Abstract
Minocycline and candesartan have both shown promise as candidate therapeutics in ischemic stroke, with multiple, and somewhat contrasting, molecular mechanisms. Minocycline is an anti-inflammatory, antioxidant, and anti-apoptotic agent and a known inhibitor of matrix metalloproteinases (MMPs). Yet, minocycline exerts antiangiogenic effects both in vivo and in vitro. Candesartan promotes angiogenesis and activates MMPs. Aligning these therapies with the dynamic processes of injury and repair after ischemia is likely to improve success of treatment. In this study, we hypothesize that opposing actions of minocycline and candesartan on angiogenesis, when administered simultaneously, will reduce the benefit of candesartan treatment. Therefore, we propose a sequential combination treatment regimen to yield a better outcome and preserve the proangiogenic potential of candesartan. In vitro angiogenesis was assessed using human brain endothelial cells. In vivo, Wistar rats subjected to 90-min middle cerebral artery occlusion (MCAO) were randomized into four groups: saline, candesartan, minocycline, and sequential combination of minocycline and candesartan. Neurobehavioral tests were performed 1, 3, 7, and 14 days after stroke. Brain tissue was collected on day 14 for assessment of infarct size and vascular density. Minocycline, when added simultaneously, decreased the proangiogenic effect of candesartan treatment in vitro. Sequential treatment, however, preserved the proangiogenic potential of candesartan both in vivo and in vitro, improved neurobehavioral outcome, and reduced infarct size. Sequential combination therapy with minocycline and candesartan improves long-term recovery and maintains candesartan’s proangiogenic potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is one of the most common and undertreated conditions worldwide. Each year, more than 700,000 stroke cases occur in the USA, resulting in significant disability and mortality as well as an estimated cost of $36 billion [1]. Tissue plasminogen activator (tPA) remains the only FDA-approved pharmacologic/biologic treatment option [2]. With only 2–3 % of patients receiving tPA nationwide [3], there is an urgent need to develop new therapies for acute ischemic stroke.
Cerebral ischemia promotes an angiogenic response in the brain [4] that is considered to be a key component of recovery [5, 6]. Angiogenic genes are upregulated within minutes of the incidence of an ischemic insult. Endothelial cell proliferation starts as early as 12 hours and continues for several weeks in a mouse model of focal cerebral ischemia [7]. In humans, evidence of active angiogenesis was detected 3–4 days after stroke [8, 9]. The role played by angiogenesis in mediating recovery goes beyond creating a shunt to reperfuse ischemic tissues [10]. Newly formed vessels help clean up the ischemic site in preparation for neurovascular remodeling [11]. In addition, blood vessels upregulate neuron guidance cues to recruit neural stem cells to the site of injury [12]. Due to the tight relationship between angiogenesis and neurogenesis in addition to the dramatic failure of neuroprotectants in stroke clinical trials, therapies that aim at promoting angiogenesis are now being attempted in experimental models of focal cerebral ischemia [13–15].
Decades of preclinical research have led to improved understanding of the timing and molecular mediators of injury and repair after ischemic brain insult [16, 17]. It has been acknowledged that the mediators of initial injury (e.g., matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF)) contribute to the repair and remodeling process in the long term [17]. Sequential therapy, although extensively studied in oncology [18] and gastroenterology [19], has been understudied in stroke.
Minocycline, a tetracycline antibiotic with anti-inflammatory, anti-apoptotic, antioxidant, and MMP-inhibitory effects, has shown promising results in stroke preclinical studies. Our group, as well as others, has reported reduced infarct size and intracerebral hemorrhage, together with improved neurobehavioral outcome in response to minocycline treatment [reviewed in [20]]. Minocycline treatment, however, suppressed in vivo and in vitro angiogenesis via MMP-dependent and MMP-independent mechanisms [21–25]. Therefore, combining the benefits of minocycline with a proangiogenic approach represents an attractive therapeutic option.
Candesartan, an angiotensin II type-1 receptor blocker, has long been studied in experimental stroke settings. Candesartan treatment improved neurobehavioral outcome, reduced infarct size [26–29], and increased cerebrovascular density, an effect that was associated with enhanced MMP activity, especially MMP-2 [29]. Clinical utility is, however, complicated by candesartan’s blood pressure lowering effect. Recent studies have shown a lack of benefit, if not a deleterious effect, of blood pressure lowering in the acute phase after ischemic stroke [25, 30] and current guidelines advise against blood pressure lowering within the first 24 h [31]. In this study, we propose a combination of early minocycline treatment followed by 7-day low-dose candesartan treatment to improve functional recovery after focal cerebral ischemia. Due to opposite actions of minocycline and candesartan on angiogenesis, we employ a sequential approach designed to preserve candesartan’s proangiogenic effect.
Materials and Methods
All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Veterans Affairs Medical Center.
