Abstract
Remote ischemic conditioning (RIC) is a promising neuroprotective therapy for ischemic stroke. Preclinical studies investigating RIC have shown RIC reduced infarct volume, but clinical trials have been equivocal. Therefore, the efficacy of RIC in reducing infarct volume and quality of current literature needs to be evaluated to identify knowledge gaps to support future clinical trials. We performed a systematic review and meta-analysis of preclinical literature involving RIC in rodent models of focal ischemia. This review was registered with PROSPERO (CRD42019145441). Eligibility criteria included rat or mice models of focal ischemia that received RIC to a limb either before, during, or after stroke. MEDLINE and Embase databases were searched from 1946 to August 2019. Risk of bias was assessed using the SYRCLE risk of bias tool along with construct validity. Seventy-two studies were included in the systematic review. RIC was shown to reduce infarct volume (SMD − 2.19; CI − 2.48 to − 1.91) when compared to stroke-only controls and no adverse events were reported with regard to RIC. Remote ischemic conditioning was shown to be most efficacious in males (SMD − 2.26; CI − 2.58 to − 1.94) and when delivered poststroke (SMD − 1.34; CI − 1.95 to − 0.73). A high risk of bias was present; thus, measures of efficacy may be exaggerated. A limitation is the poor methodological reporting of many studies, resulting in unclear construct validity. We identified several important, but under investigated topics including the efficacy of RIC in different stroke models, varied infarct sizes and location, and potential sex differences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is the second leading cause of mortality worldwide [1]. Although more individuals are surviving stroke due to advances in acute care interventions (i.e., tissue plasminogen activator, thrombectomy), the number of people living with stroke-induced disabilities has significantly increased, making it the second leading cause of disability-adjusted life years [1]. With more than 80 million survivors worldwide, it is predicted that the incidence of stroke will be more than double between 2010 and 2050 due to changes in population demographics [1,2,3]. Since the number of individuals experiencing stroke and stroke-induced disability continues to rise, it is necessary to continue to investigate novel stroke therapeutics including neuroprotective strategies.
To date, neuroprotective drugs that showed efficacy in animal models have failed in clinical trials [4] likely because these compounds targeted single molecular pathways associated with cell death (e.g., calcium influx, excess glutamate) [5,6,7]. However, neuroprotective strategies that target multiple molecular pathways associated with cell death, such as hypothermia or prestroke exercise, have demonstrated the most clinical efficacy [4, 5]. Remote ischemic conditioning (RIC) is a therapy that involves repetitive cycles of occlusion and reperfusion of blood flow to a limb. A myriad of studies have found that it conveys protection against ischemia of a distant organ (e.g., brain and heart) and therefore may be a promising neuroprotective intervention for acute stroke [8, 9]. Similar to clinically induced hypothermia and exercise, RIC acts upon multiple neuroprotective pathways and is highly tolerable and safe, with no reported adverse side effects [10].
Over the last decade, the potential of RIC as a neuroprotectant for stroke has generated considerable interest. RIC can be delivered prophylactically before surgery or in high-risk populations (preconditioning), as well as during (per-conditioning) or shortly after (postconditioning) stroke. The literature suggests that RIC is neuroprotective in animal models of focal ischemia [8, 10]. Despite this early promise, clinical trials evaluating RIC efficacy in stroke have been equivocal [8]. To avoid repeating past failures in translating promising neuroprotective strategies, it is essential that the efficacy and quality of the preclinical evidence for RIC be evaluated [5,6,7]. Identifying issues in the existing literature is necessary to better inform future clinical trials.
The failed pharmacological neuroprotective approach for stroke did not meet international guidelines for designing and reporting preclinical research aimed at informing clinical trials [11, 12]. It is important that preclinical RIC research does not follow the same path. Three important sets of guidelines for preclinical stroke research are the stroke therapy academic industry roundtable (STAIR) [11, 13], stroke recovery and rehabilitation roundtable (SRRR) [14], and stem cell therapies as an emerging paradigm in stroke (STEPS) [11, 15, 16]. These guidelines advocate that novel stroke therapies should be evaluated in a randomized and blinded fashion in at least two species, and in both sexes. In addition, administration of the therapy should also be delivered during a clinically relevant time window using long-term functional outcomes (i.e., at least 1-month poststroke) in multiple stroke models [11, 14].
Although there have been previous reviews of RIC as a neuroprotective therapy, a systematic review and meta-analysis of the preclinical stroke literature has not been conducted [8, 17]. As such, we aimed to systematically determine the efficacy of RIC in reducing infarct volumes in rodent models of focal ischemic stroke. In addition, subgroups including sex, species, stroke model, and timing of RIC were examined in relation to the neuroprotective efficacy of RIC. Another major aim was to identify knowledge gaps in the RIC research literature considering the STAIR, SRRR, and STEPS recommendations. Addressing knowledge gaps in RIC literature and aligning with these guidelines are essential to increase the likelihood of successful translation of RIC to clinical populations.
