Abstract
While preclinical stroke studies have shown that mesenchymal stem cells (MSCs) promote recovery, few randomized controlled trials (RCT) have assessed cell therapy in humans. In this RCT, we assessed the safety, feasibility, and efficacy of intravenous autologous bone marrow-derived MSCs in subacute stroke. ISIS-HERMES was a single-center, open-label RCT, with a 2-year follow-up. We enrolled patients aged 18–70 years less than 2 weeks following moderate-severe ischemic carotid stroke. Patients were randomized 2:1 to receive intravenous MSCs or not. Primary outcomes assessed feasibility and safety. Secondary outcomes assessed global and motor recovery. Passive wrist movement functional MRI (fMRI) activity in primary motor cortex (MI) was employed as a motor recovery biomarker. We compared “treated” and “control” groups using as-treated analyses. Of 31 enrolled patients, 16 patients received MSCs. Treatment feasibility was 80%, and there were 10 and 16 adverse events in treated patients, and 12 and 24 in controls at 6-month and 2-year follow-up, respectively. Using mixed modeling analyses, we observed no treatment effects on the Barthel Index, NIHSS, and modified-Rankin scores, but significant improvements in motor-NIHSS (p = 0.004), motor-Fugl-Meyer scores (p = 0.028), and task-related fMRI activity in MI-4a (p = 0.031) and MI-4p (p = 0.002). Intravenous autologous MSC treatment following stroke was safe and feasible. Motor performance and task-related MI activity results suggest that MSCs improve motor recovery through sensorimotor neuroplasticity.
ClinicalTrials.gov Identifier NCT 00875654.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a leading cause of acquired disability, affecting 70% of survivors. After the acute stage, no treatments other than rehabilitation reliably facilitate recovery [1]. Experimental stroke studies have shown that mesenchymal stem cell (MSC) administration may lead to statistically significant improvements in functional outcome [2, 3]. Nevertheless, the clinical use of MSCs has raised safety concerns [4,5,6], as they may sometimes promote subsequent inflammation [7], tumor growth, metastasis, and unwarranted differentiation [8].
In subacute ischemic stroke, the few RCTs assessing cell therapy have shown good safety [9,10,11]. Among them, only one RCT examined intravenous (IV) autologous MSC effects, showing good short- and long-term safety, but questionable feasibility, as only one third of patients received MSCs and the group mortality rate was 48% [9, 12].
Regarding efficacy, while a recent meta-analysis showed that cell therapy may be beneficial in stroke [13], individual trials have not shown statistically significant results. It is possible that the use of global clinical outcome measures accounts for some of the observed poor efficacy. While motor performance has been widely used in experimental studies to test cell therapy effects, motor behavior outcomes are not usually tested in stroke recovery RCTs. We thus hypothesized that using motor performance measures would result in more sensitive detection of treatment effects.
The mechanisms by which the MSC secretome may promote recovery during the subacute phase of stroke include inflammation modulation, increased angiogenesis and endogenous neurogenesis, and decreased apoptosis, all contributing to brain repair [3]. Brain repair based on the reorganization of damaged brain networks [14, 15] can be captured by functional MRI (fMRI) activity measures [16]. In fact, there is strong evidence that primary motor cortex (MI) activity can serve as a motor recovery biomarker, and that fMRI can provide objective, precise and accurate measures of outcome, as compared with quantitative motor behavior measurements [16,17,18,19].
We did a 2-year randomized controlled trial (RCT) using autologous IV bone marrow-derived MSCs in patients with subacute ischemic stroke with two aims: (1) to assess safety and feasibility of IV autologous MSCs administered 1 month after stroke and (2) to perform exploratory analyses of MSC treatment effects on global and sensorimotor behavioral outcomes and MI activity assessed longitudinally during a 2-year follow-up period.
Methods
Study Design and Intervention
The trial was a single-center (Grenoble Alpes University Hospital (CHUGA), France), prospective, open-label RCT with blind outcome evaluation (PROBE design) assessing the effects of a single IV injection of autologous bone marrow-derived MSCs. The trial included both a clinical study, Intravenous Stem cells After Ischemic Stroke (ISIS) RCT and an MRI substudy “heuristic value of multimodal MRI to assess mesenchymal stem cell therapy in stroke” (HERMES).
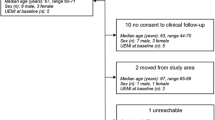
Patients were randomized 2:1 to receive an IV injection of MSCs coupled with rehabilitation (treated group) or rehabilitation alone (control group). All patients followed a 3- to 6-month rehabilitation program including 5 days each week of both intensive physiotherapy and occupational therapy in a neurologic rehabilitation center. The rehabilitation program was planned by a multidisciplinary team including several physicians, physiotherapists, and speech-language and occupational therapists who were not aware of treatment status. The MSC group received two different doses: the first ten patients assigned to treatment received low-dose MSCs (100 million) and the next ten patients received high-dose MSCs (300 million) (Fig. 1). The rationale for these doses was based on previous pre-clinical work in rats [20,21,22] and a previous clinical trial in humans [9]. The treatment delay, designed to target the subacute stroke period during which MSCs may exert immunomodulatory effects, was constrained by the time required for autologous cell expansion (i.e., 3–4 weeks).