In Vitro Experiments
Oxygen and Glucose Deprivation and Treatments
To mimic oxygen and glucose deprivation conditions that occur during stroke, human cerebral microvascular endothelial cells (hCMECs) were incubated in a hypoxia chamber (Bioshperix Proox Model C21, Lacona, NY) at 0.1 % O2 and 5 % CO2 at 37 °C and switched to glucose-free Neurobasal-A medium (Life Technologies, Carlsbad, CA). After 2 h, cells were returned to normoxic conditions and switched to serum-free medium with or without different treatments. To study the dose-response relationship of individual and simultaneous treatments, cells were treated with either minocycline (6 μg/ml) or candesartan (0.1, 1, and 10 μg/ml) alone or in combination. To compare the effect of simultaneous and sequential treatments on in vitro angiogenesis, cells were treated with minocycline (6 μg/ml) for 12 h, followed by 24-h treatment with candesartan (10 μg/ml) for the sequential treatment group. Cells were washed twice with warm PBS between the treatments to avoid physical interaction between the two agents. The simultaneous treatment group was exposed to serum-free medium in the first 12 h, followed by 24-h treatment with both minocycline and candesartan. The tested concentration of minocycline (6 μg/ml) is the peak serum concentration achieved in humans after intravenous administration of the FDA-approved dose of 200 mg [32]. Candesartan concentrations have been shown to exert an angiogenic effect in vitro on human brain microvascular endothelial cells [33].
Angiogenic Assays
We conducted cell proliferation, migration, and tube formation assays to mimic the different steps of angiogenesis.
Cell Proliferation
In vitro, endothelial cell proliferation was assessed by BrdU colorimetric assay kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s protocol. Cells were plated in density of 5,000 cells/well in 96-well plate and kept overnight in complete medium. The cells were serum starved, exposed to 2-h oxygen and glucose deprivation (OGD). Upon reoxygenation, cells were treated with candesartan (0.1, 1, or 10 μg/ml), minocycline (6 μg/ml), or the combination of minocycline and different concentrations of candesartan. The BrdU labeling solution was then added. At 24 h, absorbance was measured colorimetrically at 450 nm.
Cell Migration
Cell migration was assessed by the in vitro wound healing assay as described previously [33]. Cells were seeded at high density in six-well plates. Confluent cells were serum starved overnight and two perpendicular scratches were made using a 1,000-μl tip (Thermofisher Scientific, Waltham, MA). Treatments or serum-free medium were added and 5× micrographs were taken at 0, 18, and 24 h using phase contrast microscopy on an inverted microscope. Width of the scratch was measured at 20 points (5 points/arm) and the average width was calculated for each well using the formula: Percent migration = [(average width at 0 h − average width at × hours) / average width at 0 h] × 100.
Tube Formation
The ability of cells to form tubes was measured by Matrigel tube formation assay (Castellon et al. 2001). Confluent cells were serum starved overnight. Cells were subjected to serum-free medium with or without treatment for 24 h. Cells were then harvested and resuspended in serum-free medium: matrigel (BD Biosciences, Franklin Lakes, NJ) in a ratio of 70:30. The mixture was quickly transferred to 96-well plate (100 μl/well) and matrigel was allowed to solidify. Approximately 3 × 104 cells in 100 μl were seeded into each quadruplicate well. At 24 h, 10× micrographs were taken from the center of each well and tube-like structures were quantified in a blinded fashion.
Lactate Dehydrogenase Release
Lactate dehydrogenase (LDH) released into the cell supernatant was used as a marker of cell death. LDH concentration was measured using the cytotoxicity detection (LDH) kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s protocol. Cells were serum starved overnight and exposed to OGD conditions for 2 h followed by 24-h reoxygenation and treatment with either minocycline alone or in combination with 10× increments of candesartan concentration. Culture medium was then collected and cell-free supernatant was used for this assay by centrifugation of the samples at 13,000 rpm at 4 °C for 10 min. A volume of 100 μl was then transferred in quadruplicates to a 96-well plate from each test group. Reaction mixture was prepared according to the manufacturer’s instructions and a volume of 100 μl was added to each well. LDH released from dead cells converts the tetrazolium INT salt into a colored formazan that was measured at 500 nm using an ELISA plate reader. Results were represented as fold-increase relative to the control group.
In Vivo Experiments
Experimental Cerebral Ischemia and Treatments
Adult (9–10 weeks old) male Wistar rats (Charles River Breeding Company, Wilmington, MA, USA), weighing between 280–300 g, were subjected to 90-min middle cerebral artery occlusion (MCAO) using intraluminal suture model as described previously [28]. Briefly, animals were anesthetized using 2–5 % isoflurane inhalation. Temporary MCAO was achieved using 4–0 silicon-coated nylon suture (Doccol, Sharon, MA), advanced into the internal carotid artery to block the origin of the middle cerebral artery. After 90 min, animals were re-anaesthetized and sutures were removed to allow reperfusion of ischemic brain areas. Animals were randomly assigned into four treatment groups by a blinded researcher: saline, minocycline only (Mino), candesartan only (Cand), and the sequential combination of minocycline and candesartan (Sequential Comb), and received treatments as shown in Table 1. The dose of minocycline (20 mg/kg) was shown to exert neuroprotective effects in vivo [34] and the dose of candesartan (0.3 mg/kg) was determined to be subhypotensive in our previous experiments [35]. The timing of intervention was controlled such that, in all treatment groups, the protective therapy was initiated at reperfusion as an intravenous (i.v.) dose to achieve rapid drug delivery. Subsequent treatments were given intraperitoneally (i.p.) to achieve longer-lasting drug levels in blood. Candesartan was a kind gift from AstraZeneca Pharmaceuticals (Wilmington, DE). Minocycline was purchased from Sigma-Aldrich (St. Louis, MO). All animals were singly housed before and after surgery, with free access to food and water.
