Abstract
In patients with malignant middle cerebral artery (MMCA) stroke, a vital clinically relevant question is determination of the speed with which infarction evolves to select the time for decompressive hemicraniectomy [DHC]. A retrospective, multicenter cross-sectional study of patients referred for DHC, based on the criteria of randomized controlled trials, was undertaken to identify factors for selecting the timing of DHC in MMCA stroke, stratified by time [< 48, 48–72, > 72 h]. Infarction volume and infarct growth rate [IGR] were measured on all CT scans. One hundred eighty-two patients [135 underwent DHC and 47 survived without DHC] were included in the analysis. After multivariate adjustment, factors showing the strongest independent association with DHC were patients < 55 years of age, septum pellucidum deviation, temporal lobe involvement, MCA with additional infarcts, and IGR on second CT. Of the five factors identified, different combinations of determining factors were observed in each subgroup. Both first and second IGRs were highest in the < 48, 48–< 72, and > 72 h [p < 0.001]. Patients who survived without surgery had the slowest IGRs. There was no association between time to DHC and infarct volume, although infarct volume was lower in patients who survived without DHC compared to the DHC subgroups. We identify the major risk factors associated with DHC in time-stratified subgroups of patients with MMCA. Evaluation of IGRs between the first and second scan and when possible second and third scan can help in selecting the timing of hemicraniectomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In patients with malignant middle cerebral artery (MMCA) stroke, an important and clinically relevant question is determination of the speed with which the infarction evolves, as it is important in determination of the best timing for decompressive hemicraniectomy [DHC]. Recent studies utilizing multimodal imaging have shown that the speed of infarction growth rate (IGR) following occlusion of a major cerebral vessel varies widely in patients with MMCA [1].
Clinicians are very frequently faced with the dilemma of deciding on the best time for DHC in patients with MMCA stroke. Some reports support the concept of early surgery [< 24 h] [2,3,4], while others have failed to show any benefit with such an approach [5,6,7]. Systematic reviews regarding time to surgery and outcome also show conflicting results [6, 8, 9]. Indeed, a recent survey and retrospective data reviews have shown widely varying attitudes and recommendations for DHC in relation to age, preoperative GCS, and extent of infarction among neurosurgeons and stroke physicians [7, 10, 11].
We hypothesized that combining key clinical characteristics with IGR on imaging can be used as a guide in selecting the timing of hemicraniectomy.
Patients and Methods
DHC databases from three tertiary referral centers in three countries [Hamad General Hospital, Qatar; Rashid Hospital, Dubai, UAE; and Shifa International Hospital, Pakistan] collected between 2007 and 2014 were analyzed. All patients referred for DHC were included. The selection of patients was based on the following criteria: National Institutes of Health Stroke Scale score [NIHSS] ≥ 15 including, a score of 1 for item 1a (decreased level of consciousness from the beginning or progressive deterioration], brain computed tomography [CT] evidence of ischemia involving two third of the middle cerebral artery [MCA] or 50% MCA with additional anterior cerebral artery [ACA] or posterior cerebral artery [PCA] infarction and signs of local swelling. Patient’s records were reviewed for demographics, risk factors, imaging studies, hyperosmolar therapy, neurological signs and time of herniation, and time to surgery. Measurement of the infarct volume [IV] was made using open source image analysis software OsiriX version 5.6 [12]. Maximum infarct volume [MIV] was calculated using the last CT before DHC. For the type of vessel occlusion, CT angiography (CTA), MR angiography (MRA), or a conventional digital angiogram were utilized. Patients were excluded if only a single imaging study was performed, with parenchymal hematoma grade II [13], or hemorrhage with ventricular extension and missing surgical details.
Infarct Growth Rate Calculation
For the first infarct growth rate [IGR 1] calculation, we assumed the stroke volume to be zero prior to stroke onset.
Second infarct growth rate [IGR2] was measured on the second CT [CT2] using the following formula:
Experienced clinical physicians/neurosurgeons based upon the individual clinical condition and brain imaging made the decision for DHC. Patients were generally taken for surgery if there was progressively deteriorating level of consciousness with or without early clinical signs of herniation.
Outcome was assessed with the modified Rankin score (mRS) dichotomized as favorable [mRS ≤ 4] and unfavorable [mRS > 4] at 3 months by patient examination in the outpatient clinics. We also looked at the outcome in subgroup stratified by time [< 48, 48–72, > 72 h] to DHC.