The inclusion visit occurred 10 ± 5 days following stroke onset. After the time required for cell expansion (3–4 weeks), the baseline visit (M0) occurred 1 day before MSC injection, 31 ± 7 days following stroke onset. Follow-up visits were performed after 15 ± 2 days (M0.5), 60 ± 7 days (2 months (M2)), 120 ± 7 days (4 months (M4)), 180 ± 15 days (6 months (M6)), 365 ± 30 days (12 months (M12)), and 730 ± 30 days (24 months (M24)) following M0.
Participants
Patients aged 18–65 years with an MRI confirmed carotid ischemic stroke less than 2 weeks previously were enrolled in the study if they fulfilled all inclusion criteria (Supplementary Table 1). All patients had a National Institute of the Health Stroke Scale (NIHSS) score above 10 at the time of cell injection. Because possible participants frequently exhibited spontaneous recovery in the first month after stroke, the protocol was amended in July 2013, after 20 patients had been included, extending the upper age limit to 70 years and reducing the minimum baseline NIHSS to 7. Patients were screened for eligibility in the Stroke Units of CHUGA, Annecy and Chambery Hospitals (France). All patients were transferred to the CHUGA Stroke Unit for treatment and follow-up visits, received standard medical care, and were admitted to a stroke rehabilitation center. All patients gave written informed consent. The trial and the amendments were approved by the local ethics committee (“Comité de Protection des Personnes”). ISIS was monitored by an independent data and safety monitoring board (DSMB) and was registered with ClinicalTrials.gov NCT00875654.
Randomization
Using the “Clininfo” program, we randomly assigned patients in a 2:1 distribution to receive MSCs (treated group) or no MSCs (control group) (Fig. 1). Real-time dynamic randomization included three stratification criteria: lesion side (right or left hemisphere), age, and stroke severity (NIHSS score).
Cell Manufacturing
Patients were included and randomized during an inclusion visit that occurred less than 2 weeks after stroke onset. After inclusion, patients assigned to the treatment group underwent 20 mL bone marrow sampling from the iliac crest to harvest cells for MSC expansion. For ethical reasons, only treated patients underwent bone marrow aspiration. MSCs were intravenously administered 3 weeks after inclusion, at baseline (M0), to allow time for MSC expansion.
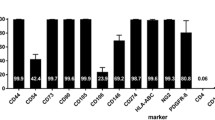
All of the isolation and culture procedures were conducted in the authorized Cell Therapy and Engineering Unit of EFS Auvergne Rhône Alpes (Agreement TCG/04/O/008/AA) according to Good Manufacturing Practices for Cell Therapy products and French regulations. MSCs were expanded in a semi-closed system. Quality controls were performed on the bone marrow aspirate, after the first passage, and on the final harvested MSCs, with measurements of cell viability, MSC identity (phenotype), MSC functionality (colony-forming fibroblast unit), tumorigenicity (soft-agar test and telomerase activity), and cytogenetic stability (karyotype). MSCs were isolated following plastic adhesion, and then cultured at 37 °C in a humidified atmosphere containing 5% CO2. Alpha Minimum essential medium (Macopharma, Mouvaux, France) was supplemented with ciprofloxacin 0,01 mg/mL, bFGF 1 ng/mL (CellGenix Technologie Transfer GmbH, Germany) and 10% fetal calf serum (Hyclone, USA)).
After two cell passages for expansion, autologous MSCs were injected in patients allocated to treatment if the results of quality controls allowed batch release. The dose of injected MSCs for each treatment group was constant, requiring cell expansion duration from 20 to 29 days in different individuals, thereby minimizing the risk of incomplete doses. We administrated MSCs intravenously by gravity at 8–10 mL/min.
Clinical Assessment
All patients underwent serial functional and physiotherapy assessments, including NIHSS (0 to 42, with higher scores indicating greater stroke severity) [23], Barthel Index (0 to 100, with higher scores indicating greater ability to complete activities of daily life) [24], and a modified Rankin scale (mRS; 0 as no symptoms to 6 as death) [25] to assess independence and handicap. The motor component of the NIHSS (motor-NIHSS, range 0–10), and the motor Fugl-Meyer Score (motor-FMS, range 0–100) [26], were used as motor outcome measures, as previously described [27]. Behavioral assessments were performed at each visit by a stroke neurologist, and the motor-FMS was administered at M0, M6, and M24 by a physiotherapist, all blind to treatment assignment. We also recorded rehabilitation time, defined as the total number of hours of motor rehabilitation from stroke onset to the end of follow-up, including walking and hand physiotherapy.
Structural and Functional MRI Assessment
The regional fMRI BOLD-contrast signal is monotonically related to underlying neural activity in primary sensory and motor cortices. Comparing movement and rest periods, it is possible to measure changes in sensorimotor system activity reflecting motor recovery after stroke [19, 28]. During the last decade, fMRI has been widely used in clinical applications [29] and has been recommended for use as a clinical trial biomarker [30]. In patients who are not able to perform voluntary movements on command, passive motion fMRI tasks can evoke sensorimotor cortical activity in most patients [31], with activity patterns similar to those observed during voluntary movement [32,33,34,35]. As most participants were not able to produce voluntary hand movements in the subacute phase following stroke, we used a passive wrist flexion/extension task [19]. An examiner standing inside the room administered timed movements by moving a forearm splint with an axis of rotation through the wrist. Movements were visually cued using a screen placed in front of the examiner. The patients’ affected hand was moved with alternating 20 s epochs of 1 Hz 40° passive wrist flexion/extension and rest during 8 cycles over 340 s (Fig. 2a). The fMRI data were collected on an Achieva 3.0T-TX Philips MRI system at the IRMaGe MRI facility (Grenoble, France) with a 32-channel head coil, using echo-planar imaging (TR 3 s, voxel size 2.2*2.2*2.5 mm3). High resolution (1 mm3) sagittal 3D-T1-weighted and 3D-FLAIR images were acquired for lesion delineation to compute lesion volume and obtain lesion masks. Both T1 images and lesion masks were used for segmentation preprocessing before spatial normalization.
