Abstract
Sex dimorphism has been demonstrated after experimental intracerebral hemorrhage (ICH). Decreased mortality and improved neurobehavioral outcomes occur in female compared to male mice after intrastriatal autologous blood or collagenase injection. Sex-specific differences in post-ICH gene and protein expression may provide mechanistic insight into this phenomenon. Ten- to 12-week-old C57BL/6 male (M) and female in high estrous state (HE-F) underwent left intrastriatal collagenase injection. We assessed neurobehavioral outcomes over the first 30 days, hematoma volume and cerebral edema evolution over the first 24 h, and transcriptomic gene and protein expression at pre-selected time points during the acute phase of injury. Genome-wide expression profiling was performed with Affymetrix GeneChip Mouse Genome 2.0 Probes, and proteomics analyses were performed using mass spectroscopy. Sex does not affect hemorrhage evolution, but female sex is associated with improved neurobehavioral recovery after ICH. A total of 7037 probes qualified for our filtering criteria, representing 5382 mapped genes and 256 unmapped genes. Female-unique pathways involved cell development, growth, and proliferation, while male-unique pathways involved molecular degradation. At 6 and 24 h post-ICH, differential expression was observed in 850 proteins vs baseline in males, 608 proteins vs baseline in females, and 1 protein in females vs males. Female sex is associated with improved neurobehavioral recovery, and differential gene and protein expression after intrastriatal collagenase injection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sex differences in incidence and outcome after ischemic stroke, traumatic brain injury, and subarachnoid hemorrhage are well known. However, sex differences after intracerebral hemorrhage (ICH) remain unclear. Understanding sex differences in brain responses to ICH and recovery after ICH may lead to new therapeutic targets.
In recent years, pre-clinical models have contributed to mechanistic understanding of injury after ICH. While neurobehavioral recovery is clearly more complete in female animals [1], mechanisms of sex differences are only beginning to emerge. Improved recovery in female animals may be related to an inflammatory response that favors recovery [2, 3]. While many seemingly sex-specific reactions after ICH are related to presence or absence of gonadal hormones [4,5,6,7,8,9], other mechanisms are possible. Further, sex-specific pharmacogenomic interactions have been demonstrated in both humans and animals [10]. Understanding basic sex differences in brain responses to ICH will inform further investigation of these interactions.
The purpose of this study was to discover new, potential gene and protein targets associated with improved recovery after ICH in females. To achieve this objective, transcriptomic gene expression and proteomics analyses were performed in a model of experimental ICH.
Methods
All animal procedures were designed to minimize animal discomfort and numbers, conformed to international guidelines on the use of animals, and were approved by the Duke University Institutional Animal Care and Use Committee. Ten- to 12-week-old male and female C57BL/6 mice were used in all experiments. Mice were randomly selected for injury, and all operators were blinded to group assignment.
Experimental Groups
Cohort 1. Neurobehavioral Recovery
A separate cohort of age-matched male and female mice were tested on the rotarod (RR) over the first 7 days and in the Morris water maze (MWM) over days 28–31 after ICH (n = 15/group).
Cohort 2. Hemorrhage Volume and Edema Measurements
On separate cohorts of age-matched male and female mice, hematoma volume was measured at 6 and 24 h after ICH using magnetic resonance imaging (MRI) (n = 5/group), and brain water content was determined at 24 h after ICH (n = 8/group).
Cohort 3. Differential Transcriptomic Gene Expression
Gene expression analysis was performed in a separate cohort of age-matched male and female mice at 0, 2, 6, and 24 h after ICH, and again at 5 days after ICH (n = 3/group per time point with three female and three male baseline samples, i.e., non-injured).
Cohort 4. Proteomic Assessment
Protein expression analysis was performed in a separate cohort of age-matched male and female mice at 0, 6, and 24 h after ICH (n = 3/group per time point with three female and three male baseline samples, i.e., non-injured).