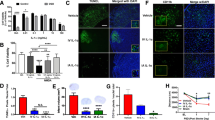
Simultaneous combination of minocycline and candesartan decreases candesartan-induced proangiogenic effect in vitro. a, e Quantification of endothelial cell proliferation (a, n = 4–6) and tube formation (e, n = 6) after 2-h OGD and 24-h reoxygenation. b Determination of LDH concentration in cell culture media collected from brain endothelial cells exposed to 2-h OGD and 24-h reoxygenation (n = 3); asterisk, significantly different from untreated control; number sign, significantly different from the corresponding candesartan concentration without minocycline co-treatment; ns, non significant. c Representative micrographs of endothelial cell migration at 18 h in individual treatments or simultaneous combination and compared to the untreated control (scale bar, 50 μm). d Quantification of endothelial cell migration after 2-h OGD and 18- or 24-h reoxygenation (n = 4–9/group; asterisk, significantly different from untreated control at the same time point; number sign, significantly different from the corresponding candesartan concentration without minocycline co-treatment at the same time point)
Sequential combination of minocycline and candesartan preserves candesartan-induced proangiogenic effect in vitro. a Representative micrographs of endothelial cell migration at 18 h in simultaneous and sequential combination treatments, compared to the untreated control (scale bar, 25 μm). b Quantification of endothelial cell migration after 2-h OGD, followed by 12-h minocycline treatment and 18-h candesartan treatment (Sequential) or 12-h serum-free medium and 18-h concomitant minocycline and candesartan treatments (Simultaneous). c Representative micrographs of endothelial cell alignment into tube-like structures in simultaneous and sequential combinations, compared to the untreated control (scale bar, 100 μm). d Quantification of endothelial cell alignment into tube-like structures after 2-h OGD, followed by 12-h minocycline treatment and 24-h candesartan treatment (Sequential) or 12-h serum-free medium and 24-h concomitant minocycline and candesartan treatments (Simultaneous) (n = 4/group, * P < 0.05)
Sequential combination of minocycline and candesartan preserves candesartan-induced proangiogenic response in vivo. Early minocycline treatment does not inhibit MMP-2 or MMP-9 activity at 14 days. a–c Quantification of MMP-2 (a), MMP-9 (b), and VEGF (c) in the ipsilateral hemispheres of brains collected 14 days after MCAO from different treatment groups (n = 3–4). d Diagrammatic illustration of the regions assessed for laminin staining. e Representative micrographs of laminin-stained vessels in the ischemic border zone 14 days after MCAO (scale bar, 50 μm). f Quantification of laminin-stained vessels in the cortex, ischemic border zone, and contralateral hemisphere 14 days after MCAO (n = 4/group; asterisk, significantly different from saline group; Greek small letter psi, significantly different from minocycline only group, P < 0.05;ns, non significant)
Combining minocycline and candesartan sequentially decreases infarct volume more than each agent alone. a Representative images of cresyl violet-stained coronal sections 14 days after MCAO, collected from animals treated with saline, minocycline, candesartan, or a sequential combination of minocycline and candesartan. b Quantification of infarct volume 14 days after MCAO (n = 4/group; asterisk, significantly different from saline group; Greek small letter psi, significantly different from minocycline group, P < 0.05)
Sequential combination of minocycline and candesartan improves neurobehavioral outcome more than each agent alone. a–d Assessment of neurobehavioral and motor function 1, 3, 7, and 14 days after MCAO using modified Bederson score (a), beam walk (b), rotarod performance (c), and grip strength tests (d) in animals treated with saline, minocycline, candesartan, or a sequential combination of minocycline and candesartan (n = 6–10/group; asterisk, significantly different from saline group; Greek small letter psi, significantly different from minocycline only group; Greek small letter final sigma, significantly different from candesartan only group, P < 0.05)
Neurobehavioral Testing
Neurobehavioral assessment was done 1, 3, 7, and 14 days after the onset of MCAO in a blinded fashion using modified Bederson score, beam walk, rotarod performance, and grip strength tests.
Modified Bederson Score
Animals were assigned a score from 0 to 3, with lower scores indicating better performance. The animal was given one point for each of the following: forelimb flexion; diminished resistance to lateral push; and contralateral circling [36].
Beam Walk
Animals were placed on a beam (60 cm long and 4.5 cm wide) for 60 s and assigned a score from 0 to 6 as follows: balances on the beam with a steady posture = 0; grasps side of the beam = 1; hugs the beam with one limb falling = 2; hugs the beam with two limbs falling = 3; falls off the beam within 40 to 60 s = 4; falls off the beam within 20 to 40 s = 5; falls off the beam in less than 20 s = 6 [37].
Rotarod Test
Three days prior to MCAO, animals were trained to stay on the accelerating rod as follows: on day 1, animals were habituated to stay on the rod rotating at a fixed speed of 2 rpm for 5 min. On day 2, animals perform three 5-min running sessions and allowed to rest for 10 min between sessions. Animals run at a basal speed of 2 rpm that was accelerated by 1 rpm every 10 s to a final speed of 15 rpm. On day 3, animals follow the same training schedule as day 2 but reaching a final speed of 30 rpm. Baseline performance was recorded before MCAO surgery. Animals were then tested 1, 3, 7, and 14 days after MCAO. Maximum speed reached before the animal falls off the rod was recorded for the three sessions and averaged. Rotarod performance was calculated as percentage of baseline performance [38].
Grip Strength
Grip Strength Meter (Columbus instruments, Columbus, OH) was used to measure baseline and post-stroke neuromuscular function. Animals were suspended by the tail and allowed to hold to a sensor bar. The animals were then pulled by the tail away from the sensor bar till they release their grip. An electronic digital force gauge was used to measure the peak pulling force exerted by the animal on the sensor bar. Three readings were recorded for each animal and averaged. Results were expressed as percentage of baseline grip strength.