a fMRI paradigm: the movement task involved alternating passive flexion and extension of the paretic wrist and rest. b ROIs including MI-4a (red) and MI-4p (blue). c–e Motor cortex activity was associated with passive movement. Axial MRI slices z = 60 mm above AC-PC axis showing flexion/extension task activity in the canonical motor areas (p < 0.001 uncorrected for multiple comparisons) for c healthy participants (healthy). d Stroke control group (no MSC) at 6-month follow-up and e stroke-treated group (MSC) at 6-month follow-up. R, contralesional hemisphere
For safety assessments (recurrent stroke, hemorrhage, tumors, and inflammation), we acquired additional 4 mm axial images including T1-weighted with gadolinium contrast, T2-weigthed FLAIR, diffusion and MRA scans. Chest radiographs were also obtained. To assess long-term effects of autologous MSCs, appropriate biological tests and imaging were performed when other pathology, such as cancer, was suspected from clinical signs or symptoms.
MRI sessions were done at M0, M0.5, M2, M6, and M24 months after baseline. Functional MRI was performed at each session unless severe wrist spasticity developed.
Functional MRI data analysis was performed using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/). Preprocessing included: (1) rigid body realignment for head motion correction, (2) slice timing correction, (3) rigid body co-registration of EPI with high resolution anatomical data, (4) lesion masked spatial normalization to the Montreal Neurological Institute (MNI) anatomical space, and (5) spatial smoothing (5 mm full width at half maximum). Outliers in EPI time series were identified using a scan-to-scan movement threshold of 1 mm and global signal scan-to-scan changes > 3 SD (https://www.nitrc.org/projects/artifact_detect). Statistical modeling of movement-related effects involved a summary statistics approach. At the first level, for each subject, signal variation was predicted with a set of regressors using a general linear model (GLM). The wrist movement timing vector was convolved with a canonical hemodynamic response function, resulting in explanatory regressors for each participant (first level analysis). Then, d effect size estimates were derived from the FE-task SPM-t images (https://sourceforge.net/projects/spmtools). We measured task-related activity within MI-4a and MI-4p subregions of the damaged MI provided by SPM Anatomy toolbox (http://www.fz-juelich.de/inm/inm-1/DE/Forschung/_docs/SPMAnatomyToolbox/SPMAnatomyToolbox_node.html) and used MI-4a and MI-4p regional activity measures to assess MSC effects (Fig. 2b). Second level group analyses were also performed in the control and treated groups (Fig. 2c-e). An extended description of MRI acquisition, preprocessing and analysis procedures is reported elsewhere [19].
Outcomes
The primary study outcomes were safety and feasibility. Safety was defined as adverse events or changes in deficit and disability scores assessed using clinical evaluation, NIHSS, mRS, and the Barthel Index. Short-term safety was assessed based on the monitoring of patients’ clinical condition (blood pressure, heart rate, oxygenation, fever, rash, shock, and thromboembolic events) every 10 min during the first hour, then every 2 h for the first 24 h, and then every day for the first week following IV MSC administration. Long-term safety was assessed at each clinical visit, focusing on signs and symptoms of malignant disease, as stem cell therapy may promote tumor growth [8]. Feasibility was defined as the proportion of treatment allocated patients who received MSC injection. The secondary outcomes of the ISIS RCT were global behavioral recovery assessed using NIHSS, mRS and the Barthel Index, and motor recovery assessed using motor-FMS and motor-NIHSS. The main outcome of the MRI HERMES substudy was ipsilesional MI fMRI activity measured at M6 and M24. Recovery was assessed from baseline (M0) to the end of follow-up (M24) with repeated measurements.
Statistical Analysis
Sample Size
The clinical study (ISIS) was designed to assess IV autologous bone marrow derived MSC safety and feasibility and was not specifically powered to detect MSC effects on behavior. The only previous study of IV MSC stroke therapy included 30 participants and did not report any safety issues. Thus, without an empirical estimate for the expected low rate of MSC therapy complications, a sample of 30 participants was again used. In the MRI part of the trial (HERMES), the assessment of MSC treatment effects on motor outcome was based on MI activity, serving as a neurophysiological biomarker of motor system recovery [16]. Using a previous fMRI dataset, we calculated that a sample size of 13 patients per group would allow detection of 50% MI task-related activity treatment effects, with 90% power and 10% alpha.
Univariate Analysis
To measure the effect of the experimental treatment relative to the control condition, as-treated analyses were performed. The treated group included patients who received MSC doses (100 or 300 million MSCs). Patients who were initially assigned to treatment, but did not receive MSCs, were included in the control group.
Group Comparisons
Comparisons between the as treated and control groups for safety and efficacy endpoints were explored at M6 and M24 using Mann Whitney and chi-squared tests. As recommended, we reported 95% confidence intervals, U values, p values and effect sizes to assess both the statistical significance and magnitude of MSC effects [36, 37], Cohen’s d effect sizes were calculated with the formula d = (Mean1 − Mean0) / √(n1 – 1)*SD12 + (n0 – 1)*SD02) / (n1 + n0 – 2) [38, 39]. For reference purposes, we also performed intent to treat (ITT) analyses.
