studies. The risk of bias of the included cohort studies was assessed with the Newcastle–Ottawa Scale, with a total score of 0–9 [64]. Scores of 7–9 and 0–6 respectively indicated low and high risk of bias.
Methods: Data Synthesis
Mantel–Haenszel random-effects models were used to estimate the associations between the collateral status (good versus poor) and the outcomes, presented in RRs and the 95% CI. Publication bias of the primary studies was assessed by visual inspection of the funnel plot. Between-study heterogeneities were tested by Cochran’s Q (χ2) and the I2 statistics. Two-sided p values < 0.05 and < 0.10 were considered statistically significant, respectively in the estimation of the RRs and the between-study heterogeneities. Cochrane Review Manager (version 5.4) was used for all the analyses.
Results: Study Selection and Description
Of 1412 records retrieved from literature search, 9 studies were eligible in the systematic review [32, 65,66,67,68,69,70,71,72]. A flow chart is provided in Supplementary Fig. 1, and the characteristics of these included studies are summarized in Table 1. The primary studies include post hoc analysis of the WASID [65] and SAMMPRIS [72] trials, and data from the Chinese IntraCranial AtheroSclerosis (CICAS) cohort [68], while the remaining studies are single-center studies conducted in East Asia [32, 66, 67, 69,70,71]. Most studies used the ASITN/SIR collateral grading system, or a modified version, to assess leptomeningeal collaterals in DSA [65, 66, 69, 70, 72]. All of the included studies had a low risk of bias, with the Newcastle–Ottawa Scale of 7–9 (Table 1).
Results: Collateral Circulation and Recurrent Stroke in Medically Treated ICAS Patients
Among the 9 included studies, 2 studies reported the association between leptomeningeal collateral status at baseline by the ASITN/SIR collateral flow grading system in DSA and recurrent relevant ischemic stroke in patients receiving medical treatment in WASID and SAMMPRIS [65, 72]. In 287 symptomatic ICAS (50–99%) patients in WASID, leptomeningeal collaterals were associated with recurrent ischemic stroke risk with a mean follow-up of 1.8 years (HR none versus good collaterals, 1.62; 95%CI 0.52–5.11; poor versus good, 4.78; 95%CI 1.55–14.7; p = 0.002), independent of demographics, NIHSS, luminal stenotic severity, and stroke onset-to-enrollment time [65]. In the medical arm of SAMMPRIS, among 82 patients with symptomatic 70–99% ICAS and acute infarct(s) in the anterior circulation, the 1-year risks of recurrent relevant ischemic stroke were 21.7 versus 6.1% in those with impaired and complete leptomeningeal collaterals, and 3-year risks were 30.6 versus 6.1% in the two subgroups (log-rank p = 0.014). However, confounders were not adjusted in the analyses with the small sample size [72]. There were two other studies reporting the association between leptomeningeal collaterals and recurrent stroke in symptomatic ICAS patients [32, 66].
Overall, in these 4 studies of 517 medically treated patients with symptomatic ICAS [32, 65, 66, 72], good leptomeningeal collaterals at baseline were associated with a lower risk of recurrent stroke or TIA during follow-up (RR 0.39; 95%CI 0.21–0.74; p = 0.004; random-effects model), with no between-study heterogeneity (p = 0.55 for Cochran’s Q test; I2 = 0%) on the effect sizes (Fig. 2). There was no apparent publication bias by visual inspection of the funnel plot.
Forest plot. Good leptomeningeal collaterals at baseline was associated with a lower risk of recurrent stroke or TIA (RR 0.39; 95%CI 0.21–0.74; p = 0.004; random-effects model), in 517 medically treated symptomatic ICAS patients from 4 studies. There was no significant between-study heterogeneity (p = 0.55 for Cochran’s Q test; I.2 = 0%) on the effect sizes. The outcome measures and durations of follow-up were different between the primary studies, which are provided in the footnotes. TIA, transient ischemic attack; ICAS, intracranial atherosclerotic stenosis
Yet, the collateral status may exert different effects on the stroke risks in moderate (50–69%) vs severe (70–99%) ICAS. In post hoc analysis of the WASID data, interestingly, extensive collaterals diminished the stroke relapse rate in severe ICAS (HR none versus good collaterals, 4.60; 95%CI 1.03–20.56; poor versus good, 5.90; 95%CI 1.25–27.81; p = 0.043), but was associated with a higher or similar risk of stroke recurrence in patients with moderate ICAS (HR none versus good collaterals, 0.18; 95%CI 0.04–0.82; poor versus good, 1.78; 95%CI 0.37–8.57; p < 0.001), compared with patients with poor or none collaterals [65]. It is plausible that in severe ICAS with minimal or trickle antegrade flow, robust retrograde collaterals secured distal perfusion and might reduce antegrade thromboembolism. Moreover, apart from the stenotic severity, other morphological features of the ICAD plaques could be associated with stroke recurrence [73]. In a computational fluid dynamics (CFD) model that simulated flow across ICAS lesions, we found that a larger translesional pressure gradient (and hence a reduced antegrade flow) is a driving force to recruit retrograde collaterals [40]. Consistently, a CFD and CT perfusion study also showed complementary effects of residual antegrade flow and leptomeningeal collateral flow in sustaining cerebral perfusion distal to ICAS [4].