Methods
Our systematic review was registered on PROSPERO (CRD42019145441) and is reported in accordance with PRISMA guidelines (Supplemental Table 1).
Eligibility Criteria
Preclinical studies using adult mice or rats were required to include an experimental group that underwent RIC in at least one limb and received a focal ischemic stroke. RIC must have exclusively occurred either before, during, or after stroke. Studies where RIC was delivered before and after stroke in the same animal or did not have a stroke control group that did not receive RIC were excluded since administering RIC before and after stroke is not clinically plausible. Studies conducted in models of global ischemia or hemorrhage, and those using neonates, non-rodent models, ex vivo and in vitro preparations, or humans were excluded. In addition, studies in which RIC was delivered to a remote organ (i.e., not a limb) or only had groups that RIC was delivered in conjunction with another therapy were excluded.
Outcomes
The primary outcome for this systematic review was infarct volume because it is the most widely used measure of neuroprotection [18]. Infarct volume was recorded as percentage of hemisphere, percentage of whole brain, or in mm3. All methods of infarct volume measurement were accepted (e.g., magnetic resonance imaging (MRI), tetrazolium chloride (TTC), cresyl violet). If infarct volume was measured at multiple timepoints, the last timepoint was recorded for analysis to assess the long-term effects of RIC. Secondary outcomes included mortality and adverse events. When provided, mortality was recorded as number of animals that died in each experimental group.
Literature Search Strategy
The literature search was performed using the Embase and MEDLINE databases from 1946 to August 14, 2019. The search strategy was developed by an information specialist (Risa Shorr, MLS, Ottawa Hospital Library Services) in conjunction with preclinical experts in the field of stroke recovery (MJ, MM, DC). Keywords related to RIC in rodent models of stroke were used to develop a search strategy. The search strategy can be located in the supplementary material. No date or language restrictions were applied to the search.
Study Selection Process
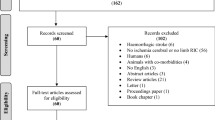
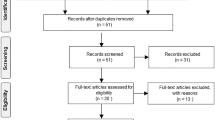
Studies identified from this search strategy were screened for duplicates. After removal of the duplicates, the remaining studies were uploaded into Distiller Systematic Review Software (DistillerSR). Study selection was documented using a PRISMA selection process flow diagram (Fig. 1). Initially, study titles and abstracts were screened by two independent researchers (AR, MM) based on the predetermined list of criteria. Studies that did not meet these criteria were excluded from the systematic review. Full-text articles were obtained for all articles determined to be relevant and the full-texts were screened by the same two reviewers (AR, MM). Studies that did not meet the eligibility criteria were removed from the review. Remaining studies that met eligibility criteria were included in the systematic review. Discrepancies were mediated through discussion with a third reviewer (MJ) to ensure consensus of inclusion/exclusion. Chinese language articles were run through Google Translate services and followed the same data extraction procedures as other articles. However, a fourth reviewer, a native Chinese reader (Junzheng Wu), also extracted data from the original (untranslated) text and verified the results obtained from Google Translate. Although review articles were not eligible for inclusion, their reference lists were reviewed to identify additional relevant articles. In the case of missing papers, articles were requested from authors.
Data Extraction
Following determination of eligible articles, data extraction was performed by two independent reviewers (AR, MM). Collected data included variables relating to study characteristics, RIC intervention, stroke induction, outcomes, risk of bias, and construct validity. Mean infarct volume was extracted from the text and supplementary data or in cases when not explicitly reported, extracted from figures using Engauge Digitizer. Discrepancies were mediated through discussion with a third reviewer (MJ) to ensure consensus. Missing data items were not requested from study authors as the data has not been peer reviewed.
Risk of Bias Assessment and Construct Validity
Risk of bias was assessed by using an adapted version of the SYRCLE risk of bias tool [19]. Each prompt was rated as a high, low, or unclear risk of bias. To assess the clinical generalizability of experimental conditions, the construct validity was assessed for each study. Construct validity items included the following: (1) use of adult animals, (2) temperature regulation during RIC, (3) confirmation of limb ischemia during RIC, (4) confirmation of successful stroke surgery, (5) monitoring of physiological conditions during stroke, (6) regulation of temperature during or after stroke, and (7) assessment of whether infarct volume was proportional to typical human stroke size. Each requirement was rated as either “good,” “poor,” or “unclear” by two independent reviewers (AR, MM). Discrepancies were mediated through discussion with a third reviewer (MJ) to ensure consensus of ratings.