The hospitals included in the study are tertiary referral centers with well-established comprehensive stroke services including acute stroke diagnostic, vascular interventional services, stroke units, and rehabilitation services. An acute stroke team provides a rapid assessment service 24 h a day, 7 days a week. Each hospital has a neurosurgical program, actively participating in the vascular neurology service.
Data Analysis
Descriptive results are presented as mean ± standard deviation for normally distributed data, or median with range for data that are not normally distributed. Number (percentage) is reported for all qualitative variables (gender). Bivariate analysis was performed using analysis of variance (ANOVA), the Kruskal-Wallis test, the Pearson chi-square, or the Fisher exact test whenever appropriate to compare all independent variables (e.g., age, gender) among the DHC subgroup stratified by time [< 48, 48–72, > 72 h]. A multinomial logistic regression model was used to identify significant independent factors associated with the DHC subgroup stratified by time [< 48, 48–72, > 72 h]. Participants who did not undergo DHC were used as the outcome reference category. Using goodness of fit test assessed the fit of the final model to the data. Adjusted odds ratios (AOR) and 95% CI for each level of DHC subgroup are reported for the final model. A two-sided P value < 0.05 was considered statistically significant. Data analysis was performed using SPSS version 22 (IBM Corporation: Armonk, NY, USA).
Results
Two hundred and twelve patients were selected for DHC based on the above criteria. One hundred and forty-six patients underwent DHC and 66 patients initially selected for surgery were not operated on (19 refused surgery and died and 47 stabilized without further deterioration and the treating physician/surgeon decided not to operate on them). Eleven patients were excluded from the DHC analysis (2 with incomplete data, 6 with hemorrhage and ventricular extension, and 3 with hemorrhage deemed to have caused acute worsening (PH II)). Nineteen patients who refused surgery and died were also excluded from further analysis. The final analysis included 135 patients who underwent DHC and 47 who survived without DHC. Median time to DHC was 51.33 h (range 12 to 312 h: l < 48 h, 54 (40%); 48–72 h, 44 (32.6%); and > 72 h, 37 (27.4%)).
Factors Associated with Surgical Timing [Table 1]
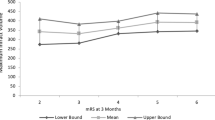
The demographics and clinical and radiological variables among DHC subgroups stratified by time to surgery [< 48, 48–72, > 72 h] are given in Table 1. In bivariate multinomial logistic regression analysis, there was a significant association between patient age and the timing of surgery in each subgroup of time to DHC. There were more males in each subgroup due to the expatriate population in Qatar and UAE being predominantly male. There was no difference in admission NIHSS [p = 0.083] or preoperative GCS [p = 0.597] in the patients with and without DHC. There was no statistically significant difference in the first and final infarction volume in patients who underwent DHC but was higher than in those who survived without surgery [Fig. 1]. While the first infarction volume did not show any significant difference, the second infarction volume was significantly higher in patients who underwent DHC [p = 0. 001] [Fig. 1]. There was no significant difference in second infarct volume between each DHC subgroup except >72 h which was similar to the non-DHC group. The final infarct volume in the >72 h group was also significantly higher than the non-DHC group, reflecting an increased growth in this subgroup (untreated for > 72 h) [Fig. 1]. Both first and second infarct growth rates were highest in the <48 h group followed by 48– < 72, and > 72 h [p < 0.001]. The third infarct growth rate showed a decline in all remaining subgroups except > 72 h where it showed an increase [Fig. 1]. Though the 3-month outcome was better in the non-DHC group compared to the DHC group, there was no statistically significant difference in the outcome of DHC subgroups stratified by time to surgery (P = 0.747).