Concerning stroke prognostication by the competence of the Circle of Willis (via anterior/posterior communicating arteries), there have been more studies in patients with proximal carotid artery stenosis or occlusion, but data were limited in symptomatic ICAS. In CICAS study, among 2864 stroke/TIA patients (1,335 with ICAS), a complete Circle of Willis was paradoxically associated with a higher risk of ischemic or hemorrhagic stroke within 12 months. Nevertheless, the inclusive definition of ICAS in CICAS (i.e., the presence rather than being the culprit lesion of the index stroke/TIA) limited the result interpretation [68]. In the other small-scale study, good (versus poor) integrity of the Circle of Willis was associated with a lower risk of a composite endpoint, symptomatic ischemic or hemorrhagic stroke within 30 days, and ischemic stroke or TIA beyond 30 days (0 versus 17%; log-rank p = 0.059), with a median follow-up of 36 months in medically treated patients with symptomatic 70–99% ICAS [69].
Results: Collateral Circulation and Functional Outcome of Medically Treated Symptomatic ICAS Patients
In 4 small-scale studies (344 patients) [32, 66, 67, 70], good collateralization at baseline was associated with a higher chance of achieving a favorable functional outcome (mRS 0–2) at 3 months in medically treated symptomatic ICAS patients (RR 2.94; 95%CI 1.58–5.48; p < 0.001; random-effects model). There was no apparent publication bias by visual inspection of the funnel plot. However, there was significant between-study heterogeneity (p = 0.03 for Cochran’s Q test; I2 = 67%) on the effect sizes (Fig. 3). The beneficial effect of good collaterals on the 3-month functional outcome may be explained by the protective effect against recurrent stroke/TIA as mentioned above.
Forest plot. Good leptomeningeal collaterals at baseline was associated with a higher chance of achieving a favorable functional outcome (mRS 0–2) at 3 months (RR 2.94; 95%CI 1.58–5.48; p < 0.001; random-effects model), in 344 medically treated symptomatic ICAS patients from 4 studies. There was significant between-study heterogeneity (p = 0.03 for Cochran’s Q test; I.2 = 67%) on the effect sizes. mRS, modified Rankin Scale; ICAS, intracranial atherosclerotic stenosis
Summary: Collaterals and Prognosis of Medically Treated Symptomatic ICAS
Synthesis of published data supported a protective role of good leptomeningeal collaterals against recurrent stroke, along with a higher chance in achieving a favorable functional outcome, in medically treated symptomatic ICAS patients. Of note, subgroup analysis of WASID revealed possibly different effects of good leptomeningeal collaterals on the stroke risks, by the degree of luminal stenosis (moderate vs severe) in symptomatic ICAS. However, these previous studies were mostly retrospective analyses, or single-center, small-scale studies, which used different imaging modalities/methods in assessing the collateral status. The prognostic value of collaterals via the Circle of Willis in symptomatic ICAS patients remains unclear, which may partly depend on the location of the ICAS lesion (proximal or distal to the Circle of Willis).
