Abstract
Mesenchymal stromal cells (MSCs) can differentiate into multiple tissues. Preclinical studies have shown that MSC-based therapy is a potential new treatment approach for ischemic stroke. These results support the urgent need for further studies of MSC transplantation in the treatment of ischemic stroke in humans. Here, we develop a prospective, randomized, controlled, observer-blinded phase II trial to assess the clinical safety, feasibility, and therapeutic mechanisms of allogenic bone marrow-derived MSCs (BM-MSCs) by intrathecal infusion in the treatment of patients with cerebral infarction within the middle cerebral artery and with a National Institutes of Health Stroke Scale (NIHSS) score from 15 to 25. Sample size calculation has determined that a patient population of 118, with ischemic stroke between 30 and 90 days following onset, will be randomly divided into experimental (n = 59) and control (n = 59) groups. Then eligible patients will receive four intrathecal infusions of allogenic BM-MSCs (1 × 106 cells/kg body weight) once a week. All patients have detailed functional assessments and magnetic resonance imaging prior to cell infusion and at intervals up to 1 year after. The primary outcome is the score on the modified Rankin Scale at 90 days after treatment, and the second outcomes include multiple indicators of safety and feasibility. And this trial has been registered as ChiCTR-INR-16008908 (25 July 2016).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke, a leading cause of death and disability worldwide, is associated with high mortality, disability, and recurrence rate. Severe ischemic stroke patients usually suffer neurological deficits and severe complications (i.e., hypostatic/aspiration pneumonia, bedsore, deep vein thrombosis, etc.), despite acute therapy that protects them from death. When neurological deficits persist, there is limited treatment to enhance recovery currently. As a result, severe stroke remains a significant unmet clinical need, imposing the urgent hope for novel treatments.
Laboratory studies have shown that cell-based therapy is a potential new treatment approach of regenerating the injured brain beyond the acute phase of ischemic stroke [1, 2]. Various cell types have been used to provide functional and structural benefits after stroke, including embryonic stem cells (ESCs), immortalized pluripotent stem cells (iPSCs), neural stem/progenitor cells (NSCs), and non-neuronal adult stem cells such as mesenchymal stem cells (MSCs) and bone marrow mononuclear cells (MNCs) [3]. MSCs are not ethically controversial and have no risk of tumor formation as the case in ESCs and iPSCs. Among the various stem cells, MSCs have been most commonly used in the clinical trials for patients with stroke.
MSCs, originally isolated from bone marrow, can differentiate into multiple tissues, including the bone, fat, cartilage, neurons, hepatocytes, and cardiocytes. Also, MSCs can be isolated from other tissues, such as the umbilical cord, peripheral blood, adipose tissue, endometrial polyps, and menses blood [4]. MSCs are found to play multiple roles in the treatment of cerebral ischemia. Transplanted MSCs can improve the outcome through differentiating into neurons and astrocytes, increasing cytokines and neurotrophic factors, promoting angiogenesis and cerebral blood circulation, facilitating a proliferation in endogenous neurogenesis, reducing apoptotic cells, and encouraging axonal sprouting, myelin remodeling, and restoration of neural circuits. Moreover, MSCs play an important role in immunomodulatory function [5].
A meta-analysis examined preclinical studies of MSCs in the treatment of ischemic stroke and found that this cellular therapy improves outcome, with very large effect sizes. Effects were robust across species, delivery route, time of administration in relation to stroke, MSC immunogenicity, and MSC dose [6]. These results support the urgent need for further studies of MSC transplantation in the treatment of ischemic stroke in humans. In recent years, several clinical studies have administrated MSCs in stroke through intracerebral injection or intravascular injection [7,8,9,10,11,12,13,14]. These trials varied in terms of the patient characteristics, timing, and dose of cell therapy (Table 1). Moreover, the assessments of functional improvement, adverse effects, and pretreatment screening tests for safety have varied greatly among the studies. None of the studies aimed to determine the efficacy of MSC therapy in patients with stroke. All of the studies aimed to assess the feasibility and safety of stem cell treatments, and most were small series and did not include a control group [15].