Data Analysis
Studies were pooled using Comprehensive Meta-Analysis (CMA) (version 3; Biostat Inc., USA). For continuous outcomes, a standardized mean difference (SMD) was calculated using random effects inverse variance meta-analyses and presented with 95% confidence intervals. SMD was chosen due to the different measurement techniques for our primary outcome of infarct size. Statistical heterogeneity was assessed using the I2 statistic. An I2 value of > 50% was considered to indicate important heterogeneity requiring additional exploration. Where sufficient data were available, we performed subgroup analyses to determine whether the effect of RIC on infarct size varied by species, sex, number of limbs, which limb, type of RIC, number of sessions, number of cycles, duration of occlusion and reperfusion, RIC onset relative to stroke (preconditioning vs. postconditioning), time of RIC delivery relative to stroke, stroke model, and type of anesthesia (post hoc), randomization (post hoc), and blinding (post hoc). Significance of a subgroup interaction was determined by a z-test using CMA. For studies using a single control group and multiple experimental groups, control group sample sizes were split to avoid double counting control animals. Where experimental arms outnumbered control group animals, the review team made decisions on the most relevant experimental arms, in consultation with stroke experts. Publication bias was examined using funnel plots and Egger’s regression test for trials reporting our primary outcome. Studies with missing data items for specific subgroups were not included in the corresponding subgroup analysis.
Deviations from Protocol
For continuous variables (i.e., number of RIC cycles, number of RIC sessions, duration of RIC occlusion, duration of RIC reperfusion, time of RIC onset), a univariate random effects meta-regression analysis was performed using the method of moments approach to investigate potential underlying relationships that may explain some of the variability in effect sizes. Subgroups included in this analysis were number of RIC cycles, number of RIC sessions, duration of RIC occlusion/reperfusion, duration of RIC occlusion/reperfusion during preconditioning/postconditioning, latest infarct measurement timepoint, and time of RIC onset in preconditioning and postconditioning. We included an additional subgroup analysis with regard to the type of anesthesia used. This subgroup is importantly included to assess if there are any differences in the neuroprotective benefits of different anesthetics when in conjunction with RIC.
Results
Characteristics of Included Studies
Included studies were published between 2007 and 2019 from 8 countries (Supplemental Table 2). Sample sizes in the studies ranged from 5 to 54 with a mean of 6.7 ± 1.27 animals. Of the species used in each study, 86.1% and 13.9% were rats and mice, respectively (Table 1). The majority of the studies used solely male (87.5%) animals, 5.6% of studies used solely female animals, and 1.4% of studies used both sexes. A small number of studies (5.6%) did not report the sex of the animals used. At least one behavioral outcome was included in 79.2% of studies. Comorbidities were reported in 4.2% of the studies and 2.8% involved a comorbidity (type II diabetes mellitus) commonly associated with stroke. Excluded animals were reported in 13.9% of studies (Supplemental Table 2).
Stroke Model Characteristics and Infarct Volume Assessment
Reperfusion models were the most common model for stroke (84.7%). The intraluminal suture and embolism models accounted for 81.9% and 2.8% of all strokes respectively. The most widely used occlusion time for reperfusion models of stroke was 90 min (33.3%) followed by 120 min (27.8%), 60 min (16.7%), 45 min (2.8%), and 100 min (1.4%). Few studies (2.8%) did not report the occlusion time during stroke. Permanent occlusion models accounted for 15.3% of stroke models used. More specifically, 9.7% of studies used a cauterization model and 1.4% of studies used either a permanent clip, permanent distal MCA ligation, permanent intraluminal suture, or a modified 3 vessel occlusion (Table 1).
The method of infarct volume assessment was collected from the included studies (Table 1). The majority of studies used TTC staining (84.7%), followed by cresyl violet (2.8%), and MRI (2.8%). Some studies (9.7%) did not report how infarct volume was measured. Infarct volume assessment was most commonly measured 1 (52.8%), 2 (15.3%), and 3 days (12.5%) after stroke onset. Infarct measured at shorter and longer time intervals was only performed in 1.4% of all studies. Infarct volume data was not provided in 11.1% of studies.
Remote Ischemic Conditioning Intervention Characteristics
The most widely used method for remote ischemic conditioning was invasive femoral artery occlusion (52.8%), followed by tourniquet (36.1%), and blood pressure cuff (5.6%). It was unclear how RIC was performed in 6.9% of studies. During the RIC procedure, 22.2% used chloral hydrate anesthesia, 20.8% used isoflurane, 12.5% used sodium pentobarbital, and 6.9% used enflurane. Less commonly, 1.4% used sevoflurane, urethane, or Zoletil. Of the included studies, 1.4% explicitly stated that anesthesia was not used and 8.3% did not report using anesthesia. The most common occlusion times for RIC were 5 (34.7%), 10 (48.6%), and 15 (15.3%) minutes. The majority of studies performed a single session of RIC (93.1%) with 3 cycles of occlusion/reperfusion during a session of RIC (84.7%). Occluding both limbs during RIC was more common (52.8%) than one limb, where the ipsilesional hindlimb was used more (23.6%) than the contralateral hindlimb (9.7%). Details of limb occlusion were not reported by 8.6% of RIC studies. Finally, 69.4% of studies implemented RIC poststroke (postconditioning) compared to 34.7% who applied RIC before stroke (preconditioning). A total of 68.2% of the studies initiated RIC within 2 h after cerebral ischemia onset. No adverse events related to RIC were reported in any of the studies (Table 1).