Effects of Treatment on Outcome Measures Over Time
The effects of treatment on behavioral scores were analyzed using longitudinal linear mixed models (LMM) with repeated measures. Mixed modeling expands the general linear model to accommodate effects of correlated and non-constant variability. The mixed linear model, therefore, provides the flexibility of modeling not only the means of the data but their variance and covariance as well. We chose a LMM with a normal distribution link function because of the longitudinal structure of our data, accommodating missing time-points, and non-equidistant intervals between time points [40,41,42].
For each behavioral score, we modeled the effects of time from M0 to M24, MSC treatment, and the treatment by time interaction. Participants were included as random effects and time and treatment group as fixed effects. The NIHSS collected at inclusion was entered as a covariate to adjust for initial severity for mRS, NIHSS, motor-NIHSS, and motor-FMS models. The baseline Barthel was entered for the Barthel Index model. The effects of demographic and clinical variables that could influence stroke recovery, including risk factors and MSC dose, were selected using LASSO regression and kept if significant in the final LMMs. A critical threshold of (p < 0.05) was used. We employed robust estimation to ensure consistent inferences from the LMMs even if the correlation strength between repeated observations varies from patient to patient [42]. Estimated means at each time point were contrasted with the last time point (M24) with the sequential Bonferroni method for test significance adjustment.
Treatment effects on fMRI activity in ipsilesional MI were assessed using a LMM as described above. The fixed effects of time, MSC treatment, and NIHSS at inclusion were included in the model. The time by treatment interaction was tested and kept in the model if significant. The effects of age, gender, thrombolysis, and lesion volume were tested for each model and included if significant and if the model fit was improved. The model fit was estimated with the Akaike Information Criterion (AIC), and R2 to assess prediction accuracy. R2 was computed by regression diagnostics included plotting predicted versus observed values for the behavioral scores [43]. The stability of model parameters was assessed using residual plots [43]. The residual histogram and residual probability plot (residuals versus their expected values) examined whether the data include outliers or showed violations of the assumption of constant residual variance. SPSS 20.0 and R were used for data analysis.
Results
Thirty-one patients were recruited between 31 Aug 2010 and 31 Aug 2015. Twenty patients were randomized to the MSC group and 11 to the control group (Fig. 1). There were no baseline clinical differences between as-treated groups, including thrombolysis treatment, except for atrial fibrillation being more frequent in the control than in the treated group (p = 0.045) (Table 1). No patient was lost to follow-up.
The duration of rehabilitation was collected for all but one patient. Median duration (IQR) was 90 days (150) in the treated group and 145 (112.5) days in the non-treated group. No significant difference was observed between the two groups (p = 0.195).
Individual characteristics of the 31 patients are reported in Supplementary Table 2. The overlap of stroke lesions is shown in Fig. 3 and individual lesions in Supplementary Fig. 1.
Primary Feasibility and Safety Outcomes
Among the 20 autologous MSC cultures begun, four did not meet quality specifications for batch delivery, resulting in 16 injections performed. Non-conformity for cell delivery included karyotype abnormalities (patients 6 and 14), cell death and weak culture amplification (patient 15), and infection of the bone marrow sample (patient 31). These non-conformities were officially reported to the sponsor and to the French authorities. These four patients did not receive MSC injections, indicating 80% overall feasibility.
Regarding short-term safety, there were no adverse events during bone marrow sampling, and no adverse event was attributable to MSC injection during the first week. Regarding long-term safety, one control group patient died by drowning after a fall 10 months following stroke onset (Tables 2 and 3). Half of the adverse events occurred within 6 months after baseline, with no significantly higher rate in the control group. Structural MRI did not reveal evidence of expanding intracerebral processes or inflammatory reactions between baseline and study end. However, diffusion MRI showed a small hyperintensity in the right insular cortex of a control group patient, indicating a new cerebral infarct that occurred between V2 and V3. This patient had no additional clinical symptoms related to this new event.
Secondary Efficacy Outcomes
Group comparisons are presented in Table 4 for the as treated analysis. There were no significant differences in global scales at 6-month and 2-year follow-ups. Regarding the interpretation of treatment effect on motor outcomes [38, 39] at the 2-year follow-up, MSCs showed a significant effect on the motor-NIHSS with a large effect size (0.81), while there was a non-significant trend for the motor-FMS, with a medium effect size (0.66). MI-4a and MI-4p fMRI measures were significantly increased in the treated compared with the control group at both times with large effect sizes at 2 years (1.41 and 1.60, respectively). As expected, results of ITT analyses did not show any cell therapy effects.
Regarding global scales, LMM analyses did not show significant influences of MSC on NIHSS (estimate = − 1.566, − t = − 1.354; p = 0.177), Barthel Index (estimate = −2.431, − t = 0.296; p = 0.768), or mRS (estimate = − 0.355, t = 1.205; p = 0.230) measures, even after controlling for MSC dose, age, gender, thrombolysis, and lesion volume (Fig. 4). The MSC by time interactions were not significant.