Intrathecal injection of autologous or allogenic MSCs has been administrated in many kinds of human neurological disorders, such as basilar artery dissection [16], progressive multiple sclerosis [17, 18], spinal cord injury [19, 20], amyotrophic lateral sclerosis [21], spinal muscle atrophy [22], progressive supranuclear palsy [23], cerebral hemorrhage [24], and spinocerebellar ataxia and multiple system atrophy [25]. No serious stem cell-related adverse effect was reported and some patients had functional improvement in the studies above. At present, there is no clinical report on the treatment of cerebral infarction with MSCs through intrathecal injection. Although intracerebral transplantation may allow more directly cell homing, local injection may lead to poor cell distribution in the injured brain, and it is an invasive method that may result in bleeding, seizures, and other complications. So there are many hurdles for getting intracerebral injection into clinical application. Intra-arterial injection, delivering cells to the infarcted brain by intra-carotid injection, may lead to a better distribution of stem cells compared with intracerebral transplantation. However, a potential problem of this approach is that MSCs might be unable to pass the blood-brain barrier [26, 27]. Additionally, several laboratorial and clinical studies have shown that MSCs can adhere to each other and form microemboli after intra-arterial administration (IA), which aggravate the brain damages [28, 29]. Intravenous injection (IV) represents the least invasive method of delivery, but it has the same problem as the case in IA that MSCs might be unable to pass the blood-brain barrier. And injected cells by IV also migrate to perivascular locations in other organs, and there is a potential risk of leading to ectopic growth or elaboration of secreted proteins in other organs [30]. What’s more, cells delivered by IV have to first pass through the lungs before they can be distributed throughout the body. This presents a major problem with what has been termed the pulmonary “first-pass” effect, which results in significant entrapment of active stem cells and the greatly increasing of the treatment dose [31, 32]. Furthermore, intravenous injection of a large dose of stem cells may lead to pulmonary embolism [33, 34]. Intrathecal injection is a safe and feasible approach that infuses stem cells into the subarachnoid space of the patient by lumbar puncture. It allows higher concentrations of stem cells to migrate to the lesion site. Moreover, it is safer than intracerebral injection. So, MSC transplantation via intrathecal injection may be the best routine of stem cell therapy in patients with ischemic stroke.
Autologous MSCs have some disadvantages compared with allogenic MSCs although they are the best safe cells. First of all, the culture of MSCs usually takes 4–7 weeks in view of cell amount, and the cell therapy may fail because the number of cells is insufficient, while it is easy to get enough allogenic MSCs within 2 weeks following admission, which has been isolated from healthy donors. Second, patients with cerebral infarction usually take antiplatelet or anticoagulant drugs, so the extraction of MSCs from bone marrow may lead to local bleeding. Third, patients with cerebral infarction are mostly elderly, the proliferation and differentiation capacity of stem cells is greatly decreased, and it is difficult to obtain sufficient stem cells. Fourth, MSCs will not pose any immunological problems because MSCs express low levels of HLA class I major histocompatibility complex (MHC) molecules and no class II MHC or costimulatory molecules [35]. So, allogeneic MSC transplantation is more suitable for the treatment of cerebral infarction patients, especially elderly patients.
In addition, mechanisms of MSC therapy in patients with ischemic stroke are unknown, and the researches on the mechanisms are limited to animal experiments [5, 6]. Moreover, whether there is a difference between human and animal important immune systems is still under controversy [36, 37]. Hence, it is necessary to explore the therapeutic mechanism of MSC transplantation in clinical trials.
The aim of the study is to determine the safety, efficacy, and therapeutic mechanisms of allogenic intrathecal MSC therapy in severe ischemic stroke (phase II).