Comparison of infarct volume and IGR according to DHC time subgroups vs. non-DHC group. Colored solid lines represent infarction volume changes with time and corresponding colored dashed lines IGR changes. While the first infarction volume did not show any significant difference, the second infarction volume was significantly higher in patients who underwent DHC [p < 0. 002]. There was no significant difference in second infarct volume between each DHC subgroup except > 72 h which was similar to the non-DHC group. The final infarct volume in the > 72 h group was also significantly higher than the non-DHC group, reflecting an increased growth in this subgroup. Both first and second infarct growth rates were highest in the < 48 h group followed by 48–< 72 and > 72 h [p < 0.001]. The third infarction growth rate showed a decline in all remaining subgroups except > 72 h where it increased and underwent DHC after the increase in the IGR. Time to CT in hours, average infarct volume in cm3, and IGR in milliliters/hour
Multivariate multinomial logistic regression analysis [Table 2] identified the following significant independent factors to be associated with time to DHC in the subgroups [< 48, 48–72, > 72 h]: patients < 55 years of age, septum pellucidum deviation ≥ 0.75 cm, patients with temporal lobe involvement, MCA with additional infarcts, and infarct growth rate on second CT. Of the five factors identified, different combinations of predictive factors were identified in each subgroup. Infarction growth rate on second CT showed a highly significant [p < 0.01] relation to surgery in < 48 [21% chance of early surgery with each unit ml/h increase in the IGR] and a significant [p < 0.01] association in 48– < 72 h [14% chance of surgery with each unit ml/h increase in the IGR] subgroups and no association in the > 72 h subgroup [Fig. 1]. Though clinical herniation was significantly associated with surgery in each subgroup in the bivariate analysis, in multivariate stepwise regression analysis in the presence of temporal lobe involvement and septum pellucidum deviation, clinical signs lost their significance. Temporal lobe involvement retained its significance compared to uncal herniation when adjusted for other confounder variables. MCA with additional infarction was significantly associated with surgery in each subgroup except 48– < 72 h compared to the non-DHC group. This could be related to septum pellucidum displacement in the 48– < 72 h group [more than twelve times the odds of surgery] leading to additional ACA infarcts in the > 72 h group where MCA with additional infarcts again regains its significant relationship to DHC [nearly five times the odds of surgery]. In the multivariable regression constructs evaluating time to surgery after including infarct volume, type of infarct, and infarct growth rate in addition to other covariates, only infarct growth rate and type of infarct retained a significant association.
Discussion
In the present analyses, we identified five factors that may assist with the determination of the best timing for DHC in patients with MMCA stroke. Our analysis shows that the rate of change in infarction size between two CT scans can be helpful in selecting the timing of hemicraniectomy. Our data shows no statistically significant difference in the first, second, and final infarction volumes across DHC subgroups stratified by time, but the IGRs [first, second, and third] were significantly higher in patients operated earlier [Table 1, Fig. 1]. The analysis of the two IGRs shows an initially rapid and approximately linear growth in each DHC subgroup and subsequent continued growth at a slower pace [Fig. 1]. While studies have reported a natural logarithmic pattern, human stroke may grow initially in a linear pattern followed by slower growth due to space limitation from the cranium and dural attachments [14, 15].
The process of edema formation and progression, after an acute ischemic insult, is a complex interplay between distinct fluid compartments within the cranium. This results in ionic gradients leading to a stepwise temporal progression from cytotoxic (cellular) to ionic [intact blood brain barrier] and finally to vasogenic edema [with disruption of blood brain barrier] [16], causing an increase in brain volume and elevation of intracranial pressure [ICP]. These changes in turn compromise cerebral microcirculation and failure of collateral circulation with expansion of the infarction and eventually herniation of cerebral tissue. A plethora of clinical, laboratory, and radiological predictors of malignant edema after large MCA infarction has been reported [7, 17,18,19,20,21]. Among patients with MMCA infarctions, an increased possibility of DHC is associated with younger age, MCA with additional infarction, midline shift, diabetes, infarction volume, and temporal lobe involvement [17, 22, 23].
The wide range of IGR in patients with MMCA stroke suggests that an individualized approach utilizing clinical and imaging information to select the time for DHC may offer an alternate approach in selecting time of DHC. Post stroke edema progresses during the first 24 to 48 h and often peaks later than 48 h [24]. Therefore, the time window within which DHC may be beneficial for such patients might be wider. Currently, DHC is limited to less than 48 h, based on the results of the European randomized control trials. These trials were neither designed nor powered to evaluate the optimal timing of intervention. Although some studies have reported improved outcome with early treatment, as compared with treatment after clinical deterioration, [25, 26] others could not confirm this finding [6]. In a recent nationwide inpatient sample analysis, early surgery, within 24 or 48 h, was not associated with differential outcomes. However, surgery after 72 h increased the odds of poor outcome [9]. A major limitation was time to surgery was calculated from hospital admission and not symptom onset. Despite the current recommendations, majority of neurosurgeons and stroke physicians [60%] would recommend surgery between 48 and 72 h and 27% beyond 72 h [10]. In the real world beyond randomized trials, DHC is still being performed after 48 h window [7, 9, 11, 27, 28]. The timing for DHC has been an issue that foments considerable debate. What triggers surgery for large MCA stroke is unknown. American Heart Association recommends using a “decrease in level of consciousness and its attribution to brain swelling as selection criteria” (strength of evidence is moderate or Class IIa) [29]. On the other hand, delaying DHC may lead to clinical herniation. The relevance of signs of herniation before surgery has not been addressed in the controlled trials, as all patients were operated on prior to the development of signs of herniation [30]. Delaying surgery may lead to clinical herniation compromising the outcome of DHC [7, 9, 11]. Therefore, DHC should be performed prior to the occurrence of this clinical sign of herniation. The current study provides an alternative approach of using the speed of infarct growth instead of waiting for clinical deterioration to operate. Since the first and second IGR showed a linear relationship, patients with rapid IGR could be operated earlier [Fig. 1]. The convincing results of the European trials and their pooled analysis should be applied to the patients with rapid IGR [< 48 h].