Conclusions and Future Directions
ICAD is globally an important ischemic stroke subtype. The higher ICAS prevalence in those of Asian, African, and Hispanic ancestries than Caucasians has long been established. With global population aging and evolution on the profile and management intensities over cardiovascular risk factors in the past years, such ethnic disparity in ICAS prevalence has been narrowed. This on one hand corroborates the effectiveness of cardiovascular risk factor management in preventing development and progression of ICAS and on the other hand indicates a necessity of screening for ICAS as a stroke etiology in older Caucasian patients with multiple cardiovascular risk factors. Moreover, the remaining difference in ICAS prevalence across populations signifies differences in the genetic backgrounds, warranting further investigations. The risk of stroke relapse in symptomatic ICAS has also been declining in the last 2 decades with a “best” medical treatment, composed of antiplatelet treatment and stringent vascular risk factor control. However, better understanding of the stroke mechanisms in symptomatic ICAS, which entail different stroke relapse risks despite medical treatment, is needed, for more effective secondary stroke prevention in these patients.
The collateral circulation plays an important role in mediating the stroke mechanisms and prognosis of symptomatic ICAS patients. When an ICAS impedes antegrade flow, the collateral circulation will develop over time and help maintain cerebral perfusion, through the Circle of Willis (communicating bilateral anterior circulations or anterior–posterior circulations via anterior/posterior communicating artery), pial collaterals (connecting the distal territories of cerebral arteries, e.g., ACA-MCA or PCA-MCA), or new vascular channels (capillaries or arterioles) in the periphery of the ischemic core. Effective collaterals also facilitate clearance of thromboemboli in the distal vascular bed from a ruptured ICAD lesion. In general, a large pressure gradient across an arterial stenosis/occlusion, altered shear stress, and subsequent changes in cytokines may underlie the recruitment of pre-existing collateral routes and development of new collateral channels. In terms of predictors of collateralization, some previous studies revealed associations of cardiovascular risk factors such as hypertension and metabolic syndrome with poor collaterals in acute LVO, but findings were inconsistent between studies. This may partly be explained by the differences in pathophysiology of collateral recruitment in LVO of atherosclerotic versus embolic etiologies, i.e., more slowly progressive and time-dependent fashion in atherosclerotic LVO with long-standing ischemia. More studies are needed to clearly delineate the dynamic evolution of collateral circulation in ICAS, and to further clarify the mechanisms and associated factors. Currently, various assessment methods using noninvasive (e.g., ultrasound-, CT-, and MR-based vascular imaging and CT- and MR-based perfusion imaging) and invasive imaging modalities (e.g., DSA) may assess the anatomy and/or function of cerebral collaterals in stroke and ICAS patients. However, validation and comparisons of these imaging methods in assessing collateralization, as well as a consensus over the grading methods for future large-scale, multicenter, or cross-population studies, are needed.
In the acute phase of ischemic stroke ± LVO irrespective of the stroke etiology, previous systematic reviews have demonstrated the associations of good pre-treatment collateralization with higher reperfusion/recanalization rates, a lower risk of symptomatic intracranial hemorrhage, and better functional outcomes, after intravenous/intra-arterial reperfusion treatment. However, there are differences in patient characteristics, pathophysiology, and temporal patterns of collateral recruitment, the pre-treatment collateral status, and the endovascular procedures, in acute strokes of atherosclerotic versus embolic etiologies. Hence, the associations between collateral status and outcomes after acute reperfusion treatment in these etiology subgroups could differ, based on limited data in the literature. Further investigations in ICAD-related stroke patients receiving acute reperfusion therapy are warranted. The prognostic value of collateral circulation in secondary prevention of symptomatic ICAS patients have been investigated in more studies, although most of these studies were retrospective analyses or of a small scale. In a systematic review and meta-analysis, we have associated good leptomeningeal collaterals with a lower risk of stroke relapse and a higher chance of a favorable functional outcome, in medically treated symptomatic ICAS patients. Yet, the prognostic value of collaterals via the Circle of Willis and the possibly different prognostic values of collaterals by the location of ICAS or the degree of luminal stenosis need further verifications, preferably in prospective, larger-scale studies. In future studies, the collateral status could be an inclusion criterion in identifying high-risk, symptomatic ICAS patients, in studies exploring for more effective therapeutic interventions in the acute and chronic settings. Last but not least, tremendous efforts are needed to delineate the effects and mechanisms of therapeutic methods for collateral augmentation in ischemic stroke patients with or without ICAS, such as induced hypertension [74], sphenopalatine ganglion stimulation [75], external counterpulsation [76], remote limb ischemic preconditioning [77], and encephaloduroarteriosynangiosis, an indirect extracranial-intracranial bypass surgery [78] (more details covered in another article under this special issue [52]).
Data Availability
Data are available upon reasonable request to the corresponding author.
References
Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet. 2014;383:984–98.