Effect of RIC on Infarct Volume
Overall, RIC was shown to have a protective effect by reducing infarct volume (Fig. 2). Experimental groups that received RIC showed a significant reduction in infarct volume compared to the control groups (SMD − 2.19; 95% CI − 2.48 to − 1.91, I2 = 74%).
Efficacy of RIC under Varying Parameters
The efficacy of RIC between different subgroups was assessed across studies (Fig. 3). There was a significant difference in the efficacy of RIC between male (SMD − 2.26; CI − 2.58 to − 1.94, I2 = 75%) and female animals (SMD − 1.34; CI − 1.95 to − 0.73, I2 = 42%) when compared to their respective control groups. One study that included both male and female animals found a large reduction in infarct volume in the RIC animals compared to stroke but had large variance when examining the 95% confidence interval (SMD − 3.53; CI − 4.94 to − 2.13). There was no significant difference in the efficacy of RIC between permanent (SMD − 2.33; CI − 2.98 to − 1.68, I2 = 62%) and reperfusion (SMD − 2.17; CI − 2.49 to − 1.85, I2 = 75%) stroke models. Similarly, there was no significant difference in the efficacy of RIC in terms of type of RIC used such as invasive occlusion (SMD − 2.35; CI − 2.71 to − 2.00, I2 = 65%), tourniquet (SMD − 1.83; CI − 2.35 to − 1.31, I2 = 82%), and blood pressure cuff (SMD − 2.48; CI − 2.98 to − 1.43, I2 = 0%). However, there was a significant difference in the efficacy of RIC between preconditioning and postconditioning where postconditioning (SMD − 2.40; CI − 2.75 to − 2.06, I2 = 72%) was significantly more effective at reducing infarct volume compared to preconditioning (SMD − 1.77; CI − 2.27 to − 1.27, I2 = 76%). There were no significant differences in the efficacy of RIC in terms of number of limbs, specific limb(s) used for RIC, number of cycles, number of sessions, time between sessions, duration of occlusion/reperfusion, and species (SFig. 1–7). Overall, studies that used anesthesia had a larger reduction in infarct volume than the study that did not use anesthesia (SMD − 0.04; CI − 1.01 to 0.93). Anesthetics included were chloral hydrate (SMD − 3.39; CI − 4.34 to − 2.44), sodium pentobarbital (SMD − 1.86; CI − 2.55 to − 1.18), enflurane (SMD − 3.39; CI − 4.34 to − 2.44), sevoflurane (SMD − 1.32; CI − 2.59 to − 0.05), both urethane and isoflurane (SMD − 1.62; CI − 2.35 to − 0.89), and Zoletil (SMD − 0.76; CI − 1.79 to 0.26). Some studies were unclear (SMD − 2.42; CI − 2.92 to − 1.92) in which anesthesia was used. No significant findings were found in the meta-regression analysis of the subgroups (SFig. 8–18).
Forest plots of the effect of remote ischemic conditioning in subgroups. Reported as standardized mean difference and a 95% confidence interval for (a) sex, (b) stroke type, (c) type of RIC, (d) preconditioning versus postconditioning, and (e) type of anesthethic. n is the number of groups that fall within the category per subgroup. Meta-analysis of RIC literature suggests that postconditioning is significantly more neuroprotective than preconditioning, and there is no difference in efficacy in male rodent models compared to female or model of stroke or RIC type
Risk of Bias
Regarding the risk of potential bias of the included studies (Fig. 4), the overall quality of the studies was poor. Most studies (91.7%) did not report if group allocation was randomized nor described the method of randomization. Similarly, most studies (95.8%) did not report the randomization of brains selected for infarct volume assessment. Only 1.4% reported ensuring that the experimental groups were similar before surgery/intervention. A small proportion of the included studies explicitly reported blinding of group allocation (8.3%) and blinding of the personnel to stroke status of subjects in the RIC intervention group (8.3%). Furthermore, only 11.1% of the studies reported blinding of personnel during infarct volume assessment. All studies omitted reporting if animals were randomly housed during the experiment. There was no difference in the efficacy of RIC at reducing infarct volume in terms of reported blinding or randomization (SFig 19–20). Finally, 27.8% of studies had no other potential sources of bias, with 18.1% and 54.2% have an unclear and high risk of bias, respectively. Visual inspection of the funnel plot (Fig. 5) and Egger’s regression test (p < 0.0001) both indicate the possible presence of publication bias in our results.
Construct Validity
Construct validity, or the clinical generalizability, was determined for the included studies (Fig. 6). Adult animals were clearly identified in 52.8% of studies and 1.4% of studies reported the use of immature animals. Monitoring and/or regulating temperature during RIC was reported in 29.2% of studies. Confirmation of ischemia was reported in 29.2% and 43.1% of studies during RIC and stroke surgery, respectively. During stroke, 76.4% reported monitoring temperature and 19.4% reported physiological monitoring. Finally, 62.5% produced very large infarct volumes akin to malignant infarction in humans (> 40% of one hemisphere). No studies produced an infarct size proportional to that typically observed clinically (4.5–14% of the ipsilateral hemisphere) [20].