ICH Model
The murine intrastriatal collagenase injection ICH model [11, 12] was adapted from a previously described model in rats [13]. Mice were anesthetized with 4.6% isoflurane in 30% O2/70% N2. After anesthetic induction, the trachea was intubated, and the lungs were mechanically ventilated with 1.5% isoflurane in 30% O2/70% N2. Rectal temperature was maintained at 37.0 ± 0.2 °C by an underbody circulating waterbed. The animal’s head was secured in a stereotactic frame. The scalp was incised and a burr hole was created 2.2 mm left lateral to bregma. A 1.0-μL syringe (Hamilton, Reno, NV) with a 25-gauge needle was mounted on the stereotactic frame. The eye of the needle was advanced to a depth of 3 mm from the cortical surface. Type IV-S clostridial collagenase (Sigma, St. Louis, MO, USA; 0.075 U in 0.4 μL 0.9% NaCl) was injected over 2 min, and the needle was held motionless for an additional 5 min. After slowly withdrawing the needle, the incision was closed, and animals were allowed to recover spontaneous ventilation with subsequent extubation. Following recovery in a warm, non-stimulating environment, mice were allowed free access to food and water.
Determination of Estrous Stage
Prior to injury, morning vaginal smears were obtained from female mice to determine the reproductive stage [14]. Smears from mice in the estrous stage contained a cluster of irregularly shaped, cornified squamous epithelia cells that lacked nuclei. These mice were used in the experiments. Subsequent vaginal smears were obtained on consecutive days from females in other reproductive stages until they could be classified as estrus, and added to the experimental pool.
Neurobehavioral Testing
Rotarod
An automated RR (Ugo Basile, Comerio, Italy) was used to assess the effects of therapeutic intervention on vestibulomotor function [15]. On the day prior to injury, mice underwent two consecutive conditioning trials at a set rotational speed (16 rev/min) for 60 s, followed by three trials with accelerating rotational speed. The average time to fall from the rotating cylinder in the latter three trials was recorded as baseline latency. On days 1, 3, 5, and 7 after ICH, mice underwent every other day testing for RR with three trials of accelerating rotational speed (4–40 rpm, inter-trial interval of 15 min) for up to 300 s. Average latency to fall from the rod was recorded. Mice unable to grasp the rotating rod were given a latency of 0 s.
Morris Water Maze
MWM was evaluated in a black aluminum pool (105 cm in diameter, 60 cm deep) filled with water opacified with powdered milk, and containing a platform (7.5 cm in diameter) submerged 1 cm below the water surface (22 ± 0.5 °C). The maze was kept in a room dedicated to behavioral testing, to decrease stress. Mice were tested on days 28–31 after ICH with four trials per day and an inter-trial interval of 1 h. For each trial, mice were placed in one of four quadrants. Mice were allowed to search for the platform for 90 s. If unable to locate the platform, mice were guided to the platform where they remained for 15 s. A computerized video tracking system (KeilSoft LLC, Chapel Hill, NC) recorded latency from entering the maze to finding the platform, as well as swimming speed. On the final day, after hidden platform testing, a probe trial was conducted for which the escape platform was removed, and the mouse was released at a point diagonally opposite the previous location of the platform. The time spent searching all four quadrants and the number of crossings into the previous platform quadrant were recorded.
MRI
Mouse brain imaging was performed on a 7-T Bruker Biospec 70/30 horizontal bore system (Billerica, MA). Animals were lightly anesthetized with isoflurane, and physiologic parameters were continuously monitored and maintained throughout the imaging session (~60 min/animal). Axial 2D T2-weighted fast spin echo images (TURBO-RARE, TE/TR = 11/4200 ms with 1-mm-thick slice, matrix = 256 × 256 and FOV of 2.4 × 2.4 cm, five averages, 0.0-mm interslice gap) were obtained first for screening purposes and supplemental anatomic information. For directed hemorrhage volumetric analysis by a blinded observer, 64 contiguous 500-μm-thick 3D FSE proton density images (TURBO-RARE, TE/TR = 9/1500 ms, matrix = 256 × 256 × 64 and FOV of 2.2 × 2.2 × 2.2 cm, 25-min duration) were acquired.
Hemorrhage Volume Measurement
Volumetric analysis of MRI data sets was performed using Osirix software, an open-source image-processing application developed and maintained by Pixmeo (Geneva, SUI). Hemorrhages were manually segmented in each animal by an investigator blinded to background. Selected areas were reviewed for consistency on coronal and sagittal representations, and cross-correlated with axial 2D FSE images. Two separate striatal segmentations were obtained for each animal, and the average volume was recorded. Intrarater reliability (kappa value) was 0.97.