By contrast, LMM showed significant treatment effects on motor-FMS and motor-NIHSS (Fig. 5). Significantly higher scores were found for the motor-FMS (t = 2.242, p = 0.028). Compared with the 24-month follow-up FMS, there was a significant effect of time at baseline but not at 6 months, indicating that recovery occurred mainly during the first 6 months after stroke. The NIHSS measured at inclusion had a significant effect on motor-FMS (t = − 3.768, p < 0.001), indicating that initial severity influenced motor recovery. Significant gains in motor NIHSS scores were also found for the MSC group during follow-up (t = 3.379, p = 0.001), with a significant treatment by time interaction from baseline to 3 months after stroke, showing gains after M6. As for the FMS LMM, there was a significant effect of NIHSS at inclusion (t = − 3.768, p = 0.001).
LMM showing significant effects of MSC treatment over the 24-month follow-up on motor behavioral scores: a motor FMS, b motor NIHSS, and fMRI measures in c MI-4a and d MI-4p. Note that there was no significant difference on FMS at baseline between the treated and non-treated groups. The red line indicates MSC treatment and the blue line No-MSC treatment
Treatment effects on MI-4a and MI-4p activity were significant with an effect of initial severity and time but no significant time by treatment interaction (Fig. 5). Higher t values were observed for MI-4p (t = 3.922, p = 0.002) than for MI-4a (t = 3.121, p = 0.031). Furthermore, we found no effect of MSC dose on behavior scales and fMRI activity, as well as no significant effect of age, gender, thrombolysis treatment, or lesion side as covariates. All the models showed a significant effect of time, indicating that some recovery occurred in patients, independently on other factors. The results for motor outcomes, including AIC, R2, estimates and 95% CI, and t and p values, are presented in Supplementary Table 3.
Discussion
In this RCT, we assessed safety and feasibility of IV autologous MSCs in 31 patients with subacute ischemic stroke, with a 2-year follow-up. Consistent with other results, we found that IV autologous MSC administration was safe [9,10,11], with similar adverse event rates in treated and control groups. Although clinical use of MSCs has raised safety concerns [8], we observed no tumor appearance, pro-inflammatory effects, or other adverse events related to MSCs, in accordance with the previous stroke study using IV MSCs using a 4-year follow-up [12], and with recent meta-analyses [13, 44]. While patients had moderate to severe stroke and one patient expired, adverse events were much lower than in previous RCTs using MSCs [12]. Feasibility reached 80%, indicating good feasibility relative to previous RCTs using IV autologous MSC [9, 12]. Nevertheless, feasibility could have been improved, since autologous cell therapy was not administered in two patients with karyotype abnormalities, which is no longer considered to be a contraindication for cell therapy. Moreover, patients with severe stroke were included since the upper limit for the NIHSS was 24. We observed weak culture amplification in one of these patients. It is possible that an upper limit of 18–20 would allow higher feasibility. In contrast, culture infection was more difficult to prevent based on our protocol.
Secondary efficacy outcomes tested the effect of MSCs on independence scores, disability scores, and motor performance measures. No significant effects were found for the NIHSS, Barthel Index, and mRS measures. These results are consistent with previous RCTs assessing MSCs and other cell therapies using the IV route [9, 10], although significant improvements have been noted in post hoc analyses using mRS and/or Barthel Index categories [10, 12]. The delay before MSC administration may be relevant, since the Barthel Index at 1 year was improved in the treated group, which had cell therapy administered 36 h after stroke onset [10].
As hypothesized, we noted improvements in clinical motor performance measures. Our findings are supported by previous experimental evidence showing that cell therapy improves motor recovery in rats with middle cerebral artery occlusion [2].
The dissociation between global and motor outcome measures could be related to their differing variance, with the motor outcome measures exhibiting less variability [45]. Motor behavior assessment based on continuous scores may have resulted in precise and accurate recovery predictors. In contrast, global outcomes capture other dimensions such as social and emotional components that may not be influenced by cell therapy in the same way.
According to consensus-based guidelines concerning the development of cell therapies for stroke, entitled “Stem Cells as Emerging Paradigm in Stroke” (STEPS), we combined behavioral and MRI measures to monitor safety and provide information on surrogate MRI markers of treatment effects [30]. We measured passive wrist movement-related fMRI activity in MI to assess the effect of MSCs. This is the first time that fMRI has been used as a biomarker in association with behavioral measures in a cell therapy RCT. MI activity was significantly increased in the treated compared with the control group for both 4a and 4p subregions, confirming the better clinical motor recovery. Increased MI activity has previously been associated with functional motor improvement in subacute and chronic stroke [16, 18, 39, 46] and is a potentially robust biomarker of motor system recovery [17, 19]. There is a body of neuroimaging evidence in the literature, showing that fMRI (using either active or passive hand motor tasks) can predict outcome [16, 19, 46,47,48], including three meta-analyses [17, 18, 49]. In this study, we used the same passive wrist movement task as Loubinoux et al. [16, 48], which can be considered as an external validation of using fMRI activity related to a passive hand task to measure stroke recovery.
The observed effect sizes were larger in MI-4p than in MI-4a, suggesting that MI-4p and MI-4a, which differ in terms of chemo- and cytoarchitectonic characteristics [50] and functional specialization [51], may respond differently to MSC therapy.
There is some evidence that motor cortex neuroplasticity, reflected by increased task-related MI activity, is accompanied by changes in dendritic and synaptic structure [52, 53], highlighting one of the possible pathophysiological mechanisms by which MSC paracrine secretion may enhance brain repair [3, 54]. The current literature consensus is that the MSC secretome may act during the subacute phase of stroke through inflammation modulation that promotes more delayed mechanisms such as angiogenesis and neurogenesis [3]. In our study, MSCs were administered with a median delay of 32 days, during the subacute stage of stroke, within a time window that might have allowed the MSC secretome to exert its immunomodulatory effects [55], support brain repair, and improve stroke recovery.
