Abstract
Two randomized control trials demonstrated that transcatheter aortic valve implantation was associated with 1–2 year clinical outcomes comparable or even superior to surgical aortic valve replacement (SAVR) in low surgical risk patients with severe aortic stenosis (AS). However, no previous study has reported the clinical outcomes after SAVR in Japanese patients with low surgical risk. From 3815 consecutive patients enrolled in the CURRENT AS registry, we retrieved 220 patients who underwent SAVR in reference to the inclusion and exclusion criteria of the PARTNER 3 trial. Age and surgical risk score in the current study population were comparable to those in the PARTNER 3 trial (Age: 75 years versus 74 years, and STS-PROM score: 2.3 versus 1.9). The cumulative incidence of a composite all-cause death or stroke was comparable between the current study population and the SAVR patients in the PARTNER 3 trial both at 30-day (2.3% versus 3.3%), and at 1-year (4.1% versus 4.9%). The clinical outcomes of SAVR in low surgical risk patients with severe AS selected from a real world Japanese registry according to the inclusion and exclusion criteria of the PARTNER 3 trial was favorable and numerically comparable to those of SAVR patients in the PARTNER 3 trial.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last decade, treatment of aortic stenosis (AS) has dramatically changed [1]. The only option to improve prognosis of patients with severe aortic stenosis had been surgical aortic valve replacement (SAVR) [2,3,4,5,6,7]. However, transcatheter aortic valve implantation (TAVI) has been introduced, and had demonstrated clinical outcomes superior to conservative management in inoperable patients, and comparable to SAVR in intermediate to high surgical risk patients [8,9,10,11,12,13,14,15,16]. More recently, two randomized control trials demonstrated that TAVI was associated with 1–2 year clinical outcomes comparable or even superior to SAVR in low surgical risk patients [17, 18]. Importantly, TAVI, which is of course less invasive to patients, was actually associated with consistently decreased operative complications such as death or stroke, major bleeding, and new-onset atrial fibrillation with shorter hospital stay. Furthermore, 6-min walking distance significantly more increased after TAVI than after SAVR at 30-day [17]. Given these favorable results, the indications of TAVI in patients with severe AS would be expanded to low surgical risk patients with severe AS. However, we might be cautious about extrapolating these trial results to Japanese patients, because only a few Japanese patients were enrolled in these trials comparing TAVI with SAVR in low surgical risk patients [17, 18]. Furthermore, no previous study has reported the clinical outcomes after SAVR in Japanese patients with low surgical risk. We should know the clinical outcomes of SAVR in low surgical risk Japanese patients relative to those in US/European patients, to extrapolate the low surgical risk trial results to Japanese patients. With these backgrounds, the aim of this study was to evaluate the clinical outcomes of SAVR in low surgical risk patients with severe AS in Japan using data from a large Japanese multicenter registry.
Methods
Study population
The CURRENT AS registry was a multicenter, retrospective registry that enrolled consecutive patients with severe AS irrespective of the treatment modalities from 27 centers (on-site surgical facilities: 20 centers) in Japan from January 2003 to December 2011 (Supplementary Appendix A and B). Severe AS was defined as peak aortic jet velocity (Vmax) > 4.0 m/s, mean aortic pressure gradient (PG) > 40 mmHg, or aortic valve area (AVA) < 1.0 cm2. The detailed design and results of the registry have been previously published [19]. The relevant institutional review boards at all participating hospitals approved the study protocols, and we performed the study in accordance with the Declaration of Helsinki. Written informed consent was waived in the CURRENT AS registry, because of the retrospective study design.
In the present analysis, we sought to evaluate the clinical outcomes of SAVR patients in the CURRENT AS registry who fulfilled the inclusion and exclusion criteria in reference to the Placement of Aortic Transcatheter Valves (PARTNER) 3 trial (Fig. 1) [17]. Among 3815 patients enrolled in the CURRENT AS registry, initial aortic valve replacement (AVR) strategy was chosen in 1197 patients. After excluding 34 patients who did not undergo SAVR, 1163 patients actually underwent SAVR. We identified 448 patients who met all the following 3 inclusion criteria: (1) AVA ≤ 1.0 cm2 or AVA index ≤ 0.6 cm2/m2, and Jet velocity ≥ 4.0 m/s or mean gradient ≥ 40 mmHg; (2) symptomatic patients or asymptomatic patients with left ventricular ejection fraction (LVEF) < 50%; (3) Society of Thoracic Surgeons (STS)-predicted risk of mortality (PROM) score < 4 (Supplementary Appendix C). Furthermore, we excluded 228 patients in reference to the exclusion criteria of the PARTNER 3 trial [17]. Details of the exclusion criteria in this study were described in Supplementary Appendix C. Finally, the current study population consisted of 220 low surgical risk Japanese patients who underwent SAVR from January 2003 to December 2011 (Fig. 1).