Materials and Methods
Design
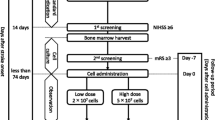
This is a prospective, randomized, controlled, observer-blinded phase II trial. The study will be held in the neurology department of the Sun Yat-sen Memorial Hospital, Sun Yat-sen University, China. The included subjects (n = 118) will be randomly divided into experimental (n = 59) and control (n = 59) groups according to a random number table, which is generated by computer. Patients with ischemic stroke at the subacute phase (30 to 90 days following onset) will receive four intrathecal infusions of allogenic BM-MSCs (1 × 106 cells/kg body weight) once a week. The primary objective will be assessed in 90 days after treatment. After four infusions, follow-up evaluations will be performed in 7, 30, 90, 180, and 360 days. So this trial includes ten visits, namely from V1 to V10. Details of patient follow-up are summarized in Table 2 and Fig. 1.
Patient Population—Inclusion and Exclusion
The ischemic stroke included in this trial is based on the diagnosed standards of guidelines from the American Heart Association/American Stroke Association [38]. The detailed inclusion and exclusion criteria are shown in Table 3.
Randomization
The statistics office of Sun Yat-sen University will use a computer to generate a randomized sequence table for subjects. The subjects will be randomly divided into the experimental (n = 59) and control (n = 59) groups at a ratio of 1:1. To ensure the allocation concealment, the third party will place paper strips with black characters on a gray background into non-transparent envelopes which will be sealed with adhesive tape. Care providers and patients will not be masked; however, the outcome assessors will be masked to treatment allocation.
Interventions
Preparation of Allogenic BM-MSCs
All procedures were approved by the Ethics Committee and are accomplished at the Center for Biotherapy, Sun Yat-sen Memorial Hospital, Sun Yat-sen University (Guangzhou, China). All healthy donors were informed of the scientific contributions, possible risks and complications, and the corresponding prevention and treatment measures for bone marrow aspirations and signed the informed consent form. The protocols for isolation, expansion, passaging, and storing of BM-MSCs were performed as described by our previous works [39, 40]. After identifying MSC immunophenotype markers by flow cytometry, passages three to five will be used for the clinical trial.
Intervention Dose and Route
Intrathecal injection of allogenic BM-MSCs will be administrated in patients with ischemic stroke. The allogenic MSCs (1 × 106 cells/kg body weight) in 10 ml normal saline are slowly injected over approximately 10 min after the mixture with 2 mg (0.4 ml) dexamethasone and 0.6 ml normal saline (to prevent aseptic chemical meningitis) is injected. After the infusion of the MSCs, 2 ml of normal saline is injected to flush the syringe and spread the MSCs.
Clinical Outcomes
The primary outcome and secondary outcomes have been summarized in Table 4.
Sample Size Estimates
Sample size is calculated for superiority hypothesis on the percentage of effective treatment. The effective treatment is defined as modified Rankin Scale (mRS) ≤ 3 in 90 days after cell transplantation, while mRS > 3 means an effective treatment. According to the two previous trials, sample size is calculated [8, 41] with a standard formula [42] to yield a sample size of 53 per group. To allow approximately 10% of patients to be excluded from the population, a total of 118 subjects (59 per arm) should be randomized to provide a study power of 90% with an alpha risk of 5%.
Statistical Analyses
Statistical analysis will follow the intention-to-treat (ITT) principle. The primary effect parameter is defined as the relative risk for improvement on the mRS and will be compared between the MSC treatment group and the control group using ordinal logistic regression [43]. The analysis will be repeated after adjustment for sex, age, basic scale scores, time since onset, previous stroke, hypertension, diabetes mellitus, hyperlipemia, atrial fibrillation, and history of smoke and alcoholic intemperance using multivariable regression analysis. For the analysis of the secondary outcomes, Student’s t test, Mann–Whitney tests, chi-square test, analysis of variance, and multivariable linear and logistic regression models will be used, where appropriate. All of the statistical tests will adopt a two-tailed test and P values < 0.05 are considered statistically significant.