Surprisingly, we observed clinical recovery until the late chronic period of recovery, suggesting that recovery might be profitably assessed longer than the usual 90 day time point, at least for studies including patients with severe stroke during the subacute period.
The moderating role of rehabilitation needs be considered, as it might have influenced the outcome [56]. In this study, similar efforts were made for rehabilitation in the treated and non-treated groups, since the main criteria for rehabilitation duration and intensity were related to neurological deficits and patient’s abilities. As a result, no significant difference was observed between the two groups in terms of rehabilitation duration. In addition, rehabilitation time showed no significant effect in the LMMs modeling stroke recovery, suggesting that the maximum useful time of rehabilitation was reached in our patients.
Methodological Considerations
A main limitation of this study is related to the use of autologous MSCs, which imposed several constraints. First, we performed bone marrow aspiration in the treated group, but not in the control group for obvious ethical reasons, resulting in an open-label design, as patients knew the treatment to which they were assigned. To compensate for this potential bias, patients’ therapists and investigators assessing clinical and MRI outcome measures were blind to MSC treatment. Second, patients with MSC culture abnormalities did not receive cell therapy. While the culture abnormalities were due to karyotype abnormalities or technical contamination of the culture, and were not related to stroke severity or recovery, our results are not likely to have been biased by feasibility limitations. Adopting a pragmatic approach, we assessed safety and efficacy effects of MSC through “as-treated” rather than with an “intent-to-treat” analysis. Of note, we obtained similar results when performing per-protocol analyses by excluding patients who were assigned to MSC treatment and did not receive MSC (results available on demand). Third, delays in MSC administration were constrained by the variable cell expansion times required to reach the target dose. In this context, we could not treat patients at the early subacute phase, during which potentially greater effects might have been observed on global scales, as suggested by a recent RCT using allogenic cells within a time window of 48 h after stroke onset [10]. These limitations related to the use of autologous MSCs encourage the use of allogenic cells in future RCTs.
Also, there is no sample size justification for the primary endpoints (safety and feasibility). At the time of the protocol submission (2007), safety of autologous stem cells was reported to be excellent, with no side effect in humans and the literature on MSC in experimental studies had not reported any side effects or feasibility issues. Therefore, it was not possible to compute a sample size based on empirically derived estimates. In this study, we chose to assess safety and feasibility in a group 30 patients in line with a previous autologous stem cell study [9], which was ethically acceptable.
Another limitation of this study is related to the small sample size, which does not provide the sensitivity to detect treatment effects based on relatively variable global behavior measures. Nevertheless, we observed a significant effect of treatment on motor behavioral scores and fMRI measures with associated medium-large effect sizes, illustrating that our sample size was adequate for assessing motor recovery effects. As the effect size measures the treatment effect strength, we can infer from our data that autologous MSC have medium to large effects on motor recovery [39]. The combination of behavioral motor scales with fMRI activity biomarkers in a longitudinal design demonstrates the effect of MSC treatment on motor recovery after stroke. Moreover, employing a 2-year follow-up with multiple assessments allowed utilization of longitudinal linear mixed models to analyze treatment effects on both behavioral and fMRI measures. This approach better models the trajectory of recovery, compared with contrasting outcomes between groups at fixed time points, and allows incorporation of potential confounding effects such as age and baseline group differences (i.e., initial severity and atrial fibrillation) that might be expected in small samples.
Conclusions
Autologous MSC treatment is safe and feasible for treating moderate to severe stroke. Although our results need to be replicated in further studies, both behavioral and physiological motor outcomes showed effects of cell therapy. This initial IV MSC stroke recovery study provides important preliminary data that will be useful to plan subsequent studies, incorporating better estimates of expected behavioral and physiological effects, allowing more accurate justification of the sample size required to detect treatment effects. In addition, we found that passive wrist movement was associated with regional task-related fMRI activity changes in MI related to cell therapy, suggesting that physiological measures of sensorimotor cortex activity may be sensitive recovery biomarkers that can be used in future studies exploring novel therapies for stroke. The observation of steadily increasing behavioral and physiological effects of stem cell therapy suggest that recovery might be profitably assessed longer than the usual 90-day time point in future trials.
References
Sarraj A, Grotta JC. Stroke: new horizons in treatment. Lancet Neurol. 2014;13(1):2–3.
Moisan A, Favre I, Rome C, De Fraipont F, Grillon E, Coquery N, et al. Intravenous injection of clinical grade human MSCs after experimental stroke: functional benefit and microvascular effect. Cell Transplant. 2016;25(12):2157–71.
Cunningham CJ, Redondo-Castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab. 2018:271678X18776802.
Dhere T, Copland I, Garcia M, Chiang KY, Chinnadurai R, Prasad M, et al. The safety of autologous and metabolically fit bone marrow mesenchymal stromal cells in medically refractory Crohn’s disease—a phase 1 trial with three doses. Aliment Pharmacol Ther. 2016;44(5):471–81.
Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59(12):1662–9.
Kuriyan AE, Albini TA, Townsend JH, Rodriguez M, Pandya HK, Leonard RE 2nd, et al. Vision loss after intravitreal injection of autologous “stem cells” for AMD. N Engl J Med. 2017;376(11):1047–53.