Study flowchart. CURRENT AS, Contemporary outcomes after surgery and medical treatment in patients with severe aortic stenosis; SAVR surgical aortic valve replacement; TAVI transcatheter aortic valve implantation; STS score Society of Thoracic Surgeons (STS)-predicted risk of mortality (PROM) score; AVA aortic valve area; LVEF left ventricular ejection fraction; PARTNER3 trial Placement of AoRTic TraNscathetER Valves trial; IE infectious endocarditis; WBC white blood cell; Hb hemoglobin; CPAP continuous positive airway pressure; Bilevel PAP bilevel positive airway pressure; IABP intraaortic balloon pumping; PCPS percutaneous cardiopulmonary support; eGFR estimated glomerular filtration rate; FEV1.0 forced expiratory volume in 1 s as percent of forced vital capacity; TRPG tricuspid regurgitation pressure gradient; BMI body mass index
The follow-up was commenced on the day of SAVR in the current analysis. Follow-up was censored at 5-year. We obtained clinical follow-up data from the medical records and/or through mail exchanges and/or telephone interviews with the patients, families, or referring physicians.
Study outcomes
The primary outcome measure in the current analysis was a composite of all-cause death or stroke at 30-day, 1-year and 5-year. The secondary outcome measures included all-cause death, cardiovascular death, aortic valve-related death, aortic valve procedure death, stroke, heart failure hospitalization, myocardial infarction, and infectious endocarditis at 30-day, 1-year and 5-year. Procedural complications included stroke, re-thoracotomy, mediastinitis, acute kidney injury (AKI), new-onset atrial fibrillation, new-onset complete left bundle branch block, new-onset advanced/complete atrioventricular block, and pacemaker implantation. AKI and cause of death were defined based on the Valve Academic Research Consortium (VARC)-2 classification [20]. Stroke was defined as ischemic or hemorrhagic stroke either requiring or prolonging hospitalization with symptoms lasting > 24 h. Definitions of other clinical events are described in the Supplementary Appendix D. Clinical events were adjudicated by the clinical event committee in the CURRENT AS registry (Supplementary Appendix A).
Statistical analysis
We expressed continuous variables as mean ± standard deviation (SD) or median with interquartile range (IQR), and categorical variables as numbers and percentages. We used Kaplan–Meier method to estimate the cumulative incidences and their 95% confidence interval. We also performed subgroup analyses in terms of SAVR with and without coronary artery bypass grafting (CABG) or other concomitant surgical procedures.
All analyses were performed using JMP 14.0.0 (SAS Institute, Cary, NC, USA).
Results
Characteristics and procedural outcomes of SAVR
Mean age of the study population was 75 years, and 56.8% of patients were women. The mean STS-PROM score was 2.3% (Table 1). Regarding the distributions of STS score and age, 45.5% of patients had STS scores ≥ 2 and < 3, and 65.9% of patients were ≥ 70 and < 80 years of age (Supplementary Fig. 1).
Regarding the procedure characteristics, 19 mm or 21 mm valves were used in 77% of patients, and Carpentier Edwards PERIMOUNT valves (Edwards Lifescience, Irvine, CA, USA) were used in 58.2% of patients (Table 2, and Supplementary Table 1).
The incidence of new-onset atrial fibrillation after SAVR was 20.4%, while those of stroke was 1.4% at perioperative period (Table 2, and Supplementary Table 1). With respect to the concomitant surgical procedures with SAVR, 45.9% of patients underwent concomitant procedures including CABG in 28.6% of patients, Maze surgery in 9.1% of patients, mitral valve surgery in 8.6% of patients, and tricuspid valve surgery in 5.5% of patients (Table 2, and Supplementary Table 2).
Regarding the procedural complications, the incidences of stroke, AKI, new-onset atrial fibrillation, and pacemaker implantation were 1.4%, 5.5%, 20.4%, and 0.9%, respectively (Supplementary Table 1).
Clinical outcomes
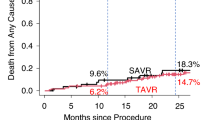
The cumulative incidences of the primary outcome measure (all-cause death or stroke) was 2.3% (95%CI 0.3–4.2%) at 30-day, 4.1% (95%CI 1.5–6.7%) at 1-year, and 13.9% (95%CI 8.3–19.1%) at 5-year, respectively (Fig. 2, and Table 3). The cumulative incidence of all-cause death was 0.9% at 30-day, 2.8% at 1-year, and 11.3% at 5-year, respectively (Fig. 3a, Table 3). The cumulative incidence of stroke was 1.4% at 30-day, 1.4% at 1-year, and 3.2% at 5-year, respectively (Fig. 3b, Table 3). The cumulative incidences of other secondary outcome measures are described in Table 3, Supplementary Figs. 2, and 3.