Brain Water Content Measurement
Brain water content was measured at 24 h after injury. After anesthesia, mice were euthanized by decapitation, and brains were immediately harvested. The cerebellum and brain stem were discarded. Right and left hemispheres were separately weighed (“wet” weight). Brains were allowed to dehydrate over 24 h at 105 °C, and then reweighed (“dry” weight). Water content was calculated as a percentage of wet weight ([wet weight − dry weight / wet weight] × 100).
Tissue Processing, Messenger RNA Extraction, and Protein Extraction
Tissue Processing
After anesthesia induction, mice were intubated, and perfused transcardially with 30 mL PBS. After brains were removed, ipsilateral and contralateral hemispheres were dissected, flash frozen in liquid nitrogen, and then stored at − 80 °C.
Messenger RNA Extraction and Microarray Processing
Frozen, pulverized ipsilateral hemisphere tissue was processed for RNA extraction (RNA Lipid Tissue Mini Kit, Qiagen, Hilden, Germany). RNA quantity and quality were assessed with the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE) and by agarose gel electrophoresis. Only samples with a 260/280 ratio of 1.9–2.1 and a 260/230 ratio > 2.0, were further processed. Hybridization targets were prepared from 2 μg of total RNA, and hybridized according to standard Affymetrix protocols, using Affymetrix GeneChip Mouse 2.0 arrays (Affymetrix, Santa Clara, CA). Arrays were scanned on the Affymetrix GeneChip scanner, and probe-set expression values (percent present and 3′/5′ probe-set ratios for actin and GAPDH [for microarray quality control]) were calculated using the Affymetrix Microarray Analysis Suite v5.0.
Protein Isolation
Protein isolation and proteomics analysis were performed by the Duke Proteomics Core Facility (DPCF). Mouse brain tissue was solubilized using a standard MS-compatible surfactant/burst sonication procedure in which brains were suspended in 750 μL 50 mM ammonium bicarbonate (pH 8) with 0.25% ALS-1 (standard MS-compatible surfactant), and then subjected to three 10-s probe sonication bursts at 30% power. Samples were spun at 15,000 rpm for 5 min, and insoluble material was discarded. A Bradford assay (mini-Bradford, Bio-Rad Inc., Hercules, CA) of all samples was taken after protein isolation to determine protein yield. Using 50 mM ammonium bicarbonate, 30 μg from each sample was normalized to 1.0 μg/μL for reduction (10 mM DTT), cysteine alkylation (20 mM iodoacetamide), and trypsin digestion according to standard protocol (http://www.genome.duke.edu/cores/proteomics/samplepreparation/documents/InsolutionDigestionProtocol_012309.doc). After digestion, all samples were spiked with ADH1_YEAST digest (Massprep standard, Waters Corporation) as a surrogate standard (50 fg ADH/μg total brain lysate), dried, and resuspended in 2% acetonitrile and 0.1% TFA (pH 2.5) to a concentration of 0.6 μg/μL prior to analysis. A “QC pool” was generated by removing an equal quantity (1 μg) from each of the 28 samples (2 samples were discarded due to poor quality).
Statistical Analysis
RR and WM performance were compared with repeated measures analysis of variance, with time as the repeated variable. Hemorrhage volume and interhemispheric water weight differences were compared using Student’s t test. P < 0.05 was considered statistically significant, and data were expressed as mean ± SD.
Gene Expression Analyses
Data analysis from individual mouse samples was performed using Bioconductor (http://bioconductor.org/). Probe sets that were significantly different (adjusted P value < 0.05) in each compared group were analyzed using Ingenuity Pathway Analysis (IPA; http://www.ingenuity.com/products/ipa). IPA allows development of gene and gene-disease networks by incorporating multiple source databases, including major NCBI databases (EntrezGene, RefSeq, and OMIM disease associations), microRNA-mRNA target databases, GWAS databases, and Kyoto Encyclopedia of Genes and Genomes (KEGG).