An interesting finding was the rapid increase in the third IGR in patients operated beyond 72 h compared to the non-DHC group while first and second IGR were comparable between the two groups [Table 1, Fig. 1]. Since these patients may not have high IGR, therefore, early surgery cannot be suggested in this group. This may reflect delayed failure of the collateral circulation, related to increasing mass effect. We did not measure the collateral status in our patients but research suggests that the presence of good collaterals may extend the time treatment window of acute stroke by slowing down the loss of penumbral tissue [31]. Our results are similar to DEFUSE-2 where10% of patients with distal MCA occlusion were slow progressors [32] and to the significant variation in IGR as evident on serial MRI study of untreated acute stroke [33]. Our data is also supported by previously reported penumbral loss rate of 8.9 ml/h without collateral flow [34]. The IGR in patients who survived without surgery was significantly slower in comparison with DHC subgroups [Table 1, Fig. 1]. IGR also varied considerably for stroke with occlusions of the same vessel [type of infarct], likely related to the collateral circulation. There was no significant difference in the final infarct volume in DHC subgroups suggesting that the time to repeat the CT scan will depend on the IGR calculated on the first CT scan. This is supported by the MRI-based infarction volume of more than 82 ml within 6 h [21] or more than 145 ml within 14 h of stroke onset [corresponding IGR 13.66 ml/h and 10.35 ml/h respectively] as predictors of malignant transformation. In turn, the second IGR can help select surgical timings or if IGR is slow, the time for repeat imaging.
Limitations
Our study has several limitations including the retrospective nature of the study with the lack of a randomized comparison between groups. Another limitation relates to the assessment of the IGR on CT scans. DW-MRI would have been a more sensitive tool but patient’s condition and logistical and financial reasons precluded repeated MRI imaging in our patients. Moreover, the DECIMAL trial excluded patients who were unable to undergo MRI. The MRI imaging-based data may not be generalizable to many centers that do not have access to MR imaging. Non-contrast CT of the brain remains the mainstay of imaging in acute stroke, as it is fast, inexpensive, and readily available. Although early ischemic changes within 6 h of stroke onset may be difficult to recognize on CT scans, the reported sensitivity for detection of early signs of infarction within 3 h of an MCA stroke is 75% [35, 36]. Moreover, using variable window width and center level settings can increase the sensitivity.
Other limitations include imaging not performed at uniform time points and the assumption that CT changes were present when the stroke symptoms began. It is possible that the hypodensity developed at a later time interval; hence, the first IGR may have a different value. However, the IGR on second CT did not show any significant difference presuming that infarct growth is linear in the acute stage [1, 15]. The smaller sample size in DHC subgroups may have led to overfitting the model with five predictors and wide confidence intervals for the odds ratios in our data sound a note of caution for the interpretation of these values. However, this is a retrospective data review only, identifying factors that might help determine the DHC timings. The criteria for surgery may have differed between physicians/surgeons and centers with widely applied standardization protocol for DHC; thus, a selection bias cannot be excluded. Finally, a major limitation is the short-term (3-month) follow-up and type of rehabilitation received. The difference in rehabilitation facilities could be another factor impacting the outcome. Because of most significant functional recovery happening within the first 6 months after stroke, a minimal observation period of 6 months is recommended [37]. Since most expatriates leave the country [Qatar and UAE] after treatment, long-term follow-up was not available. The sample size of time-stratified subgroups was small with differences in the demographic, clinical, and radiological characteristics. The multivariate analyses performed may not have adequately addressed this issue due to sample size limitations.