Assessment of Infarct Size and Immunohistochemistry
On day 14 post-stroke, animals were deeply anaesthetized with ketamine/xylazine mixture (85 and 15 %, respectively) and transcardially perfused with ice cold PBS followed by 10 % formalin (Thermofisher Scientific, Waltham, CA). Animals were then decapitated and their brain tissue collected and stored in 10 % formalin overnight. The brains were then embedded in OCT blocks and cryosectioned coronally into 12-μm-thick slices starting from the frontal pole at 2 mm intervals. Sections were stained with 1 % cresyl violet. Infarction areas, defined as areas showing reduced staining under light microscopy, were measured blindly using Image J software, corrected for edema, and expressed as percentage of the contralateral hemisphere using the formula: 100 × [contralateral − (ipsilateral − infarct)] / contralateral.
Frozen 10 μm sections were immunostained for laminin (Dakocytomation, Carpinteria, CA). Briefly, brain sections were incubated with primary antibody overnight at 4 °C at the dilution of 1/50, followed by 2-h incubation with 1/300 fluorescent secondary antibody. Slides were then coverslipped with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and viewed using Zeiss Axio Observer Z1 fluorescent microscope. Micrographs were taken at magnification ×20 from four different fields in the ischemic cortex, ischemic border zone, and contralateral hemisphere. Micrographs were quantified in a blinded fashion using ImageJ software (NIH).
MMP Zymography
MMP gelatinolytic activity was measured using zymography, as described previously [39]. Briefly, brains were collected at day 14 post stroke and sliced coronally into seven sections (A–G); a volume containing 100 μg total protein (from slices D and E) was loaded into 10 % zymogram gel and electrophoretically separated under non-reducing conditions. The gel was then incubated for 48 h with Zymogram Development Buffer at 37 °C. Zymogram gel was stained with Coomassie Blue R-250 (Bio-Rad, Hercules, CA) and destained with Destain Solution (Bio-Rad, Hercules, CA). The gelatinolytic bands were analyzed with ImageJ software (NIH).
Human Cerebral Microvascular Endothelial Cell Line
Cells were provided by Dr. Jason Zastre (University of Georgia, Athens, GA). The cells were grown in MCDB-131 complete medium (VEC technologies, Rensselaer, NY). Before treatments, endothelial cells were serum-starved overnight in serum-free EMEM (ATCC, Manassas, VA). This clinically relevant cell line was chosen for our experiments since we have previously shown and established the proangiogenic response of candesartan using these cells [33, 40]. Therefore, we studied whether minocycline will abolish this proangiogenic effect upon simultaneous versus sequential treatment with candesartan using the same cell type.
Western Blotting
Brain tissue was collected from a separate set of animals 14 days after the onset of MCAO. From each hemisphere, slices B–E were collected and snap frozen. Brain tissue was then homogenized and protein expression was measured as described previously [28]. Non-specific binding was blocked by incubating the membranes in 5 % milk in TBST for 60 min prior to overnight incubation with primary antibody against VEGF (Millipore, Billerica, MA). β-Actin (Sigma-Aldrich, St. Louis, MO) was used as an endogenous loading control. Densitometric measurements were done using ImageJ software (NIH).
Statistical Analysis
All statistics were carried out using NCSS2007 software. Results are expressed as mean ± standard error of the mean (±SEM). Data were statistically analyzed using one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparison post-hoc test. Tests of interaction were done using two-way ANOVA. A value of P < 0.05 was considered statistically significant.
Results
Simultaneous but Not Sequential Minocycline and Candesartan Treatment Abolishes Candesartan-Induced Proangiogenic State In Vitro
We have previously demonstrated enhanced angiogenic steps in human brain endothelial cells in response to candesartan treatment in vitro [33]. Here, we sought to optimize the combination timing to preserve candesartan-induced proangiogenic state. To assess the functional outcome of simultaneous treatment, we quantified different angiogenic steps in vitro in response to different candesartan concentrations with or without simultaneous minocycline treatment. Endothelial cell proliferation was reduced by 36 % in response to minocycline treatment (Fig. 1a). Candesartan treatment (0.1, 1, and 10 μg/ml) enhanced cell proliferation by 37, 59, and 47 %, respectively. Simultaneous minocycline treatment resulted in a decreased proliferative potential, as compared to their respective candesartan only treatment groups. To assess whether the reduced BrdU incorporation observed with minocycline treatment was due to an anti-proliferative or a direct cytotoxic effect of the tested minocycline concentration, we measured LDH release in the cell culture media. LDH concentration did not increase with minocycline treatment either alone or in combination with different concentrations of candesartan (Fig. 1b). Endothelial cell migration followed the same pattern as cell proliferation. Minocycline treatment reduced migration by 27 and 41 % at 18 and 24 h, respectively, as compared to the control group (Fig. 1c, d). Candesartan treatment, on the other hand, exerted a pro-migratory effect. At 18 h, candesartan, at different concentrations, enhanced endothelial cell migration by about 40 %. At 24 h, candesartan treatment displayed a dose-dependent pro-migratory response, resulting in 41, 59, and 74 % increase in endothelial cell migration in response to treatment with 0.1, 1, and 10 μg/ml of candesartan, respectively. Simultaneous treatment with minocycline significantly reduced candesartan-induced pro-migratory effects as compared to their respective candesartan only treatment groups. Although there was a trend of decreasing endothelial cell alignment into tube-like structures with minocycline treatment, this effect did not reach significance at the tested sample size (Fig. 1e). Candesartan treatment induced a profound dose-dependent increase in the number of tube-like structures at 24 h. Simultaneous minocycline treatment, however, abolished endothelial cell response to candesartan treatment. Further, we tested whether sequential treatment preserves candesartan-induced proangiogenic effect. As shown in Fig. 2a, endothelial cell migration was only slightly increased by simultaneous treatment. Sequential treatment, however, enhanced cell migration by more than twofold. Similarly, endothelial cell alignment into tube-like structures 24 h after simultaneous treatment was comparable in the untreated control group (Fig. 2b). Sequential treatment enhanced endothelial cell alignment into tubular structures by almost fivefold.