IPA attempts to map items in the gene ID column, to gene symbols and names in the Ingenuity Pathways Knowledge Base. When multiple probe sets were mapped to one gene, the probe set with the greatest standard deviation of expression values was selected for detailed analysis. The “Canonical Pathways” tab displays the most significant canonical pathways in IPA across the entire dataset. The significance values for the canonical pathways are calculated by Fisher’s exact test right-tailed. The significance indicates the probability of association of molecules from the dataset with the canonical pathway by random chance alone.
Proteomics Analyses
To assess global expression profiles, a 2D agglomerative clustering of protein expression levels (z-score-transformed to plot significance of change) was generated from individual mouse samples. As described above, the data were intensity-scaled at the peptide level to the robust mean (excluding the highest and lowest 10% of the signals) across all injections, resulting in a final quantitative dataset for 8425 peptides. Expression data for 1235 mouse brain proteins were derived by summing the intensities of all peptides within that protein for each individual sample. Proteins with only one peptide are highly likely to be correctly identified, as the peptide-level false discovery rate is < 1%. However, highest confidence should be placed on proteins with two or more peptides and a favorable fold change and P value. Protein expression values were used to generate fold-change data between treatment groups (male at each time point vs male uninjured and female at each time point vs female uninjured). Fold changes were calculated by ratioing the average intensity of the three animals between the two time points. An error-weighted Bonferroni-corrected ANOVA was also performed between these groups.
Results
Neurobehavioral Recovery After ICH
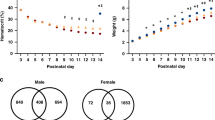
Regarding sex differences in neurological recovery after pre-clinical ICH, female mice demonstrate longer RR latencies over the first 7 days after ICH, shorter WM latencies over 28–31 days after ICH, and more time spent in the target quadrant on the last day of WM testing (Fig. 1). One female and two males died within 24 h after ICH.
Female sex is associated with improved short-term and long-term neurobehavioral recovery.a Female mice in estrus showed significantly longer rotarod latencies over the first 7 days after ICH compared to males (n = 15/group, P < 0.01, ANOVA). b Female mice in estrus demonstrated significantly shorter water maze latencies and faster learning over post-ICH days 28–31 compared to males (n = 15/group, P = 0.04, ANOVA). c Probe testing after the last water maze trail on day 31 after ICH was recorded as time spent in the quadrant previously occupied by the hidden platform. Female mice spent more time in the target quadrant than males (female vs male 34.1 ± 12.8 vs 22.3 ± 7.3 s, P = 0.02). F female, ICH intracerebral hemorrhage, M male, SHAM sham injured mice
Hemorrhage Volume and Cerebral Edema After ICH
To assess sex differences in hematoma and edema formation after pre-clinical ICH, over the first 24 h after ICH, no sex differences in hemorrhage volume and evolution were observed (Fig. 2). However, brain water content at 24 h after ICH was significantly reduced in female mice compared to males (male vs female 80.36 ± 0.2047 [n = 5] vs 79.43 ± 0.3254 [n = 5], P = 0.04).
Differential Gene Expression at Early Time Points After ICH
To assess sex differences in gene expression of the brain after pre-clinical ICH, a total of 22,275 probe sets qualified for analyses after filtering. Among these, 7037 probe sets were differentially expressed after ICH in male or female mice at pre-selected time points (adjusted P value < 0.05). Of the 7037 sets, 5382 mapped genes and 256 unmapped sets were analyzed using IPA.
In female mice, 5 significantly differentially expressed (SDE) genes were found at 2 h, 1058 at 6 h, 1513 at 24 h, and 2055 at 5 days after ICH when compared to baseline (Supplemental Table 1). Among these 4631 SDE genes, 2349 were upregulated and 2282 were downregulated.
In male mice, no SDE genes were found at 2 h, while 421 were found at 6 h, 257 at 24 h, and 3 at 5 days after ICH when compared to baseline (Supplemental Table 2). Among these 681 SDE genes, 553 were upregulated and 128 were downregulated.
In female mice, 5 unique genes were expressed at 2 h, 929 at 6 h, 1299 at 24 h, and 2061 at 5 days after ICH compared to male SDE genes at each time point. In male mice, no unique genes were expressed at 2 h, while 250 were expressed at 6 h, 40 at 24 h, and 3 at 5 days after ICH compared to female SDE genes at each time point (Fig. 3).