Discussion
Overall Efficacy of RIC
The main aim of this systematic review was to assess the preclinical evidence to determine if RIC reduces infarct volume following a focal ischemic stroke. We identified and analyzed 72 different preclinical RIC studies that showed an overall beneficial effect of RIC in reducing infarct volume.
RIC Subgroups
Remote ischemic conditioning was beneficial in both male and female animals but provided significantly more protection in males. It is unknown what may be underlying the sex-specific effect of RIC. It could be due to a number of factors not limited to the effects of anesthesia, sex-specific hormones, or differing mechanisms of cell death between sexes. One study that met our inclusion criteria did include both male and female animals but did not stratify the results based on sex [21]. The incidence of stroke is higher among women compared to men, with women experiencing poorer outcomes [22]. Given the paucity of studies using female rodents, more RIC studies using both males and females are needed.
RIC was found most effective when delivered after stroke, with the majority of studies delivering RIC at the onset of reperfusion when animals were still anesthetized. The close temporal pairing of RIC with the neuroprotective effects of anesthesia may have resulted in a greater reduction in infarct volume than when RIC is delivered prior to stroke and animals are allowed to awaken from anesthesia. In addition, the delivery of RIC at the time of reperfusion could interfere with the initiation of the ischemic cascade and thus is of limited clinical relevance since few patients can be treated in such a narrow time window [5, 23].
Only two studies looked at chronic versus acute delivery of RIC. Chronic RIC refers to the administration of multiple RIC sessions over an extended period of time (e.g., 1 session of RIC/day for a week) whereas acute is a single session of RIC, typically soon after stroke. Clinically, chronic delivery of RIC has been used to reduce the reoccurrence of stroke [24] and there are trials underway employing RIC chronically to improve recovery in patients [25]. Given the very limited evidence, further studies using chronic RIC and delivering RIC outside of the neuroprotective window should be a preclinical research priority as it is important for stroke recovery.
Most RIC studies used the intraluminal suture stroke model. While this model encompasses transient occlusion similar to most human stroke, it produces disproportionately large strokes that can cause injury in structures (e.g., hypothalamus) that normally do not occur in human stroke [14, 20]. Infarct volume was most commonly assessed 1 or 2 days following stroke with only 4 studies measuring infarct volume later than 3 days. While these studies show a significant reduction in infarct volume, a serious concern is that neurons can continue to die days and weeks following stroke treatments [7, 26], including ischemic preconditioning [27, 28]. Four studies reported infarct volume with longer survival times but the results are inconsistent [29,30,31,32]. Two of these studies measured infarct volume at both an early and late timepoint (1 day and 21 days [30]; 2 days and 7 days [31]) and showed a reduction in infarct volume at both timepoints. Contrarily, another study found that infarct volume measured at 60 days was larger than that of 2 days following stroke [32]. This finding is similar to what is seen clinically where infarct volume measured 24–36 h following stroke onset is smaller than infarct volume measured a week later [33].
Overall, our analysis shows that RIC reduces infarct volume in the short term, but whether RIC is truly neuroprotective or simply delaying cell death is unknown. Future research should include infarct volume measurements at multiple times including a very delayed timepoint as suggested in the STAIR guidelines [34]. In addition to infarct volume, behavioral outcomes at chronic time points need to be assessed as these have the greatest clinical relevance [7].
Remote ischemic conditioning was frequently delivered invasively (i.e., femoral artery occlusion), followed by the tourniquet and blood pressure cuff methods. This invasive procedure does not translate well to humans and given similar efficacy between invasive and non-invasive RIC approaches (e.g., blood pressure cuff), future work should consider use of non-invasive RIC. Subgroup analysis showed that all methods of RIC reduced infarct volume irrespective of whether one or both hindlimbs were occluded.
Risk of Bias
This systematic review uncovered substantial areas of bias due to poor methodological reporting in the included studies using an adapted version of the SYRCLE risk of bias tool [19]. A very small percentage of studies randomized animals and explicitly reported how and when randomization was completed. Time of randomization relative to stroke and intervention procedure is necessary to ensure a low risk of bias. For studies performing remote ischemic postconditioning, randomization should occur following stroke and before RIC. The incompleteness in methodological reporting exposed by this systematic review in preclinical RIC research is common among preclinical research as a whole [35, 36] and other preclinical stroke papers [37]. Interestingly, a systematic review and meta-analysis performed by Jerndal et al. [38] showed that studies that reported randomization or blinding resulted in a lower efficacy of erythropoietin at reducing infarct volume and therefore overestimating the benefits of the therapy. Future studies should follow the Animal Research: Reporting of In Vivo Experiments (ARRIVE) updated guidelines to ensure methodological reporting is complete to increase transparency and reproducibility [39].