Sequential Treatment Preserves Candesartan-Induced VEGF Upregulation and Induction of a Prolonged Proangiogenic State In Vivo
We have previously shown an enhanced VEGF expression and a proangiogenic response in vivo after candesartan treatment [28, 29, 33]. The proangiogenic response was accompanied by MMP activation, especially MMP-2 (Kozak et al. 2009). In this study, we tested whether the proangiogenic effect of candesartan was maintained in a low-dose treatment regimen and whether early minocycline treatment affected such a response. There was ∼30 % increase in MMP-2 activity in both the candesartan only and the sequential treatment groups that did not reach significance (Fig. 3a). Minocycline treatment alone or in combination with candesartan did not decrease MMP-2 or MMP-9 at 14 days (Fig. 3a, b). VEGF expression in the sequential combination group measured at 14 days was comparable to that of the candesartan only group (Fig. 3c). Both treatment regimens resulted in significantly higher VEGF levels as compared to the saline treatment. Further, we tested whether VEGF upregulation led to increased vascular density. Laminin staining showed ∼2-, 3-, and 2.3-fold increase in the cortex, ischemic border zone, and contralateral hemisphere, respectively, with candesartan treatment as compared to saline-treated group (Fig. 3d–f). Sequential combination treatment enhanced vascular density by 1.8-, 3.8-, and 2.8-fold relative to saline treatment in the respective brain regions. Of note, a single dose of minocycline enhanced vascular density at the cortex region by ∼90 %.
Sequential Minocycline and Candesartan Treatment Decreases Infarct Size and Improves Neurobehavioral Recovery 14 Days After an Ischemic Insult
To assess the neuroprotective effect of individual versus sequential minocycline and candesartan treatment, we measured infarct size 14 days after stroke. Infarct size was significantly reduced by 35 and 64 % in the minocycline only and the candesartan only treatment groups, respectively, as compared to the saline treatment (Fig. 4a, b). There was a trend of increased neuroprotective effect with the sequential combination treatment, as evident by a 78 % reduction in lesion size. Further, we tested whether the reduction in infarct size was accompanied by improved functional recovery. In Bederson test, there was a trend towards enhanced recovery with individual treatments that became significant only in the candesartan group at 14 days. However, sequential treatment resulted in significant improvement starting at day 7, as compared to the saline-treated group (Fig. 5a). Assessment of motor function and balance by the beam walk test revealed a trend of improvement as early as day 1 in the minocycline only group that continued slowly thereafter. Candesartan only treatment resulted in continuous and significant improvement starting day 3, as compared to saline treatment. Improvement with the sequential combination treatment followed the same pattern as the candesartan only treatment during the first week but became significantly superior to minocycline treatment at day 14 (Fig. 5b). We observed an early and profound enhancement of Grip strength in all treatment groups, as compared to the saline group (Fig. 5c). However, this effect plateaued at days 3 and 7 in the minocycline only and candesartan only treatment groups, respectively. At 14 days, grip strength in the sequential combination group was significantly enhanced as compared to saline as well as individual treatment groups. Similar to grip strength test, rotarod performance showed an early and significant improvement starting day 1 in all treatment groups, as compared to saline treatment (Fig. 5d). Nevertheless, improvement plateaued early in the individual treatment groups. Only the sequential combination treatment was significantly improved as compared to saline treatment on days 7 and 14 after stroke.
Discussion
The objective of this study is to test the combination of minocycline and candesartan on stroke outcome and on inherent reparative mechanisms after stroke. We sought to optimize treatment timing in order to harness the benefits and decrease the likelihood of interaction between the two agents. Our in vitro findings include the abrogation of candesartan-induced proangiogenic state by concomitant minocycline administration. Spacing of minocycline and candesartan treatments preserved the angiogenic potential of candesartan treatment both in vitro and in vivo. This was accompanied by reduced infarct size and enhanced neurobehavioral recovery 14 days after experimental focal cerebral ischemia.
Minocycline displays MMP inhibitory characteristics as well as antioxidant, anti-apoptotic, and anti-inflammatory effects, among other non-antibiotic properties [reviewed by [41]]. Minocycline treatment reduced infarct volume in suture and embolic MCAO models [42, 43] and was effective at reducing brain edema and neurological deficit in an experimental intracerebral hemorrhage model [44]. Furthermore, minocycline has shown promising neuroprotective effects in several non-stroke brain injury models, including Huntington’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and spinal cord injury [41]. Due to its well-documented non-antibiotic properties, supported by evidence of neuroprotection in different brain injury models, we attempted early minocycline treatment after focal cerebral ischemia.