Differential gene expression in male vs female mice at early time points after intracerebral hemorrhage (ICH).a In each Venn diagram, genes differentially expressed at each time point compared to baseline are stratified into female-specific genes in the left circle, male-specific genes in the right circle, and non-sex-specific genes in the center. Red arrows represent upregulation, and green arrows represent downregulation. b The time course of total number of genes differentially expressed compared to baseline. Base pre-injured baseline, d day, F female, h hour, M male, vs versus
When comparing male and female mice at each time point after ICH, 63 genes were differentially expressed at baseline. After ICH, 1 gene was differentially expressed at 2 h, 3 at 6 h, 1 at 24 h, and 1 at 5 days (Supplemental Table 3). Compared to female mice, X-inactive specific transcript (Xist) was downregulated in male mice at all time points after ICH.
Enriched Functional Category and Pathway Analysis
To systematically characterize sex differences in the functions of SDE genes, all SDE genes at each time point were included in pathway analysis using IPA. Unique pathways were selected when significant pathways appeared in only male or female mice. Shared pathways were selected when significant pathways appeared in both male and female mice. To focus the main differences at each time point, the top 10 significant pathways were selected, respectively, in male unique, female unique, and shared pathways after ICH (Supplemental Table 4).
In male mice, no pathways involving SDE genes were identified at 2 h, while 150 were identified at 6 h, 125 at 24 h, and 11 at 5 days after ICH. Among these pathways, 10 unique pathways were found at 6 h, 9 at 24 h, and none at 5 days after ICH compared to baseline. Unique male pathways consisted of “molecular degradation” at 6 h, and “cellular stress and injury” and “cellular immune response” at 24 h after ICH.
In female mice, 43 pathways involving SDE genes were identified at 2 h, 175 at 6 h, 252 at 24 h, and 255 at 5 days after ICH. Among these pathways, 43 female unique pathways were found at 2 h, 35 at 6 h, 33 at 24 h, and 244 at 5 days after ICH. Unique female pathways consisted of “cytokine signaling” and “cellular immune response” at 2 h, “growth factor signaling” and “neurotransmitter and other nervous system signaling” at 6 and 24 h, and “intracellular and second messenger signaling” and “cellular immune response” at 5 days after ICH.
Forty-four shared pathways were identified at 6 h, 70 at 24 h, 20 at both 6 and 24 h, and 9 at 5 days after ICH. Shared pathways consisted of “inflammation,” “apoptosis,” and “cellular growth and proliferation.” Further, the acute phase response signaling pathway was found to be significant in all groups except males at 5 days after ICH.
Acute Phase Response Signaling Pathway After ICH
Male and female mice shared mostly ingenuity toxicity list pathways during the acute phase after ICH. Notably, the number of enrolled SDE genes and their fold changes varied between sexes. Among these toxicity pathways, the “acute phase response signaling” pathway was the most significant pathway in male and female mice at 6 and 24 h, and is also important among cytokine signaling pathways. Therefore, we selected the acute phase response pathway to demonstrate sex differences (Supplemental Fig. 1 and Supplemental Table 5).
All genes involved in the acute phase response signaling pathway were ranked according to the P value of the most significant probe within the gene after all comparisons. The top 20 genes (best P < 0.001) were selected for the cluster analysis of this pathway. The cluster analysis revealed that expression of this subset of genes is consistent between males and females at 24 h and at 5 days after ICH. Other time points showed differences in gene expression levels (Fig. 4).
Acute phase signaling pathway cluster analysis. This heat map was created with an unsupervised clustering approach. Columns represent samples with sample names from individual mice (n = 3/group per time point) are displayed along the lower edge of the heat map. Rows represent genes with their corresponding gene symbols. Results from each mouse brain (represented by an individual column) are clustered with the results from mouse brains most similar in their gene expression across. Secondarily, each of the rows is grouped through the same process. Red depicts upregulated genes with lighter shades reflectively greater change, green depicts downregulated genes with lighter shades reflectively greater change, and black indicates no change. B baseline, d days, F female, h hours, M male
Protein Expression at Early Time Points After ICH
To assess sex differences in protein expression of the brain after pre-clinical ICH, a total of 1235 proteins qualified for analyses after filtering. In males, we identified 309 of these proteins at 6 h and 541 at 24 h after ICH with significant differential expression compared to baseline (Supplemental Table 6). In females, we identified 439 proteins at 6 h and 369 at 24 h after ICH with significant differential expression compared to baseline (Supplemental Table 7).