The cumulative 30-day incidences of all-cause death or stroke were 1.9% and 3.2% at 30-day, and 4.5% and 3.2% at 1-year in patients who underwent SAVR without and with CABG, respectively (Supplementary Table 3). Similarly, the cumulative 30-day incidences of all-cause death or stroke were 1.7% and 3.0% at 30-day, and 4.3% and 4.0% at 1-year in patients who underwent SAVR without and with concomitant surgical procedures, respectively (Supplementary Table 3).
Discussion
The main finding of the present study was that the clinical outcomes of SAVR in low surgical risk patients with severe AS selected from a real world Japanese registry according to the inclusion and exclusion criteria of the PARTNER 3 trial was favorable and numerically comparable to those of SAVR patients in the PARTNER 3 trial.
In the present analysis, we sought to evaluate the clinical outcomes of SAVR in low surgical risk Japanese patients relative to those in SAVR patients in the PARTNER 3 trial, to explore whether the trial results in low surgical risk patients conducted in US/Europe could be extrapolated to Japanese patients. We carefully selected the study population from the SAVR patients in the CURRENT AS registry based on the inclusion and exclusion of the PARTNER 3 trial. Many aspects of the baseline characteristics of the current study population including age and surgical risk score were comparable to those in the PARTNER 3 trial (Age: 75 years versus 74 years, STS-PROM score: 2.3 versus 1.9, Diabetes: 22.3% versus 30.2%, creatinine > 2 mg/dl: 0.0% versus 0.2%, coronary artery disease: 35.0% versus 28.0%, previous stroke: 6.8% versus 5.1%). However, there were several notable differences in the baseline characteristics between the 2 studies (men: 43.2% versus 71.1%, and BMI: 23.3 versus 30.3). Echocardiographic characteristics were comparable between the 2 studies (mean aortic valve gradient: 58.2 mmHg versus 48.3 mmHg, and LVEF: 64.7% versus 66.2%) (Table 2).
Regarding the procedural characteristics, the valve size was much smaller in the current study population than in the SAVR patients in the PARTNER 3 trial. Smaller valve size and prosthesis–patient mismatch have been demonstrated to have negative influence for early and late mortality [21]. We considered the main reasons for valve size difference between the two studies included the difference in physique between American and Japanese people (mean BMI: 30.3 and 23.3), and higher prevalence of women in the CURRENT AS registry than in the PARTNER 3 trial (56.8% and 26.4%). We could not adjust for the differences in valve size, which was appropriately selected in individual patients. Concomitant surgical procedures including CABG were substantially more frequently performed in the current study population than in the SAVR patients in the PARTNER 3 trial (Table 2).
In terms of clinical outcomes, the primary endpoint in the PARTNER 3 trial was a composite of death, stroke or rehospitalization. Nevertheless, we selected a composite of all-cause death or stroke as the primary outcome measure in the current analysis, because the length of hospital stay was much longer in the current study population than in the SAVR patients in the PARTNER 3 trial. In the PARTNER 3 trial, 6.5% of patients in the SAVR group had rehospitalization at 30-day, when many of the patients in the current study population were still hospitalized after the index SAVR (Table 2). Therefore, rehospitalization would not be an appropriate component of the primary outcome measure when we compare the clinical outcomes between the 2 studies.
The cumulative incidence of a composite of all-cause death or stroke was comparable between the current study population and the SAVR patients in the PARTNER 3 trial both at 30-day (2.3% versus 3.3%), and at 1-year (4.1% versus 4.9%). The cumulative incidences of the individual components of the primary outcome measure (all-cause death, and stroke) were also comparable between the current study population and the SAVR patients in the PARTNER 3 trial both at 30-day and at 1-year (Table 2). Therefore, SAVR outcomes in patients with low surgical risk were comparable between the current study and the PARTNER 3 trial, although there are some differences in patient demographics and procedural characteristics.
Only a few Japanese patients were enrolled in these trials comparing TAVI with SAVR in low surgical risk patients, which have already demonstrated that TAVI is comparable or even superior to SAVR. It is not possible to conduct another randomized controlled trial comparing SAVR and TAVI for low-risk patients in Japan. Furthermore, we could not even make an observational comparison between SAVR and TAVI for low-risk patients in Japan, because there were no data of TAVI for low-risk patients in Japan. However, TAVI outcomes in intermediate to high surgical risk patients in Japan have been reported to be comparable or even better than those in US/Europe, and it would be reasonable to assume that it would also be true for low surgical risk patients [22]. Therefore, the current study results suggesting comparable SAVR outcomes in patients with low surgical risk between Japan and US/Europe might support extrapolating the trial results in low surgical risk patients to Japanese patients, and expanding the TAVI indication for low surgical risk patients.