Future Direction
IGR calculation based on diffusion MR imaging performed at predefined time intervals is needed to further refine the concept of IGR-based DHC timings. The relationships between infarct growth and DHC timings require confirmation in larger prospective studies.
In conclusion, we report several factors associated with potential to select DHC timings in patients with MMCA. A critical variable to consider is the rapidity with which an infarction grows. The variability in IGR suggests that the timing of DHC may be individualized. Evaluation of IGRs between the first and second scan and when possible between the second and third scan can be helpful in selecting the timing of DHC. Our data was collected from multiple hospitals in three countries and reflects real-world clinical practice rather than the findings of a clinical trial.
References
Wheeler HM, Mlynash M, Inoue M, Tipirnini A, Liggins J, Bammer R, et al. The growth rate of early DWI lesions is highly variable and associated with penumbral salvage and clinical outcomes following endovascular reperfusion. Int J Stroke Off J Int Stroke Soc. 2015;10(5):723–9.
Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner HH, Jansen O, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke J Cereb Circ. 1998;29(9):1888–93.
Fandino J, Keller E, Barth A, Landolt H, Yonekawa Y, Seiler RW. Decompressive craniotomy after middle cerebral artery infarction. Retrospective analysis of patients treated in three centres in Switzerland. Swiss Med Wkly. 2004;134(29–30):423–9.
Cho D-Y, Chen T-C, Lee H-C. Ultra-early decompressive craniectomy for malignant middle cerebral artery infarction. Surg Neurol. 2003;60(3):227–32; discussion 232–3.
Uhl E, Kreth FW, Elias B, Goldammer A, Hempelmann RG, Liefner M, et al. Outcome and prognostic factors of hemicraniectomy for space occupying cerebral infarction. J Neurol Neurosurg Psychiatry. 2004;75(2):270–4.
Gupta R, Connolly ES, Mayer S, Elkind MSV. Hemicraniectomy for massive middle cerebral artery territory infarction: a systematic review. Stroke J Cereb Circ. 2004;35(2):539–43.
Kamran S, Akhtar N, Salam A, Alboudi A, Kamran K, Ahmed A, et al. Revisiting hemicraniectomy: late decompressive hemicraniectomy for malignant middle cerebral artery stroke and the role of infarct growth rate. Stroke Res Treat. 2017;2017:2507834.
McKenna A, Wilson CF, Caldwell SB, Curran D. Functional outcomes of decompressive hemicraniectomy following malignant middle cerebral artery infarctions: a systematic review. Br J Neurosurg. 2012;26(3):310–5.
Dasenbrock HH, Robertson FC, Vaitkevicius H, Aziz-Sultan MA, Guttieres D, Dunn IF, et al. Timing of decompressive hemicraniectomy for stroke: a nationwide inpatient sample analysis. Stroke. 2017;48(3):704–11.
Basu P, Jenkins H, Tsang K, Vakharia VN. National survey of neurosurgeons and stroke physicians on decompressive hemicraniectomy for malignant middle cerebral artery infarction. World Neurosurg. 2017;102:320–8.
Daou B, Kent AP, Montano M, Chalouhi N, Starke RM, Tjoumakaris S, et al. Decompressive hemicraniectomy: predictors of functional outcome in patients with ischemic stroke. J Neurosurg. 2016;124(6):1773–9.
Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17(3):205–16.
Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274(13):1017–25.
Schwamm LH, Koroshetz WJ, Sorensen AG, Wang B, Copen WA, Budzik R, et al. Time course of lesion development in patients with acute stroke: serial diffusion- and hemodynamic-weighted magnetic resonance imaging. Stroke J Cereb Circ. 1998;29(11):2268–76.
Kamran S, Akhtar N, Alboudi A, Kamran K, Ahmad A, Inshasi J, et al. Prediction of infarction volume and infarction growth rate in acute ischemic stroke. Sci Rep. 2017;7(1):7565.
Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6(3):258–68.
Kamran S, Salam A, Akhtar N, Alboudi A, Ahmad A, Khan R, et al. Predictors of in-hospital mortality after decompressive hemicraniectomy for malignant ischemic stroke. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2017;26(9):1941–7.
Kasner SE, Demchuk AM, Berrouschot J, Schmutzhard E, Harms L, Verro P, et al. Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke J Cereb Circ. 2001;32(9):2117–23.
Krieger DW, Demchuk AM, Kasner SE, Jauss M, Hantson L. Early clinical and radiological predictors of fatal brain swelling in ischemic stroke. Stroke. 1999;30(2):287–92.