We detected only one protein, XPP3, that was differentially expressed in males vs females after ICH, and it was downregulated in males at 6 h after injury compared to females.
Discussion
Robust sex differences in neurobehavioral recovery were observed in our murine model of ICH. In addition, modest sex-specific changes in gene and protein expression in brain tissue of mice after ICH occurred in both the acute and sub-acute phases of injury. Similar to human acute ischemic stroke [16], such differences in gene and protein expression could be key to the development of targeted therapeutic strategies.
Toll-like receptor-2 (TLR2), for example, was upregulated through day 5 after ICH in female mice, with increasing fold changes, but only through 24 h in males. TLR2 regulates the inflammatory response in the acute phase of ICH [17], but the role of TLR2 in the sub-acute phase after ICH is not clear. B cell lymphoma-2-related protein A1 (BCL2A1) was upregulated in female mice at all time points tested after ICH, but only at 24 h in male mice. BCL2A1 may have a cytoprotective effect as a direct transcription target of nuclear factor kappa B, which is upregulated in neuroinflammatory processes [18].
A direct comparison of SDE genes in male and female mice at each time point after ICH may be even more revealing. Only three SDE genes had a 2-fold change or more from baseline in male vs female mice: Xist was downregulated more than 8-fold in male mice at all selected time points, proteoglycan-4 (Prg4) was downregulated 2-fold in males at 6 h after ICH, and DEAD box helicase-6 (Ddx6) was upregulated 2-fold in males. XIST regulation of histone 3 (H3) may be an epigenetic mechanism of neural sex differences [19,20,21]. DDX6 is an RNA helicase found in P bodies and stress granules, and functions in translation suppression and messenger RNA (mRNA) degradation. Prg4 is connected to hemopoietic stem cell proliferation, regulation of cell proliferation, and negative feedback of interleukin-6 production. In mice lacking the beta-2 adrenergic receptor (β2AR), brain Prg4 expression is similar to its expression signature seen in a middle cerebral artery occlusion model of ischemic stroke [22]. Tendon Prg4 mRNA is upregulated in response to transforming growth factor β [23], which increases in the brain following stroke [24].
Mapping these differential gene expression data to various pathways may show powerful associations. Male and female mice shared many ingenuity toxicity list pathways at 6 and 24 h after ICH, including the apoptosis signaling pathway, acute phase response pathway, and nuclear factor kappa B signaling pathway. Although pathways were shared, the number of enrolled SDE genes and their significance and fold changes were different between male and female mice. In differential pathway expression after ICH, male gene expression mapped to metabolic pathways including biosynthesis and degradation. In contrast, female gene expression mapped to “growth” pathways including growth factor, axonal guidance, and vascular endothelial growth factor signaling (Table 1).
Interestingly, our data examined differences in gene expression up to 5 days after injury, when recovery mechanisms are believed to be optimized. At 5 days after ICH, only 3 SDE genes were found in male mice, while 2134 were found in female mice. These 2134 SDE genes were enrolled in 433 gene expression pathways: 20% related to growth and proliferation, 16% to cellular immune response, 10% to intracellular second messenger signaling, 10% to cytokine signaling, and 8% to neurotransmitter signaling. The result is that, in female mice, some pathways were significantly differentially activated in both the acute phase and at 5 days after ICH. For example, more genes mapped to axonal guidance signaling than to any other expression pathway at both 24 h and 5 days after ICH in female mice. Thus, one pathway may have different roles at different time points.
Proteomics analysis revealed only a limited number of proteins with significant differential expression in male vs female mice after ICH. XPP3 was the only protein with significant differential expression between male and female mice, and this sex difference was observed only at 6 h after ICH. The relationship of this protein to ICH is not clear.