Chronic angiotensin II type 1 (AT1) receptor blockade has been shown to increase cerebrovascular density in the presence or absence of an ischemic insult [45, 46]. Furthermore, in an animal model of focal cerebral ischemia, administration of a single candesartan dose at reperfusion induced a prolonged VEGF upregulation [33] and increased vascular density at 7 days [29]. In human brain endothelial cells, candesartan treatment induced a proangiogenic response mediated by VEGF, among other factors [33, 40]. Of note, we have previously reported enhanced MMP activity, especially MMP-2, 24 h after treatment with 1 mg/kg of candesartan, which could explain VEGF upregulation and the proangiogenic state since MMPs and VEGF are interrelated [47–50]. The benefits of candesartan treatment, however, could be masked by its blood pressure lowering effect. Current guidelines advise against blood pressure reduction in the first 24 h after stroke [31]. We, therefore, attempted a combination of minocycline at reperfusion followed by low-dose candesartan treatment starting 24 h after MCAO in our in vivo studies.
While several studies demonstrated the detrimental consequences of MMP activation early after stroke [51], caution against late MMP inhibition emerged when the latter approach was accompanied with reduced markers of neurovascular remodeling [52]. The dual “destructive-protective” nature of MMPs, depending on the timing, calls for fine-tuning of the enzymatic activity in order to improve long-term neurovascular recovery after stroke. In our treatment regimen, early minocycline administration at reperfusion, either alone or in the combination treatment group, preserved MMP activity 14 days after MCAO, allowing for neurovascular recovery. Minocycline treatment preserved vascular structure in the ischemic hemisphere, as evident by enhanced laminin staining. Vascular density was ∼90 and 40 % higher in the ischemic cortex and the ischemic border zone, respectively, in minocycline-treated animals as compared to their saline counterparts. However, this effect was not accompanied by enhanced vascular density in the contralateral hemisphere or increased cell proliferation, suggesting a vascular protective rather than a neovascularization effect of minocycline treatment. Minocycline actions as an MMP inhibitor, anti-inflammatory, anti-apoptotic, and antioxidant drug could all explain the vascular protective effect observed 14 days after a single minocycline treatment. Nonetheless, a recent report demonstrated increased neovessel formation and expression of tight junction proteins in the ischemic border zone 4 weeks after MCAO in response to a single minocycline treatment. Neovascularization was attributed to reduced tissue loss with the treatment. This is not in discord with our results, since tissue and vascular preservation at 14 days, demonstrated in our current study, could possibly lead to enhanced angiogenesis at later time points [53].
We have previously shown that candesartan (1 mg/kg) treatment enhances MMP-2 activity 24 h after the induction of focal cerebral ischemia. In this study, the 7-day, low-dose candesartan treatment induced a modest increase in MMP-2 activity that did not reach significance at 14 days. Candesartan alone or in sequential combination with minocycline induced a prolonged increase in VEGF expression that was accompanied by enhanced vascular density in both hemispheres. Interestingly, early minocycline treatment did not affect MMP activity or VEGF levels at 14 days, further supporting our treatment regimen. Therefore, early minocycline treatment at reperfusion preserved the proangiogenic response to the subsequent candesartan administration. Our data points to the importance of fine-tuning MMP activity by optimizing treatment timing in order to harness the benefits of both agents. Due to the multiple pharmacological effects induced by both agents as well as the complexity of the stroke pathophysiology, we do not exclude the involvement of other mechanisms and pathways leading to enhanced neurobehavioral recovery after stroke. This effect could be attributed to the interplay of several non-antibiotic properties of minocycline as well as AT1-dependent and AT1-independent effects of candesartan treatment.
Although there is a consistent trend of further enhancement in neurobehavioral recovery with the combination treatment at 14 days compared to individual treatments, some of the tests did not reach significance. This could be attributed to the limitation of the test, the test settings, or the selected time point. Bederson and beam walk tests use a subjective 0–3 and 0–6 scale, respectively, to assess functional recovery. The challenge of 30 rpm in the rotarod performance test did not allow for further improvement to show. Infarct size was measured 14 days after stroke. At this time point, inherent reparative mechanisms in the brain, especially when accelerated with individual treatments, could mitigate the significance of the combination treatment. Nevertheless, replacing candesartan in the first 24 h by minocycline treatment enhances the translational potential of this study by following the current guidelines that recommend against early blood pressure lowering.
With the continuous failure of stroke therapeutics in the clinical setting, there is an urgent need to develop new strategies that augment the brain’s reparative capacity. This study suggests a novel, sequential therapy with two safe and well-tolerated FDA-approved drugs as a possible tactic to enhance recovery after cerebral ischemia.
Abbreviations
- ARBs:
-
Angiotensin II type-1 receptor blockers
- hCMECs:
-
Human cerebral microvascular endothelial cells
- MCAO:
-
Middle cerebral artery occlusion
- MMPs:
-
Matrix metalloproteinases
- VEGF:
-
Vascular endothelial growth factor
References
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–292. doi:10.1161/01.cir.0000441139.02102.80.
Ramaiah SS, Yan B. Low-dose tissue plasminogen activator and standard-dose tissue plasminogen activator in acute ischemic stroke in Asian populations: a review. Cerebrovasc Dis. 2013;36(3):161–6. doi:10.1159/000354162.
Ishrat T, Soliman S, Guan W, Saler M, Fagan SC. Vascular protection to increase the safety of tissue plasminogen activator for stroke. Curr Pharm Des. 2012;18(25):3677–84.
Navaratna D, Guo S, Arai K, Lo EH. Mechanisms and targets for angiogenic therapy after stroke. Cell Adhes Migr. 2009;3(2):216–23.
Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke J Cereb Circ. 2007;38(2 Suppl):827–31. doi:10.1161/01.STR.0000250235.80253.e9.
Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11(3):298–308.
Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23(2):166–80.
Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Some remarks on the growth-rate and angiogenesis of microvessels in ischemic stroke. Morphometric and immunocytochemical studies. Patol Pol. 1993;44(4):203–9.
Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke J Cereb Circ. 1994;25(9):1794–8.
Brumm AJ, Carmichael ST. Not just a rush of blood to the head. Nat Med. 2012;18(11):1609–10. doi:10.1038/nm.2990.
Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, Schultz RL, Lyden PD. Angiogenesis after stroke is correlated with increased numbers of macrophages: the clean-up hypothesis. J Cereb Blood Flow Metab. 2001;21(10):1223–31. doi:10.1097/00004647-200110000-00011.
Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26(50):13007–16. doi:10.1523/JNEUROSCI.4323-06.2006.
Simao F, Pagnussat AS, Seo JH, Navaratna D, Leung W, Lok J, et al. Pro-angiogenic effects of resveratrol in brain endothelial cells: nitric oxide-mediated regulation of vascular endothelial growth factor and metalloproteinases. J Cereb Blood Flow Metab. 2012;32(5):884–95. doi:10.1038/jcbfm.2012.2.
Guo S, Arai K, Stins MF, Chuang DM, Lo EH. Lithium upregulates vascular endothelial growth factor in brain endothelial cells and astrocytes. Stroke J Cereb Circ. 2009;40(2):652–5. doi:10.1161/STROKEAHA.108.524504.
Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke J Cereb Circ. 2004;35(7):1732–7. doi:10.1161/01.STR.0000132196.49028.a4.
Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke J Cereb Circ. 2004;35(9):2220–5. doi:10.1161/01.STR.0000138023.60272.9e.
Hermann DM, Zechariah A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J Cereb Blood Flow Metab. 2009;29(10):1620–43. doi:10.1038/jcbfm.2009.100.
Westhoff MA, Faham N, Marx D, Nonnenmacher L, Jennewein C, Enzenmuller S, et al. Sequential dosing in chemosensitization: targeting the PI3K/Akt/mTOR pathway in neuroblastoma. PLoS One. 2013;8(12):e83128. doi:10.1371/journal.pone.0083128.
De Francesco V, Hassan C, Ridola L, Giorgio F, Ierardi E, Zullo A. Sequential, concomitant and hybrid first-line therapies for H. pylori eradication: a prospective, randomized study. J Med Microbiol. 2014. doi:10.1099/jmm.0.072322-0.
Liao TV, Forehand CC, Hess DC, Fagan SC. Minocycline repurposing in critical illness: focus on stroke. Curr Top Med Chem. 2013;13(18):2283–90.
Tamargo RJ, Bok RA, Brem H. Angiogenesis inhibition by minocycline. Cancer Res. 1991;51(2):672–5.
Gilbertson-Beadling S, Powers EA, Stamp-Cole M, Scott PS, Wallace TL, Copeland J, et al. The tetracycline analogs minocycline and doxycycline inhibit angiogenesis in vitro by a non-metalloproteinase-dependent mechanism. Cancer Chemother Pharmacol. 1995;36(5):418–24.
Yao JS, Chen Y, Zhai W, Xu K, Young WL, Yang GY. Minocycline exerts multiple inhibitory effects on vascular endothelial growth factor-induced smooth muscle cell migration: the role of ERK1/2, PI3K, and matrix metalloproteinases. Circ Res. 2004;95(4):364–71. doi:10.1161/01.RES.0000138581.04174.2f.
Jung HJ, Seo I, Jha BK, Suh SI, Suh MH, Baek WK. Minocycline inhibits angiogenesis in vitro through the translational suppression of HIF-1alpha. Arch Biochem Biophys. 2014;545C:74–82. doi:10.1016/j.abb.2013.12.023.
He J, Zhang Y, Xu T, Zhao Q, Wang D, Chen CS, et al. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA J Am Med Assoc. 2014;311(5):479–89. doi:10.1001/jama.2013.282543.
Engelhorn T, Goerike S, Doerfler A, Okorn C, Forsting M, Heusch G, et al. The angiotensin II type 1-receptor blocker candesartan increases cerebral blood flow, reduces infarct size, and improves neurologic outcome after transient cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24(4):467–74. doi:10.1097/00004647-200404000-00012.
Brdon J, Kaiser S, Hagemann F, Zhao Y, Culman J, Gohlke P. Comparison between early and delayed systemic treatment with candesartan of rats after ischaemic stroke. J Hypertens. 2007;25(1):187–96. doi:10.1097/01.hjh.0000254376.80864.d3.
Guan W, Somanath PR, Kozak A, Goc A, El-Remessy AB, Ergul A, et al. Vascular protection by angiotensin receptor antagonism involves differential VEGF expression in both hemispheres after experimental stroke. PLoS One. 2011;6(9):e24551. doi:10.1371/journal.pone.0024551.
Kozak A, Ergul A, El-Remessy AB, Johnson MH, Machado LS, Elewa HF, et al. Candesartan augments ischemia-induced proangiogenic state and results in sustained improvement after stroke. Stroke J Cereb Circ. 2009;40(5):1870–6. doi:10.1161/STROKEAHA.108.537225.
Sandset EC, Bath PM, Boysen G, Jatuzis D, Korv J, Luders S, et al. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011;377(9767):741–50. doi:10.1016/S0140-6736(11)60104-9.