Summary
This paper summarizes the methodology for a prospective, randomized, controlled, observer-blinded phase II trial. We believe that our study will provide a high level of evidence and a better understanding of allogenic MSC therapy via intrathecal injections in patients with severe ischemic stroke.
References
Misra V, Ritchie MM, Stone LL, et al. Stem cell therapy in ischemic stroke: role of IV and intra-arterial therapy. Neurology. 2012;79(13):207–12.
Chopp M, Steinberg GK, Kondziolka D, Lu M, Bliss TM, Li Y, et al. Who’s in favor of translational cell therapy for stroke: STEPS forward please? Cell Transplant. 2009;18(7):691–3.
Rosado-de-Castro PH, Pimentel-Coelho PM, da Fonseca LM, et al. The rise of cell therapy trials for stroke: review of published and registered studies. Stem Cells Dev. 2013;22(15):2095–111.
Maria Ferri ALBA, Lisini D, Boncoraglio G, et al. Mesenchymal stem cells for ischemic stroke: progresses and possibilities. Curr Med Chem. 2016;23(16):1598–068.
Wan H, Li F, Zhu L, Wang J, Yang Z, Pan Y. Update on therapeutic mechanism for bone marrow stromal cells in ischemic stroke. J Mol Neurosci. 2013;52(2):177–85.
Vu QXK, Eckert M, Zhao W, et al. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82(14):1277–86.
Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–82.
Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099–106.
Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134(Pt 6):1790–807.
Jiang Y, Zhu W, Zhu J, Wu L, Xu G, Liu X. Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transplant. 2013;22(12):2291–8.
Qiao LY, Huang FJ, Zhao M, et al. A two-year follow-up study of cotransplantation with neural stem/progenitor cells and mesenchymal stromal cells in ischemic stroke patients. Cell Transplant. 2014;23(Suppl 1):S65–72.
Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 2016;47(7):1817–24.
Bhasin A, Srivastava MV, Kumaran SS, et al. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra. 2011;1(1):93–104.
Bhasin A, Kumaran SS, Bhatia R, Mohanty S, Srivastava MVP. Safety and feasibility of autologous mesenchymal stem cell transplantation in chronic stroke in Indian patients. A four-year follow up. J Stem Cells Regen Med. 2017;13(1):14–9.
Bang OY. Clinical trials of adult stem cell therapy in patients with ischemic stroke. J Clin Neurol. 2016;12(1):14–20.
Han H, Chang SK, Chang JJ, Hwang SH, Han SH, Chun BH. Intrathecal injection of human umbilical cord blood-derived mesenchymal stem cells for the treatment of basilar artery dissection: a case report. J Med Case Rep. 2011;5:562.
Yamout B, Hourani R, Salti H, Barada W, el-Hajj T, al-Kutoubi A, et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol. 2010;227(1–2):185–9.
Mohyeddin Bonab M, Mohajeri M, Sahraian MA, Yazdanifar M, Aghsaie A, Farazmand A, et al. Evaluation of cytokines in multiple sclerosis patients treated with mesenchymal stem cells. Arch Med Res. 2013;44(4):266–72.
Chotivichit A, Ruangchainikom M, Chiewvit P, Wongkajornsilp A, Sujirattanawimol K. Chronic spinal cord injury treated with transplanted autologous bone marrow-derived mesenchymal stem cells tracked by magnetic resonance imaging: a case report. J Med Case Rep. 2015;9:79.
Liu J, Han D, Wang Z, Xue M, Zhu L, Yan H, et al. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy. 2013;15(2):185–91.
Oh KW, Moon C, Kim HY, Oh SI, Park J, Lee JH, et al. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl Med. 2015;4(6):590–7.
Andolina M. Treatment of spinal muscolar atrophy with intrathecal mesenchymal cells. Int J Stem Cells. 2012;5(1):73–5.
Choi SW, Park KB, Woo SK, Kang SK, Ra JC. Treatment of progressive supranuclear palsy with autologous adipose tissue-derived mesenchymal stem cells. J Med Case Rep. 2014;8(1):87–91.