Only a small number of studies reported mortality and infarct volume, and of those that did report mortality, few specified from which experimental groups. Given the high mortality and morbidity rate of the intraluminal suture model [40] predominantly used in these studies, the absence of such data is problematic and suggests a high likelihood of attrition bias.
Other sources of bias included differences between the control and RIC procedures and histological preparation of the brain for infarct assessment. A number of studies did not match control and intervention procedures. One glaring example is the additional anesthetic that the animals were given at the time of RIC. This is important since certain anesthetics have weak neuroprotective effects that could reduce infarct volume [41]. To circumvent such problems, both control and RIC groups should be exposed to identical anesthetic conditions [42].
Another limitation is the publication bias that was present. There are potentially some studies that had negative results that were not published. Therefore, this may lead to an overestimation of the effect of RIC on reducing infarct volume.
Construct Validity
Age is the strongest non-modifiable risk factor for stroke [43]. The majority of strokes occur in those over the age of 65 and the likelihood of having a stroke increases with age [2]. With respect to age, there is a disconnect between a typical stroke patient and preclinical stroke models where most studies use young healthy animals [12]. A large proportion of studies included in the systematic review did not report the ages of the animals used. Age-related changes could negatively impact the infarct size and other outcomes following stroke. For example, there is decreased cerebral blood flow, angiogenesis, and neurogenesis with age which may increase stroke severity or impairment. However, one study that used older animals found RIC to be efficacious [44]. Nonetheless, subtle age-related brain changes could alter the efficacy of therapies and therefore need to be taken into consideration in preclinical studies.
Similarly, the vast majority of individuals who have a stroke have existing disease comorbidities [45]. Of the studies identified, only two studies included a comorbidity (type II diabetes mellitus) which is commonly associated with stroke [46] and in these studies, RIC reduced infarct volume. While these results hold translational promise, other comorbidities such as hypertension, high cholesterol, and heart disease should also be investigated [46]. Indeed, studies investigating RIC-induced cardioprotection have found that including disease comorbidities (e.g., hypertension and hyperlipidemia) attenuates the benefits of RIC [9]. As such, further investigation into how comorbidities impact the efficacy of RIC as a neuroprotective strategy need to be performed.
Few studies explicitly reported measuring or regulating temperature during RIC while more than 75% of the studies reported measuring or regulating temperature during the stroke. Additionally, most studies did not report monitoring of other physiological variables during stroke. The underreporting of temperature regulation has the potential to skew the efficacy of RIC towards reducing infarct volume if body temperature is lower than normal physiological levels. Minor changes in body temperature, at the time of and following stroke, can affect infarct volume. For example, immediate and delayed hypothermia has been shown to decrease infarct volume in rat models of focal cerebral ischemia [47] while mild hyperthermia increases lesion volume [48]. However, RIC is effective in studies that monitored body temperature and other physiological variables, but the overall effect of RIC may be exaggerated if mild hypothermia was present. Therefore, to avoid confounding variables that may affect infarct volume, temperature should be monitored and regulated before, during, and after both stroke and RIC treatment, especially since RIC is often delivered soon following stroke.
Anesthesia during RIC delivery is another concern because it is reportedly neuroprotective in preclinical models of stroke, especially with short survival times [41] and stroke patients receiving RIC would not be anesthetized. Silachev et al. [49] examined the effects of different anesthetics on the efficacy of RIC and found that RIC in the absence of anesthesia did not reduce infarct volume. However, the considerable stress of limb ischemia in a non-anesthetized rodent may simply have exacerbated injury offsetting any benefit of RIC. Post hoc analysis does highlight that there may be an exaggeration of the neuroprotective benefits of RIC due to the administration of anesthetics (Fig. 3).
Less than 50% of studies confirmed effective ischemia during RIC or stroke. Confirmation of occlusion during stroke by Laser Doppler imaging is recommended to ensure consistent stroke injury between experimental groups [34]. Different stroke volumes between animals may bias apparent therapeutic effectiveness where the therapy may not be as efficacious in smaller strokes, such as those typically seen in humans, when compared to the large strokes often employed in preclinical studies.
It is also important to note that none of the included studies produced an infarct proportional to that commonly observed in humans. In humans, a typical infarct volume following stroke is 4.5–14% of the ipsilesional hemisphere [20]. It is unclear whether RIC efficacy varies in relation to lesion size or location; however, strokes in humans that encompass the same relative proportion of brain tissue as that destroyed by the intraluminal suture model in rodents would likely be associated with death or severe chronic impairment [20]. This further highlights the importance of choosing clinically relevant models of stroke in preclinical studies outlined by the SRRR guidelines [12]. Clearly, if the goal is to establish a novel therapy to reduce infarct volume, the stroke injury produced in preclinical studies should be similar pathologically (relative size and location) to what is observed clinically.