Leng X, Wong KS, Liebeskind DS. Evaluating intracranial atherosclerosis rather than intracranial stenosis. Stroke. 2014;45:645–51.
Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–84.
Lan L, Leng X, Ip V, Soo Y, Abrigo J, Liu H, et al. Sustaining cerebral perfusion in intracranial atherosclerotic stenosis: the roles of antegrade residual flow and leptomeningeal collateral flow. J Cereb Blood Flow Metab. 2020;40:126–34.
Kern R, Steinke W, Daffertshofer M, Prager R, Hennerici M. Stroke recurrences in patients with symptomatic vs asymptomatic middle cerebral artery disease. Neurology. 2005;65:859–64.
Tanaka M, Shimosegawa E, Kajimoto K, Kimura Y, Kato H, Oku N, et al. Chronic middle cerebral artery occlusion: a hemodynamic and metabolic study with positron-emission tomography. AJNR Am J Neuroradiol. 2008;29:1841–6.
Liebeskind DS. Intracranial collateral routes and anastomoses in interventional neuroradilolgy. In: Hurst RW, Rosenwasser RH, editors. Neurointerventional Management: Diagnosis and Treatment. 2nd ed. CRC Press; 2012. p. 59–87.
Lou X, Ma X, Liebeskind DS, Ma N, Tian C, Lyu J, et al. Collateral perfusion using arterial spin labeling in symptomatic versus asymptomatic middle cerebral artery stenosis. J Cereb Blood Flow Metab. 2019;39:108–17.
Shi M, Leng X, Li Y, Chen Z, Cao Y, Chung T, et al. Genome sequencing reveals the role of rare genomic variants in Chinese patients with symptomatic intracranial atherosclerotic disease. Stroke Vas Neurol. 2021:svn-2021–001157.
Leng X, Hurford R, Feng X, Chan KL, Wolters FJ, Li L, et al. Intracranial arterial stenosis in Caucasian versus Chinese patients with TIA and minor stroke: two contemporaneous cohorts and a systematic review. J Neurol Neurosurg Psychiatry. 2021;92:590–7.
Leung S, Ng T, Yuen S, Lauder I, Ho F. Pattern of cerebral atherosclerosis in Hong Kong Chinese. Severity in intracranial and extracranial vessels. Stroke. 1993;24:779–86.
Baker AB, Resch JA, Loewenson RB. Cerebral atherosclerosis in European populations: a preliminary report. Stroke. 1973;4:898–903.
Wong KS, Huang YN, Yang HB, Gao S, Li H, Liu JY, et al. A door-to-door survey of intracranial atherosclerosis in Liangbei County. China Neurology. 2007;68:2031–4.
Zhang S, Zhou Y, Zhang Y, Gao X, Zhang Q, Wang A, et al. Prevalence and risk factors of asymptomatic intracranial arterial stenosis in a community-based population of Chinese adults. Eur J Neurol. 2013;20:1479–85.
Wong KS, Ng PW, Tang A, Liu R, Yeung V, Tomlinson B. Prevalence of asymptomatic intracranial atherosclerosis in high-risk patients. Neurology. 2007;68:2035–8.
Elmore EM, Mosquera A, Weinberger J. The prevalence of asymptomatic intracranial large-vessel occlusive disease: the role of diabetes. J Neuroimaging. 2003;13:224–7.
Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2013;12:1106–14.
Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–9.
Hurford R, Wolters FJ, Li L, Lau KK, Küker W, Rothwell PM. Prevalence, predictors, and prognosis of symptomatic intracranial stenosis in patients with transient ischaemic attack or minor stroke: a population-based cohort study. Lancet Neurol. 2020;19:413–21.
Ip B, Au L, Chan A, Fan F, Ip V, Ma SH, et al. Evolving ischemic stroke subtypes in 15 years: a hospital-based observational study. Int J Stroke. 2022;17:444–54.
Gao S, Wang YJ, Xu AD, Li YS, Wang DZ. Chinese ischemic stroke subclassification Front Neurol. 2011;2:6.
Feng X, Chan KL, Lan L, Abrigo J, Liu J, Fang H, et al. Stroke mechanisms in symptomatic intracranial atherosclerotic disease: classification and clinical implications. Stroke. 2019;50:2692–9.
Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–16.
Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003.
Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–467.
Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11–9.
Zaidat OO, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. 2015;313:1240–8.
Alexander MJ, Zauner A, Chaloupka JC, Baxter B, Callison RC, Gupta R, et al. WEAVE Trial: final results in 152 on-label patients. Stroke. 2019;50:889–94.