Jauch EC, Saver JL, Adams Jr HP, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke J Cereb Circ. 2013;44(3):870–947. doi:10.1161/STR.0b013e318284056a.
Saivin S, Houin G. Clinical pharmacokinetics of doxycycline and minocycline. Clin Pharmacokinet. 1988;15(6):355–66. doi:10.2165/00003088-198815060-00001.
Soliman S, El-Remessy A, Ishrat T, Pillai A, Somanath P, Ergul A, et al. Candesartan induces a prolonged proangiogenic effect and augments endothelium-mediated neuroprotection after oxygen and glucose deprivation: role of VEGF-A and B. J Pharmacol Exp Ther. 2014. doi:10.1124/jpet.113.212613.
Matsukawa N, Yasuhara T, Hara K, Xu L, Maki M, Yu G, et al. Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci. 2009;10:126. doi:10.1186/1471-2202-10-126.
Ishrat T, Pillai B, Soliman S, Fouda AY, Kozak A, Johnson MH, et al. Low-dose candesartan enhances molecular mediators of neuroplasticity and subsequent functional recovery after ischemic stroke in rats. Mol Neurobiol. 2014. doi:10.1007/s12035-014-8830-6.
Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke J Cereb Circ. 1986;17(3):472–6.
Watanabe T, Okuda Y, Nonoguchi N, Zhao MZ, Kajimoto Y, Furutama D, et al. Postischemic intraventricular administration of FGF-2 expressing adenoviral vectors improves neurologic outcome and reduces infarct volume after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24(11):1205–13. doi:10.1097/01.WCB.0000136525.75839.41.
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke J Cereb Circ. 2001;32(4):1005–11.
Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006;7:56. doi:10.1186/1471-2202-7-56.
Alhusban A, Kozak A, Ergul A, Fagan SC. AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator. J Pharmacol Exp Ther. 2013;344(2):348–59. doi:10.1124/jpet.112.197483.
Garrido-Mesa N, Zarzuelo A, Galvez J. What is behind the non-antibiotic properties of minocycline? Pharmacol Res. 2013;67(1):18–30. doi:10.1016/j.phrs.2012.10.006.
Xu L, Fagan SC, Waller JL, Edwards D, Borlongan CV, Zheng J, et al. Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rats. BMC Neurol. 2004;4:7. doi:10.1186/1471-2377-4-7.
Wang CX, Yang T, Shuaib A. Effects of minocycline alone and in combination with mild hypothermia in embolic stroke. Brain Res. 2003;963(1–2):327–9.
Wu J, Yang S, Hua Y, Liu W, Keep RF, Xi G. Minocycline attenuates brain edema, brain atrophy and neurological deficits after intracerebral hemorrhage. Acta Neurochir Suppl. 2010;106:147–50. doi:10.1007/978-3-211-98811-4_26.
Forder JP, Munzenmaier DH, Greene AS. Angiogenic protection from focal ischemia with angiotensin II type 1 receptor blockade in the rat. Am J Physiol Heart Circ Physiol. 2005;288(4):H1989–96. doi:10.1152/ajpheart.00839.2004.
Munzenmaier DH, Greene AS. Chronic angiotensin II AT1 receptor blockade increases cerebral cortical microvessel density. Am J Physiol Heart Circ Physiol. 2006;290(2):H512–6. doi:10.1152/ajpheart.01136.2004.
Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169(4):681–91. doi:10.1083/jcb.200409115.
Ebrahem Q, Chaurasia SS, Vasanji A, Qi JH, Klenotic PA, Cutler A, et al. Cross-talk between vascular endothelial growth factor and matrix metalloproteinases in the induction of neovascularization in vivo. Am J Pathol. 2010;176(1):496–503. doi:10.2353/ajpath.2010.080642.
Hollborn M, Stathopoulos C, Steffen A, Wiedemann P, Kohen L, Bringmann A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Invest Ophthalmol Vis Sci. 2007;48(9):4360–7. doi:10.1167/iovs.06-1234.
Chetty C, Lakka SS, Bhoopathi P, Rao JS. MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer J Int Cancer. 2010;127(5):1081–95. doi:10.1002/ijc.25134.
Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood–brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis. 2010;38(3):376–85. doi:10.1016/j.nbd.2010.03.008.
Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12(4):441–5. doi:10.1038/nm1387.
Yang YR, Salayandia VM, Estrata EY, Thompson JF, Rosenberg GA, Yang Y. Abstract W MP42: early treatment with minocycline promotes neurovascular remodeling of tight junctions facilitating recovery after stroke in rat brain. Stroke J Cereb Circ. 2014;45 Suppl 1:AWMP42.
Compliance with Ethical Standards
Funding Source
This study was funded by the National Institute of Health (R01-NS063965) and Veterans Affairs Merit Award (BX000891) to SCF and the American Heart Association-Southeast Affiliate (12PRE12030197) to SS.
Conflict of Interest
Authors declare no conflict of interest.
Ethical Approval
All applicable institutional guidelines for the care and use of animals were followed. All experimental protocols were approved by the Care of Experimental Animal Committee of Georgia Regents University/Institutional Animal Care and Use Committee (IACUC) of the Veterans Affairs Medical Center. This article does not contain any studies with human participants performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soliman, S., Ishrat, T., Fouda, A.Y. et al. Sequential Therapy with Minocycline and Candesartan Improves Long-Term Recovery After Experimental Stroke. Transl. Stroke Res. 6, 309–322 (2015). https://doi.org/10.1007/s12975-015-0408-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-015-0408-8