Gazdic M, Volarevic V, Arsenijevic N, Stojkovic M. Mesenchymal stem cells: a friend or foe in immune-mediated diseases. Stem Cell Rev. 2015;11(2):280–7.
Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, et al. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15(1):36–45.
Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–82.
Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16(5):360–8.
Moniche F, Gonzalez A, Gonzalez-Marcos JR, Carmona M, Pinero P, Espigado I, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke. 2012;43(8):2242–4.
Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099–106.
Detante O, Moisan A, Hommel M, Jaillard A. Controlled clinical trials of cell therapy in stroke: meta-analysis at six months after treatment. Int J Stroke. 2017;12(7):748–51.
Wang LE, Fink GR, Diekhoff S, Rehme AK, Eickhoff SB, Grefkes C. Noradrenergic enhancement improves motor network connectivity in stroke patients. Ann Neurol. 2011;69(2):375–88.
Ramsey LE, Siegel JS, Baldassarre A, Metcalf NV, Zinn K, Shulman GL, et al. Normalization of network connectivity in hemispatial neglect recovery. Ann Neurol. 2016;80(1):127–41.
Loubinoux I, Dechaumont-Palacin S, Castel-Lacanal E, De Boissezon X, Marque P, Pariente J, et al. Prognostic value of FMRI in recovery of hand function in subcortical stroke patients. Cereb Cortex. 2007;17(12):2980–7.
Richards LG, Stewart KC, Woodbury ML, Senesac C, Cauraugh JH. Movement-dependent stroke recovery: a systematic review and meta-analysis of TMS and fMRI evidence. Neuropsychologia. 2008;46(1):3–11.
Favre I, Zeffiro TA, Detante O, Krainik A, Hommel M, Jaillard A. Upper limb recovery after stroke is associated with ipsilesional primary motor cortical activity: a meta-analysis. Stroke. 2014;45(4):1077–83.
Hannanu FF, Zeffiro TA, Lamalle L, Heck O, Renard F, Thuriot A, et al. Parietal operculum and motor cortex activities predict motor recovery in moderate to severe stroke. NeuroImage Clin. 2017;14:518–29.
Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174(1):11–20.
Li Y, Chen J, Chopp M. Adult bone marrow transplantation after stroke in adult rats. Cell Transplant. 2001;10(1):31–40.
Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73(6):778–86.
Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–70.
Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Med J. 1965;14:61–5.
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–7.
Sullivan KJ, Tilson JK, Cen SY, Rose DK, Hershberg J, Correa A, et al. Fugl-Meyer assessment of sensorimotor function after stroke: standardized training procedure for clinical practice and clinical trials. Stroke. 2011;42(2):427–32.
Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10(2):123–30.
Loubinoux I. Can fMRI measures of brain motor activation add significantly to other variables in the prediction of treatment response? Stroke. 2007;38(7):2032–3.
Mahdavi A, Azar R, Shoar MH, Hooshmand S, Mahdavi A, Kharrazi HH. Functional MRI in clinical practice: assessment of language and motor for pre-surgical planning. Neuroradiol J. 2015;28(5):468–73.
Savitz SI, Cramer SC, Wechsler L, Consortium S. Stem cells as an emerging paradigm in stroke 3: enhancing the development of clinical trials. Stroke. 2014;45(2):634–9.
Choudhri AF, Patel RM, Siddiqui A, Whitehead MT, Wheless JW. Cortical activation through passive-motion functional MRI. AJNR Am J Neuroradiol. 2015;36(9):1675–81.
Blatow M, Reinhardt J, Riffel K, Nennig E, Wengenroth M, Stippich C. Clinical functional MRI of sensorimotor cortex using passive motor and sensory stimulation at 3 Tesla. J Magn Reson Imaging. 2011;34(2):429–37.
Weiller C, Juptner M, Fellows S, Rijntjes M, Leonhardt G, Kiebel S, et al. Brain representation of active and passive movements. NeuroImage. 1996;4(2):105–10.
Loubinoux I, Carel C, Alary F, Boulanouar K, Viallard G, Manelfe C, et al. Within-session and between-session reproducibility of cerebral sensorimotor activation: a test–retest effect evidenced with functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2001;21(5):592–607.
Tombari D, Loubinoux I, Pariente J, Gerdelat A, Albucher JF, Tardy J, et al. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. NeuroImage. 2004;23(3):827–39.
Wilkinson L, Task Force on Statistical Inference. Statistical methods in psychology journals: guidelines and explanations. Am Psychol. 1999;54:594–604.
Middlemiss W, Granger DA, Goldberg WA. Response to “let’s help parents help themselves: a letter to the editor supporting the safety of behavioural sleep techniques”. Early Hum Dev. 2013;89(1):41–2.
Cohen J. Statistical power analysis for the behavioral science. 2nd ed. New York: Lawrence Erlbaum Associate; 1988.
Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863.
Cheng J, Edwards LJ, Maldonado-Molina MM, Komro KA, Muller KE. Real longitudinal data analysis for real people: building a good enough mixed model. Stat Med. 2010;29(4):504–20.
Maas CJM, Snijders TAB. The multilevel approach to repeated measures for complete and incomplete data. Qual Quant. 2003;37(1):71–89.
Burton P, Gurrin L, Sly P. Extending the simple linear regression model to account for correlated responses: an introduction to generalized estimating equations and multi-level mixed modelling. Stat Med. 1998;17(11):1261–91.
Steyerberg EW, Harrell FE, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–81.