Conclusion
Remote ischemic conditioning is a novel and promising therapy for ischemic stroke. Preclinical studies have shown that RIC decreases infarct volume, especially if administered following stroke. While the results are encouraging, there are many knowledge gaps and areas where evidence is weak (e.g., efficacy after long term survival). Future studies should include a more comprehensive description of methods and most importantly adhere to the STAIR, SRRR, and STEPS guidelines for stroke [11, 12]. The present study highlights the considerable promise for RIC as a potential neuroprotective treatment for stroke but cautions that the current preclinical evidence base contains many gaps that presently may limit successful clinical translation.
Data Availability
Data available in supplementary material.
References
Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Abate D, et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439–58.
Krueger H, Koot J, Hall RE, O’Callaghan C, Bayley M, Corbett D. Prevalence of individuals experiencing the effects of stroke in Canada: trends and projections. Stroke. 2015;46:2226–31.
Howard G, Goff DC. Population shifts and the future of stroke: forecasts of the future burden of stroke. Ann N Y Acad Sci. 2012;1268:14–20.
Patel RAG, McMullen PW. Neuroprotection in the Treatment of Acute Ischemic Stroke. Prog Cardiovasc Dis. Elsevier Inc. 2017;59:542–8.
Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci. 2011;14:1363–8.
Bosetti F, Koenig JI, Ayata C, Back SA, Becker K, Broderick JP, et al. Translational stroke research: vision and opportunities. Stroke. 2017;48:2632–7.
Corbett D, Nurse S. The problem of assessing effective neuroprotection in experimental cerebral ischemia. Prog Neurobiol. 1998;54:531–48.
Landman TRJ, Schoon Y, Warlé MC, De Leeuw FE, Thijssen DHJ. Remote ischemic conditioning as an additional treatment for acute ischemic stroke: the preclinical and clinical evidence. Stroke. 2019;50:1934–9.
Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–95.
Hess DC, Blauenfeldt RA, Andersen G, Hougaard KD, Hoda MN, Ding Y, et al. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat Rev Neurol Nature Publishing Group. 2015;11:698–710.
Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl Stroke Res. 2013;4:279–85.
Bernhardt J, Hayward KS, Dancause N, Lannin NA, Ward NS, Nudo RJ, et al. A stroke recovery trial development framework: consensus-based core recommendations from the second stroke recovery and rehabilitation roundtable. Int J Stroke. 2019;14:792–802.
Fisher M. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–8.
Corbett D, Carmichael ST, Murphy TH, Jones TA, Schwab ME, Jolkkonen J, et al. Enhancing the alignment of the preclinical and clinical stroke recovery research pipeline: consensus-based core recommendations from the stroke recovery and rehabilitation roundtable translational working group. Neurorehabil Neural Repair. 2017;31:699–707.
Wechsler LR. Stem cell therapies as an emerging paradigm in stroke (STEPS) bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–5.
Savitz SI, Chopp M, Deans R, Carmichael ST, Phinney D, Wechsler L, et al. Stem cell therapy as an emerging paradigm for stroke (STEPS) II. Stroke. 2011;42:825–9.
Zhao W, Li S, Ren C, Meng R, Jin K, Ji X. Remote ischemic conditioning for stroke: clinical data, challenges, and future directions. Ann Clin Transl Neurol. 2019;6:186–96.
Sutherland BA, Minnerup J, Balami JS, Arba F, Buchan AM, Kleinschnitz C. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int J Stroke. 2012;7:407–18.
Hooijmans CR, Rovers MM, De Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:1–9.
Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409.
Xiao B, Chai Y, Lv S, Ye M, Wu M, Xie L, et al. Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int J Mol Med. 2017;40:1201–9.
Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–26.
Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJB, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947.
Meng R, Ding Y, Asmaro K, Brogan D, Meng L, Sui M, et al. Ischemic conditioning is safe and effective for octo- and nonagenarians in stroke prevention and treatment. Neurotherapeutics. 2015;12:667–77.
Li XQ, Tao L, Zhou ZH, Cui Y, Chen HS. Remote ischemic conditioning for acute moderate ischemic stroke (RICAMIS): rationale and design. Int J Stroke. 2019;15:454–60.
Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;68:161.
Corbett D, Crooks P. Ischemic preconditioning: a long term survival study using behavioural and histological endpoints. Brain Res. 1997;760:129–36.
Dooley P, Corbett D. Competing processes of cell death and recovery of function following ischemic preconditioning. Brain Res. 1998;794:119–26.
Wang Y, Zhang Z, Zhang L, Yang H, Shen Z. RLIPostC protects against cerebral ischemia through improved synaptogenesis in rats. Brain Inj Taylor & Francis. 2018;32:1429–36.
Liang D, He XB, Wang Z, Li C, Gao BY, Wu JF, et al. Remote limb ischemic postconditioning promotes motor function recovery in a rat model of ischemic stroke via the up-regulation of endogenous tissue kallikrein. CNS Neurosci Ther. 2018;24:519–27.