Alexander MJ, Zauner A, Gupta R, Alshekhlee A, Fraser JF, Toth G, et al. The WOVEN trial: Wingspan One-year Vascular Events and Neurologic Outcomes. J Neurointerv Surg. 2021;13:307–10.
Wabnitz AM, Derdeyn CP, Fiorella DJ, Lynn MJ, Cotsonis GA, Liebeskind DS, et al. Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke. 2019;50:143–7.
Yaghi S, Prabhakaran S, Khatri P, Liebeskind DS. Intracranial atherosclerotic disease: mechanisms and therapeutic implications. Stroke. 2019;50:1286–93.
Gui X, Wang L, Wu C, Wang H, Kong J. Prognosis of subtypes of acute large artery atherosclerotic cerebral infarction by evaluation of established collateral circulation. J Stroke Cerebrovasc Dis. 2020;29:105232.
Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–82.
Caplan LR, Wong KS, Gao S, Hennerici MG. Is hypoperfusion an important cause of strokes? If so, how? Cerebrovasc Dis. 2006;21:145–53.
Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Cloft HJ, Chimowitz MI, et al. Collateral circulation in symptomatic intracranial atherosclerosis. J Cereb Blood Flow Metab. 2011;31:1293–301.
Heil M, Eitenmüller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55.
Liang M, Wang P, Ma Y, Zhang X, Gao J, Ma M, et al. New vessels formation in young strokes with isolated steno-occlusive MCA. Brain Behav. 2018;8:e01088.
Kanazawa M, Takahashi T, Ishikawa M, Onodera O, Shimohata T, del Zoppo GJ. Angiogenesis in the ischemic core: a potential treatment target? J Cereb Blood Flow Metab. 2019;39:753–69.
Xu YY, Li ML, Gao S, Hou B, Sun ZY, Zhou HL, et al. Non-moyamoya vessel network formation along steno-occlusive middle cerebral artery. Neurology. 2016;86:1957–63.
Leng X, Lan L, Ip HL, Fan F, Ma SH, Ma K, et al. Translesional pressure gradient and leptomeningeal collateral status in symptomatic middle cerebral artery stenosis. Eur J Neurol. 2018;25:404–10.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97.
Menon BK, Smith EE, Coutts SB, Welsh DG, Faber JE, Goyal M, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol. 2013;74:241–8.
Nannoni S, Sirimarco G, Cereda CW, Lambrou D, Strambo D, Eskandari A, et al. Determining factors of better leptomeningeal collaterals: a study of 857 consecutive acute ischemic stroke patients. J Neurol. 2019;266:582–8.
Wiegers EJA, Mulder MJHL, Jansen IGH, Venema E, Compagne KCJ, Berkhemer OA, et al. Clinical and imaging determinants of collateral status in patients with acute ischemic stroke in MR CLEAN Trial and Registry. Stroke. 2020;51:1493–502.
Sinha A, Stanwell P, Beran RG, Calic Z, Killingsworth MC, Bhaskar SMM. Stroke aetiology and collateral status in acute ischemic stroke patients receiving reperfusion therapy-a meta-analysis. Neurol Int. 2021;13:608–21.
Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109-137.
Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–8.
Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30:525–31.
Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009;40:3001–5.
Menon BK, Smith EE, Modi J, Patel SK, Bhatia R, Watson TW, et al. Regional leptomeningeal score on CT angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions. AJNR Am J Neuroradiol. 2011;32:1640–5.
Liu L, Ding J, Leng X, Pu Y, Huang LA, Xu A, et al. Guidelines for evaluation and management of cerebral collateral circulation in ischaemic stroke 2017. Stroke Vas Neurol. 2018;3:117–30.
Cipolla MJ. Therapeutic induction of collateral flow. Transl Stroke Res. Published online ahead of print on 13 April 2022.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–418.
Leng X, Lan L, Liu L, Leung TW, Wong KS. Good collateral circulation predicts favorable outcomes in intravenous thrombolysis: a systematic review and meta-analysis. Eur J Neurol. 2016;23:1738–49.
Leng X, Fang H, Leung TW, Mao C, Xu Y, Miao Z, et al. Impact of collateral status on successful revascularization in endovascular treatment: a systematic review and meta-analysis. Cerebrovasc Dis. 2016;41:27–34.