This study is exploratory, and should not be viewed as conclusive. Our findings, while informative, are limited by lack of data that demonstrate functionality of any of these genes or proteins within this model system or disease state. Ongoing studies are being conducted to verify functional importance of these genes and proteins. Further, gonadal hormone physiology is complicated and, along with age and comorbidities, would likely affect gene expression in both males and females.
Conclusion
Female sex is associated with improved neurobehavioral recovery after intrastriatal collagenase injection. Improved recovery in female mice may be associated with a number of differentially expressed genes involved in recovery pathways. The present study may inform future work in acute ICH pathophysiology directed toward developing targeted therapeutics.
References
Lei B, Mace B, Bellows ST, Sullivan PM, Vitek MP, Laskowitz DT, et al. Interaction between sex and apolipoprotein E genetic background in a murine model of intracerebral hemorrhage. Transl Stroke Res. 2012;3(1):94–101. https://doi.org/10.1007/s12975-012-0176-7.
Chen TY, Tsai KL, Lee TY, Chiueh CC, Lee WS, Hsu C. Sex-specific role of thioredoxin in neuroprotection against iron-induced brain injury conferred by estradiol. Stroke. 2010;41(1):160–5. https://doi.org/10.1161/STROKEAHA.109.562850.
Nakamura T, Xi G, Keep RF, Wang M, Nagao S, Hoff JT, et al. Effects of endogenous and exogenous estrogen on intracerebral hemorrhage-induced brain damage in rats. Acta Neurochir Suppl. 2006;96:218–21.
Regan RF, Guo Y. Estrogens attenuate neuronal injury due to hemoglobin, chemical hypoxia, and excitatory amino acids in murine cortical cultures. Brain Res. 1997;764(1–2):133–40.
Gu Y, Xi G, Liu W, Keep RF, Hua Y. Estrogen reduces iron-mediated brain edema and neuronal death. Acta Neurochir Suppl. 2010;106:159–62. https://doi.org/10.1007/978-3-211-98811-4_29.
Nakamura T, Hua Y, Keep RF, Park JW, Xi G, Hoff JT. Estrogen therapy for experimental intracerebral hemorrhage in rats. J Neurosurg. 2005;103(1):97–103. https://doi.org/10.3171/jns.2005.103.1.0097.
Nguyen AP, Arvanitidis AP, Colbourne F. Failure of estradiol to improve spontaneous or rehabilitation-facilitated recovery after hemorrhagic stroke in rats. Brain Res. 2008;1193:109–19. https://doi.org/10.1016/j.brainres.2007.11.054.
Auriat A, Plahta WC, McGie SC, Yan R, Colbourne F. 17beta-estradiol pretreatment reduces bleeding and brain injury after intracerebral hemorrhagic stroke in male rats. J Cereb Blood Flow Metab. 2005;25(2):247–56. https://doi.org/10.1038/sj.jcbfm.9600026.
Chen Z, Xi G, Mao Y, Keep RF, Hua Y. Effects of progesterone and testosterone on ICH-induced brain injury in rats. Acta Neurochir Suppl. 2011;111:289–93. https://doi.org/10.1007/978-3-7091-0693-8_48.
Gaignebet L, Kararigas G. En route to precision medicine through the integration of biological sex into pharmacogenomics. Clin Sci (Lond). 2017;131(4):329–42. https://doi.org/10.1042/CS20160379.
James ML, Warner DS, Laskowitz DT Preclinical models of intracerebral hemorrhage: a translational perspective. Neurocrit Care. 2007.
Lei B, Sheng H, Wang H, Lascola CD, Warner DS, Laskowitz DT, James ML Intrastriatal injection of autologous blood or clostridial collagenase as murine models of intracerebral hemorrhage. J Vis Exp. 2014 (89). doi:https://doi.org/10.3791/51439.
Rosenberg GA, Estrada E, Wesley M, Kyner WT. Autoradiographic patterns of brain interstitial fluid flow after collagenase-induced haemorrhage in rat. Acta Neurochir Suppl (Wien). 1990;51:280–2.
Caligioni CS Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009.
Hamm RJ, Pike BR, O'Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11(2):187–96.