Qi W, Zhou F, Li S, Zong Y, Zhang M, Lin Y, et al. Remote ischemic postconditioning protects ischemic brain from injury in rats with focal cerebral ischemia/reperfusion associated with suppression of TLR4 and NF-κB expression. Neuroreport. 2016;27:469–75.
Wei D, Ren C, Chen X, Zhao H. The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke. PLoS One. 2012;7(2):e30892.
Pantano P, Caramia F, Bozzao L, Dieler C, von Kummer R. Delayed increase in infarct volume after cerebral ischemia: correlations with thrombolytic treatment and clinical outcome. Stroke. 1999;30:502–7.
Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–50.
Fergusson DA, Wesch NL, Leung GJ, MacNeil JL, Conic I, Presseau J, et al. Assessing the completeness of reporting in preclinical oncolytic virus therapy studies. Mol Ther - Oncolytics. Elsevier Ltd. 2019;14:179–87.
Fergusson DA, Avey MT, Barron CC, Bocock M, Biefer KE, Boet S, et al. Reporting preclinical anesthesia study (REPEAT): evaluating the quality of reporting in the preclinical anesthesiology literature. PLoS One. 2019;14:1–15.
MacLeod MR, Van Der Worp HB, Sena ES, Howells DW, Dirnagl U, Donnan GA. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke. 2008;39:2824–9.
Jerndal M, Forsberg K, Sena ES, MacLeod MR, O’Collins VE, Linden T, et al. A systematic review and meta-analysis of erythropoietin in experimental stroke. J Cereb Blood Flow Metab. 2010;30:961–8.
du Sert NP, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The arrive guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18:1–12.
MacRae I. Preclinical stroke research - advantages and disadvantages of the most common rodent models of focal ischaemia. Br J Pharmacol. 2011;164:1062–78.
Archer DP, Walker AM, McCann SK, Moser JJ, Appireddy RM. Anesthetic neuroprotection in experimental stroke in rodents: a systematic review and meta-analysis. Anesthesiology. 2017;126:653–65.
Sanders RD, Ma D, Maze M. Anaesthesia induced neuroprotection. Best Pract Res Clin Anaesthesiol. 2005;19:461–74.
Roy-O’Reilly M, McCullough LD. Age and sex are critical factors in ischemic stroke pathology. Endocrinology. 2018;159:3120–31.
Zhang Y, Liu X, Yan F, Min L, Ji X, Luo Y. Protective effects of remote ischemic preconditioning in rat hindlimb on ischemia- reperfusion injury. Neural Regen Res. Wolters Kluwer -- Medknow Publications. 2012;7:583–7.
Mergenthaler P, Meisel A. Do stroke models model stroke? DMM Dis Model Mech. 2012;5:718–25.
Magwood GS, White BM, Ellis C. Stroke-related disease comorbidity and secondary stroke prevention practices among young stroke survivors. J Neurosci Nurs. 2017;49:296–301.
Xue D, Huang ZG, Smith KE, Buchan AM. Immediate or delayed mild hypothermia prevents focal cerebral infarction. Brain Res. 1992;587:66–72.
Noor R, Wang CX, Shuaib A. Effects of hyperthermia on infarct volume in focal embolic model of cerebral ischemia in rats. Neurosci Lett. 2003;349:130–2.
Silachev DN, Usatikova EA, Pevzner IB, Zorova LD, Babenko VA, Gulyaev MV, et al. Effect of anesthetics on efficiency of remote ischemic preconditioning. Biochem. 2017;82:1006–16.
Acknowledgments
We would like to acknowledge and thank Risa Shorr for performing the literature search and Junzheng Wu for verifying collected data from manuscripts written in Chinese.
Funding
This study was funded by the Heart and Stroke Foundation Canadian Partnership for Stroke Recovery and the Canadian Consortium on Neurodegeneration in Aging. MWM is supported by the Canadian Vascular Network.
Author information
Authors and Affiliations
Contributions
Conceptualization: Manoj Lalu, Dale Corbett. Data analysis: Allyson Ripley, Matthew Jeffers, Matthew McDonald, Joshua Montroy. Writing—original draft preparation: Allyson Ripley, Joshua Montroy. Writing—review and editing: Allyson Ripley, Matthew Jeffers, Matthew McDonald, Joshua Montroy, Angela Dykes, Dean Fergusson, Gergely Silasi, Lalu Manoj, Dale Corbett. Funding acquisition: Dale Corbett. Supervision: Dale Corbett.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 11216 kb)
Rights and permissions
About this article
Cite this article
Ripley, A.J., Jeffers, M.S., McDonald, M.W. et al. Neuroprotection by Remote Ischemic Conditioning in Rodent Models of Focal Ischemia: a Systematic Review and Meta-Analysis. Transl. Stroke Res. 12, 461–473 (2021). https://doi.org/10.1007/s12975-020-00882-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-020-00882-1