Leng X, Fang H, Leung TWH, Mao C, Miao Z, Liu L, et al. Impact of collaterals on the efficacy and safety of endovascular treatment in acute ischemic stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87:537–44.
Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CBLM, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018;17:895–904.
Guglielmi V, LeCouffe NE, Zinkstok SM, Compagne KCJ, Eker R, Treurniet KM, et al. Collateral circulation and outcome in atherosclerotic versus cardioembolic cerebral large vessel occlusion. Stroke. 2019;50(12):3360–8.
Matusevicius M, Cooray C, Rand V-M, Nunes AP, Moreira T, Tassi R, et al. Stroke etiology and outcomes after endovascular thrombectomy: results from the SITS Registry and a meta-analysis. J Stroke. 2021;23:388–400.
Zhang X, Zhang M, Ding W, Yan S, Liebeskind DS, Lou M. Distinct predictive role of collateral status on clinical outcome in variant stroke subtypes of acute large arterial occlusion. Eur J Neurol. 2018;25:293–300.
Malhotra K, Liebeskind DS. Collaterals Ischemic Stroke Brain Hemorrhages. 2020;1:6–12.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12.
Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 10 Jan 2022.
Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69:963–74.
Lau AY, Wong EH, Wong A, Mok VC, Leung TW, Wong KS. Significance of good collateral compensation in symptomatic intracranial atherosclerosis. Cerebrovasc Dis. 2012;33:517–24.
Liu D, Li Y, Shi Z, Davis SM, Wong KS, Leung TW, et al. Presence of anterior temporal artery associates with good outcome in acute atherosclerotic M1-middle cerebral artery occlusion. Neuroradiology. 2014;56:1023–30.
Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014;45:663–9.
Kim KM, Kang HS, Lee WJ, Cho YD, Kim JE, Han MH. Clinical significance of the circle of Willis in intracranial atherosclerotic stenosis. J Neurointerv Surg. 2016;8:251–5.
Lee WJ, Jung KH, Ryu YJ, Kim JM, Lee ST, Chu K, et al. Utility of digital subtraction angiography-based collateral evaluation in medically treated acute symptomatic basilar artery stenosis. Eur J Neurol. 2017;24:1148–55.
Shang WJ, Chen HB, Shu LM, Liao HQ, Huang XY, Xiao S, et al. The association between flair vascular hyperintensity and stroke outcome varies with time from onset. AJNR Am J Neuroradiol. 2019;40:1317–22.
Wabnitz AM, Derdeyn CP, Fiorella DJ, Lynn MJ, Cotsonis GA, Liebeskind DS, et al. Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke. 2019;50(1):143–7.
Leung TW, Wang L, Zou X, Soo Y, Pu Y, Ip HL, et al. Plaque morphology in acute symptomatic intracranial atherosclerotic disease. J Neurol Neurosurg Psychiatry. 2021;92:370–6.
Bang OY, Chung JW, Kim SK, Kim SJ, Lee MJ, Hwang J, et al. Therapeutic-induced hypertension in patients with noncardioembolic acute stroke. Neurology. 2019;93:e1955–63.
Bornstein NM, Saver JL, Diener HC, Gorelick PB, Shuaib A, Solberg Y, et al. Sphenopalatine ganglion stimulation to augment cerebral blood flow: a randomized, sham-controlled trial. Stroke. 2019;50:2108–17.
Lin WH, Xiong L, Han JH, Leung TWH, Soo YOY, Chen XY, et al. External counterpulsation augments blood pressure and cerebral flow velocities in ischemic stroke patients with cerebral intracranial large artery occlusive disease. Stroke. 2012;43:3007–11.
Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79:1853–61.
Gonzalez NR, Jiang H, Lyden P, Song S, Schlick K, Dumitrascu O, et al. Encephaloduroarteriosynangiosis (EDAS) revascularization for symptomatic intracranial atherosclerotic steno-occlusive (ERSIAS) Phase-II objective performance criterion trial. Int J Stroke. 2021;16:701–9.
Funding
This work was supported by the Kwok Tak Seng Centre for Stroke Research and Intervention.
Author information
Authors and Affiliations
Contributions
X.L. performed literature search and drafted the article; T.W.L. revised the article. All the authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable of this review article.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leng, X., Leung, T.W. Collateral Flow in Intracranial Atherosclerotic Disease. Transl. Stroke Res. 14, 38–52 (2023). https://doi.org/10.1007/s12975-022-01042-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01042-3