Tian Y, Stamova B, Jickling GC, Liu D, Ander BP, Bushnell C, et al. Effects of gender on gene expression in the blood of ischemic stroke patients. J Cereb Blood Flow Metab. 2012;32(5):780–91. https://doi.org/10.1038/jcbfm.2011.179.
Hayward JH, Lee SJ. A decade of research on TLR2 discovering its pivotal role in glial activation and neuroinflammation in neurodegenerative diseases. Exp Neurobiol. 2014;23(2):138–47. https://doi.org/10.5607/en.2014.23.2.138.
Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9(5):505–12. https://doi.org/10.1038/sj/cdd/4400998.
Toyo-oka K, Wachi T, Hunt RF, Baraban SC, Taya S, Ramshaw H, et al. 14-3-3epsilon and zeta regulate neurogenesis and differentiation of neuronal progenitor cells in the developing brain. J Neurosci Off J Soc Neurosci. 2014;34(36):12168–81. https://doi.org/10.1523/JNEUROSCI.2513-13.2014.
Shen EY, Ahern TH, Cheung I, Straubhaar J, Dincer A, Houston I, et al. Epigenetics and sex differences in the brain: a genome-wide comparison of histone-3 lysine-4 trimethylation (H3K4me3) in male and female mice. Exp Neurol. 2015;268:21–9. https://doi.org/10.1016/j.expneurol.2014.08.006.
Qureshi IA, Mehler MF. Sex, epilepsy, and epigenetics. Neurobiol Dis. 2014;72(Pt B):210–6. https://doi.org/10.1016/j.nbd.2014.06.019.
White RE, Palm C, Xu L, Ling E, Ginsburg M, Daigle BJ et al. Mice lacking the beta2 adrenergic receptor have a unique genetic profile before and after focal brain ischaemia. ASN Neuro. 2012. 4(5). doi:https://doi.org/10.1042/AN20110020
Rees SG, Davies JR, Tudor D, Flannery CR, Hughes CE, Dent CM, et al. Immunolocalisation and expression of proteoglycan 4 (cartilage superficial zone proteoglycan) in tendon. Matrix Biol. 2002;21(7):593–602.
Dhandapani KM, Brann DW. Transforming growth factor-beta: a neuroprotective factor in cerebral ischemia. Cell Biochem Biophys. 2003;39(1):13–22. https://doi.org/10.1385/CBB:39:1:13.
Acknowledgements
We acknowledge Kathy Gage’s assistance in medical editing.
Funding
This work was funded by the Duke University Department of Anesthesiology Dream Innovation Grant (MLJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Electronic Supplementary Material
Supplemental Figure 1
Differential gene expression in the acute phase response signaling pathway in female (A) and male (B) mice at 6 h after intracerebral hemorrhage compared to pre-injury baseline. Note: green = down-regulated SDE genes; red = up-regulated SDE genes; 6 h, 6 h after intracerebral hemorrhage; B, baseline; F, female; M, male. (DOCX 490 kb)
Supplemental Table 1
Differentially expressed genes in female mice at selected time points after ICH compared to baseline. (XLSX 73 kb)
Supplemental Table 2
Differentially expressed genes in male mice at selected time points after ICH compared to baseline. (XLSX 429 kb)
Supplemental Table 3
Differentially expressed genes in male vs female mice at selected time points after ICH. (XLSX 17 kb)
Supplemental Table 4
Top 10 pathways at each time point after ICH for male and female mice compared to baseline. (XLSX 12 kb)
Supplemental Table 5
Differentially expressed genes enrolled in acute phase response signaling pathway in male vs female mice at selected time points after ICH. (XLS 30 kb)
Supplemental Table 6
Differentially expressed proteins in male mice at selected time points after ICH compared to baseline. (XLS 210 kb)
Supplemental Table 7
Differentially expressed proteins in female mice at selected time points after ICH compared to baseline. (XLS 156 kb)
Rights and permissions
About this article
Cite this article
Xie, Y., Li, YJ., Lei, B. et al. Sex Differences in Gene and Protein Expression After Intracerebral Hemorrhage in Mice. Transl. Stroke Res. 10, 231–239 (2019). https://doi.org/10.1007/s12975-018-0633-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-018-0633-z