Hofmeijer J, Algra A, Kappelle LJ, van der Worp HB. Predictors of life-threatening brain edema in middle cerebral artery infarction. Cerebrovasc Dis Basel Switz. 2008;25(1–2):176–84.
Thomalla G, Hartmann F, Juettler E, Singer OC, Lehnhardt F-G, Köhrmann M, et al. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: a prospective multicenter observational study. Ann Neurol. 2010;68(4):435–45.
Ong CJ, Gluckstein J, Laurido-Soto O, Yan Y, Dhar R, Lee J-M. Enhanced detection of edema in malignant anterior circulation stroke (EDEMA) score: a risk prediction tool. Stroke. 2017 Jul;48(7):1969–72.
Maramattom BV, Bahn MM, Wijdicks EFM. Which patient fares worse after early deterioration due to swelling from hemispheric stroke? Neurology. 2004;63(11):2142–5.
Qureshi AI, Suarez JI, Yahia AM, Mohammad Y, Uzun G, Suri MFK, et al. Timing of neurologic deterioration in massive middle cerebral artery infarction: a multicenter review. Crit Care Med. 2003;31(1):272–7.
Mori K, Nakao Y, Yamamoto T, Maeda M. Early external decompressive craniectomy with duroplasty improves functional recovery in patients with massive hemispheric embolic infarction: timing and indication of decompressive surgery for malignant cerebral infarction. Surg Neurol. 2004;62(5):420–9; discussion 429–30.
Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner HH, Jansen O, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke J Cereb Circ. 1998;29(9):1888–93.
Basu P, Jenkins H, Tsang K, Vakharia VN. National survey of neurosurgeons and stroke physicians on decompressive hemicraniectomy for malignant middle cerebral artery infarction. World Neurosurg. 2017;102:320–8.
von Olnhausen O, Thorén M, von Vogelsang A-C, Svensson M, Schechtmann G. Predictive factors for decompressive hemicraniectomy in malignant middle cerebral artery infarction. Acta Neurochir 2016 Feb 29;
Wijdicks EFM, Sheth KN, Carter BS, Greer DM, Kasner SE, Kimberly WT, et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(4):1222–38.
Gupta A, Sattur MG, Aoun RJN, Krishna C, Bolton PB, Chong BW, et al. Hemicraniectomy for ischemic and hemorrhagic stroke. Facts and Controversies Neurosurg Clin N Am. 2017;28(3):349–60.
Jung S, Gilgen M, Slotboom J, El-Koussy M, Zubler C, Kiefer C, et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain J Neurol. 2013;136(Pt 12):3554–60.
Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11(10):860–7.
Lansberg MG, Thijs VN, O’Brien MW, Ali JO, de Crespigny AJ, Tong DC, et al. Evolution of apparent diffusion coefficient, diffusion-weighted, and T2-weighted signal intensity of acute stroke. AJNR Am J Neuroradiol. 2001;22(4):637–44.
Cohen-Gadol AA, Bradley CC, Williamson A, Kim JH, Westerveld M, Duckrow RB, et al. Normal magnetic resonance imaging and medial temporal lobe epilepsy: the clinical syndrome of paradoxical temporal lobe epilepsy. J Neurosurg. 2005;102(5):902–9.
Nakano S, Iseda T, Kawano H, Yoneyama T, Ikeda T, Wakisaka S. Correlation of early CT signs in the deep middle cerebral artery territories with angiographically confirmed site of arterial occlusion. AJNR Am J Neuroradiol. 2001;22(4):654–9.
Kamran S, Bhutta Z, Akhtar N, Bates V, Salam A, Shuaib A. Abstract TP59: to estimate the time of stroke onset by using Ct scan and stroke severity scale. Stroke. 2017;48(Suppl 1):ATP59.
Wade DT. Evaluating outcome in stroke rehabilitation (quality control and clinical audit). Scand J Rehabil Med Suppl. 1992;26:97–104.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study adhered to the tenets of the declaration of Helsinki and was approved by the Institutional Review Board of Hamad Medical Corporation, Qatar [15246/15], Shifa International Hospital, Pakistan 421-270-2014, and Rashid Hospital, UAE DSREC 12/2015_09.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kamran, S., Salam, A., Akhtar, N. et al. Factors that Can Help Select the Timing for Decompressive Hemicraniectomy for Malignant MCA Stroke. Transl. Stroke Res. 9, 600–607 (2018). https://doi.org/10.1007/s12975-018-0616-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-018-0616-0