There are several important limitations in this study. First, we included and excluded patients in reference to PARTNER 3 trial. However, in the CURRENT AS registry, we had missing data for some inclusion and exclusion criteria in the PARTNER 3 trial. Second, we could not make statistical comparison between the CURRENT AS registry and the PARTNER 3 trial, because we could not obtain the individual patient data of the PARTNER 3 trial. Third, there were substantial differences in the procedural characteristics such as the valve size and concomitant surgical procedures. Nevertheless, the clinical outcomes were not so much different with or without concomitant surgical procedures in both the current study population and the SAVR patients in the PARTNER 3 trial (Supplementary Table 4). Fourth, we could not evaluate the rate of patient prostheses mismatch after SAVR, because we did not have enough echocardiographic data after SAVR.
Finally, SAVR procedures in this study were performed between 2003 and 2011, while SAVR patients in the PARTNER 3 trial were enrolled between 2016 and 2017. Improved technique and technology might have improved SAVR outcomes in the latter study period.
Conclusions
The clinical outcomes of SAVR in low surgical risk patients with severe AS selected from a real world Japanese registry according to the inclusion and exclusion criteria of the PARTNER 3 trial was favorable and numerically comparable to those of SAVR patients in the PARTNER 3 trial.
References
Braunwald E. Aortic stenosis: then and now. Circulation. 2018;137:2099–100. https://doi.org/10.1161/circulationaha.118.033408.
Murphy ES, Lawson RM, Starr A, Rahimtoola SH. Severe aortic stenosis in patients 60 years of age or older: left ventricular function and 10-year survival after valve replacement. Circulation. 1981;64:184–8.
Schwarz F, Baumann P, Manthey J, Hoffmann M, Schuler G, Mehmel HC, et al. The effect of aortic valve replacement on survival. Circulation. 1982;66:1105–10.
Brennan JM, Edwards FH, Zhao Y, O'Brien SM, Douglas PS, Peterson ED. Long-term survival after aortic valve replacement among high-risk elderly patients in the United States: insights from the Society of Thoracic Surgeons Adult Cardiac Surgery Database, 1991 to 2007. Circulation. 2012;126:1621–9. https://doi.org/10.1161/circulationaha.112.091371.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–185. https://doi.org/10.1016/j.jacc.2014.02.536.
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91. https://doi.org/10.1093/eurheartj/ehx391.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252–89. https://doi.org/10.1016/j.jacc.2017.03.011.
Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. https://doi.org/10.1056/NEJMoa1008232.
Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. https://doi.org/10.1056/NEJMoa1103510.
Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–95. https://doi.org/10.1056/NEJMoa1200384.
Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–704. https://doi.org/10.1056/NEJMoa1202277.
Kapadia SR, Tuzcu EM, Makkar RR, Svensson LG, Agarwal S, Kodali S, et al. Long-term outcomes of inoperable patients with aortic stenosis randomly assigned to transcatheter aortic valve replacement or standard therapy. Circulation. 2014;130:1483–92. https://doi.org/10.1161/circulationaha.114.009834.
Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485–91. https://doi.org/10.1016/s0140-6736(15)60290-2.
Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–84. https://doi.org/10.1016/s0140-6736(15)60308-7.
Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–20. https://doi.org/10.1056/NEJMoa1514616.
Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218–25. https://doi.org/10.1016/s0140-6736(16)30073-3.
Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–705. https://doi.org/10.1056/NEJMoa1814052.
Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–15. https://doi.org/10.1056/NEJMoa1816885.
Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, et al. Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2015;66:2827–38. https://doi.org/10.1016/j.jacc.2015.10.001.
Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2). Eur J Cardiothorac Surg. 2012;42:S45–60. https://doi.org/10.1093/ejcts/ezs533.
Rao V, Jamieson WR, Ivanov J, Armstrong S, David TE. Prosthesis-patient mismatch affects survival after aortic valve replacement. Circulation. 2000;102:5–9. https://doi.org/10.1161/01.cir.102.suppl_3.iii-5.
Handa N, Kumamaru H, Torikai K, Kohsaka S, Takayama M, Kobayashi J, et al. Learning curve for transcatheter aortic valve implantation under a controlled introduction system-initial analysis of a Japanese Nationwide Registry. Circ J. 2018;82:1951–8. https://doi.org/10.1253/circj.CJ-18-0211.
Funding
CURRENT AS registry was supported by an educational grant from the Research Institute for Production Development (Kyoto, Japan). This study was supported by Edwards Lifescience.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takeji, Y., Taniguchi, T., Morimoto, T. et al. Clinical outcome after surgical aortic valve replacement in low-risk Japanese patients with severe aortic stenosis. Cardiovasc Interv and Ther 36, 121–130 (2021). https://doi.org/10.1007/s12928-020-00658-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-020-00658-2