Zhu J, Xiao Y, Li Z, et al. Efficacy of surgery combined with autologous bone marrow stromal cell transplantation for treatment of intracerebral hemorrhage. Stem Cells Int. 2015;2015:318269.
Dongmei H, Jing L, Mei X, Ling Z, Hongmin Y, Zhidong W, et al. Clinical analysis of the treatment of spinocerebellar ataxia and multiple system atrophy-cerebellar type with umbilical cord mesenchymal stromal cells. Cytotherapy. 2011;13(8):913–7.
Liu L, Eckert MA, Riazifar H, et al. From blood to the brain: can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cells Int. 2013;2013:435093.
Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, et al. Cell based therapies for ischemic stroke: from basic science to bedside. Prog Neurobiol. 2014;115:92–115.
Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2007;83(5):723–30.
Walczak P, Zhang J, Gilad AA, et al. Dual-modality monitoring of targeted intra-arterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;35(9):1569–74.
Wechsler L, Steindler D, Borlongan D, et al. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40(2):510–5.
Kean TJ, Lin P, Caplan AI, et al. MSCs: delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013;36:732–42.
Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27(1):6–13.
Boltze J. The dark side of the force—constraints and complications of cell therapies for stroke. Front Neurol. 2015;6(17):155.
Savitz SI, Cramer SC, Wechsler L, Consortium S. Stem cells as an emerging paradigm in stroke 3: enhancing the development of clinical trials. Stroke. 2014;45(2):634–9.
Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313–9.
Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–12.
Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2015;112(4):1167–72.
Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947.
Wu Y, Ren M, Yang R, Liang X, Ma Y, Tang Y, et al. Reduced immunomodulation potential of bone marrow-derived mesenchymal stem cells induced CCR4+CCR6+ Th/Treg cell subset imbalance in ankylosing spondylitis. Arthritis Res Ther. 2011;13(1):R29.
Wang P, Li Y, Huang L, Yang J, Yang R, Deng W, et al. Effects and safety of allogenic mesenchymal stem cell intravenous infusion in active ankylosing spondylitis patients who failed NSAIDs: a 20-week clinical trial. Cell Transplant. 2014;23(10):1293–303.
Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S, et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke. Stroke. 2014;45(12):3618–24.
Walter SD, Gafni A, Birch S. Estimation, power and sample size calculations for stochastic cost and effectiveness analysis. PharmacoEconomics. 2007;25(6):455–66.
Saver JL. Novel end point analytic techniques and interpreting shifts across the entire range of outcome scales in acute stroke trials. Stroke. 2007;38(11):3055–62.
Funding
The study is funded by the Science and Technology Program of Guangdong province, China (no. 2015A020212018).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Adverse events and compliance to protocol will be monitored by an independent data monitoring committee, with in-person monitoring visits and phone contacts. The study protocol and information consent forms have been approved by the Ethical Committee of the Sun Yat-sen Memorial Hospital, Sun Yat-sen University. The trial has been registered in the Chinese Clinical Trial Registry (registration number: ChiCTR-INR-16008908; date of approval 25 July 2016). And all patients will be informed verbally and provided with a written document about the study by the investigators.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Significance Statement
This trial is the first to evaluate the safety, feasibility, and therapeutic mechanisms of allogenic BM-MSCs by intrathecal infusion in patients with severe cerebral infarction. This study protocol will provide a high level of evidence and better understanding of allogenic MSC therapy via intrathecal injection in patients with severe ischemic stroke.
Lingna Deng and Qingxia Peng are co-first authors.
Rights and permissions
About this article
Cite this article
Deng, L., Peng, Q., Wang, H. et al. Intrathecal Injection of Allogenic Bone Marrow-Derived Mesenchymal Stromal Cells in Treatment of Patients with Severe Ischemic Stroke: Study Protocol for a Randomized Controlled Observer-Blinded Trial. Transl. Stroke Res. 10, 170–177 (2019). https://doi.org/10.1007/s12975-018-0634-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-018-0634-y