Veldema J, Bosl K, Nowak DA. Motor recovery of the affected hand in subacute stroke correlates with changes of contralesional cortical hand motor representation. Neural Plasticity. 2017;2017:6171903.
Hommel M, Detante O, Favre I, Touze E, Jaillard A. How to measure recovery? Revisiting concepts and methods for stroke studies. Transl Stroke Res. 2016;7(5):388–94.
Rehme AK, Volz LJ, Feis DL, Eickhoff SB, Fink GR, Grefkes C. Individual prediction of chronic motor outcome in the acute post-stroke stage: behavioral parameters versus functional imaging. Hum Brain Mapp. 2015;36(11):4553–65.
Carey LM, Abbott DF, Egan GF, Bernhardt J, Donnan GA. Motor impairment and recovery in the upper limb after stroke: behavioral and neuroanatomical correlates. Stroke. 2005;36(3):625–9.
Loubinoux I, Carel C, Pariente J, Dechaumont S, Albucher JF, Marque P, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. NeuroImage. 2003;20(4):2166–80.
Rehme AK, Eickhoff SB, Rottschy C, Fink GR, Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. NeuroImage. 2012;59(3):2771–82.
Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, et al. Two different areas within the primary motor cortex of man. Nature. 1996;382(6594):805–7.
Rathelot J-A, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci. 2009;106(3):918–23.
Heddings AA, Friel KM, Plautz EJ, Barbay S, Nudo RJ. Factors contributing to motor impairment and recovery after stroke. Neurorehabil Neural Repair. 2000;14(4):301–10.
Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001;24(8):1000–19.
Boltze J, Lukomska B, Jolkkonen J, MEMS-IRBI Consortium. Mesenchymal stromal cells in stroke: improvement of motor recovery or functional compensation? J Cereb Blood Flow Metab. 2014;34(8):1420–1.
Mays RW, Savitz SI. Intravenous cellular therapies for acute ischemic stroke. Stroke. 2018;49(5):1058–65.
Foley N, McClure JA, Meyer M, Salter K, Bureau Y, Teasell R. Inpatient rehabilitation following stroke: amount of therapy received and associations with functional recovery. Disabil Rehabil. 2012;34(25):2132–8.
Acknowledgments
We thank the other members of the ISIS-HERMES Study group (listed in alphabetical order): S. Achard, P. Antoine, E. L. Barbier C.E. Bulabois, L. Carey, A. Chrispin, M. Cucherat, P. Davoine, F. de Fraipont, C. Delon-Martin, C. Dubray, H. Egelhofer, M.C. Favrot, K. Garambois, P. Garnier, J. Gere, N. Gonnet, I Goundous, F.F. Hannanu, O. Heck, A.V. Jaillard, A. Krainik, J.F. Le Bas, S. Miguel, A. B. Naegele, A. Paris, D. Perennou, P. Pernot, C. Remy, F. Renard, M.J. Richard, G. Rodier, E. Schir A. Thuriot, I. Tropres, and J. Warnking.
Trial Registration
ClinicalTrials.gov, number NCT00875654. https://clinicaltrials.gov/ct2/show/NCT00875654?term=ISIS+stroke+stem+cells&rank=1
Protocols
French ISIS RCT and satellite MRI HERMES protocols are available on demand.
Funding
This trial was funded by an academic grant from the French Health Ministry: PHRCI Grant numbers: ISIS-2007PHR04 and HERMES-2007-A00853-50. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. MRI data acquisition was performed at the IRMaGe MRI platform, which gratefully acknowledge financial support from France Life Imaging network through the grant “ANR-11-INBS-0006.” Data monitoring was performed by the Clinical Investigation Center (CIC) INSERM UMS 002 CHU Grenoble Alpes. Data analysis was partly supported by RESSTORE project (www.resstore.eu) funded by the European Commission under the H2020 program (Grant Number 681044).
Author information
Authors and Affiliations
Consortia
Contributions
Dr. Jaillard had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis. Concept and design: A. Jaillard, M. Hommel, and O. Detante. Acquisition of data. Recruitment and/or clinical follow-up: O. Detante, I. Favre-Wiki, M. Barbieux-Guillot, W. Vadot, and S. Marcel. MRI data acquisition: A. Jaillard, M. Hommel, L. Lamalle, and S. Grand. Analysis or interpretation of data: A. Jaillard, M. Hommel, T. A. Zeffiro, and O. Detante. Drafting of the manuscript: A. Jaillard, O. Detante, M. Hommel, T.A. Zeffiro, and A. Moisan. Critical revision of the manuscript for important intellectual content: A. Jaillard, T.A. Zeffiro, M. Hommel, and O. Detante. Statistical analysis: A. Jaillard and M. Hommel. Obtaining funding: A. Jaillard and O. Detante. Administrative, technical, or material support: A. Jaillard, M. Hommel, O. Detante, L. Lamalle (MRI calibration), and A. Moisan (Autologous mesenchymal stem cell preparation). Study supervision: O. Detante (ISIS) and A. Jaillard (HERMES).
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All patients gave written informed consent. The trial and the amendments were approved by the local ethics committee (“Comité de Protection des Personnes”). ISIS was monitored by an independent data and safety monitoring board (DSMB).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 2.06 mb)
Rights and permissions
About this article
Cite this article
Jaillard, A., Hommel, M., Moisan, A. et al. Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: a Randomized Clinical Trial. Transl. Stroke Res. 11, 910–923 (2020). https://doi.org/10.1007/s12975-020-00787-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-020-00787-z