Abstract
To assess the safety and efficacy of routine use of ultrasound-guided puncture and the use of vascular closure device (VCD) in patients undergoing endovascular therapy (EVT) through femoral access. This was a single-center, non-randomized clinical study that enrolled 513 patients undergoing EVT via femoral artery access in which hemostasis was achieved using VCDs (406-patient EXOSEAL arm and 107-patient PROGLIDE arm). All cases were performed by routine use of ultrasound-guided access. The primary endpoint was the achievement of hemostasis without periprocedural and 30-day incidence of major or minor access site-related complications. The primary endpoint was achieved in 91.6 % of the cases (470/513) with a higher success rate in the EXOSEAL arm (93.6). Major complications were observed in 5 patients (0.9 %) in total cohort and 3 patients (0.7 %) treated with EXOSEAL arm vs. 2 patients (1.8 %) with PROGLIDE arm (p = 0.32). Combined treatment two VCDs with the routine ultrasound guidance access for patients who underwent the EVT procedure showed high efficacy and safety outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endovascular treatment therapy (EVT) has been increasing due to an aging society and improved diagnosis of peripheral vascular disease [1–3]. While manual compression has historically been regarded as the “gold standard” for the achievement of vascular closure after femoral artery puncture, it requires immobilization for up to 6–8 h after the procedure and is often associated with patient discomfort.
In recently, some studies shows that ultrasound guidance access reduces access site-related complication of the femoral artery puncture when compared to traditional palpation and fluoroscopy guided puncture [4, 5]. Appropriate puncture location decrease bleeding complication which has been associated with decrease morbidity, mortality and health care cost. In addition, the use of novel arterial closure devices after femoral artery access is exceedingly common due to reduced time to hemostasis, decreased patient discomfort, earlier mobilization, and shortened hospital stays [6, 7]. Vascular closure devices (VCDs) are commonly used after coronary, cerebrovascular, and peripheral arterial interventions [8], and various types of VCDs are available for management of the access site after these procedures [9–11].
In 2009, the ECLIPSE trial demonstrated the safety of the EXOSEAL VCD and its greater effectiveness than manual compression [12]. On the other hand, PROGLIDE is a suture-mediated closure device that utilizes a single monofilament polypropylene suture [13]. Otherwise, exclusion criteria for these trials were severe calcification, hemodialysis, and symptomatic leg ischemia in the target vessel limb. VCDs were applied also in EVT. To the best of our knowledge, no other study has evaluated the VCDs and ultrasound guidance access specifically for EVT. The aim of this study was to assess safety and effectiveness of the EXOSEAL and PROGLIDE VCDs in achieving hemostasis after ultrasound guidance femoral artery access EVT in daily clinical practice.
Methods
Study design and patients
This study was a single-center, non-randomized clinical investigation. Between April 2013 and December 2014, we enrolled 513 consecutive cases (571 procedures) scheduled to undergo EVT for symptomatic peripheral arterial disease with a 6Fr sheath via a common femoral artery puncture. All cases were performed by routine use of ultrasound-guided access. These included 376 males (73.5 %), and the mean age (±standard deviation) was 71.9 ± 9.5 years. Indications for EVT included aortoiliac occlusive disease (AI) and superficial femoral artery (SFA) disease. Diagnostic procedures including below the knee arteries intervention and intervention with less than 6Fr were excluded for VCD use. Also, the femoral artery after a surgically sutured groin was excluded. Symptoms were classified based on the Rutherford category classifications [14], and all patients were in categories 2–5. The study was conducted by the Declaration of Helsinki, and all patients gave their written informed consent before enrollment.
Study procedure and devices
Ultrasound guidance puncture
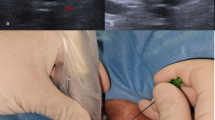
We need to realize the inferior border and upper border of the femoral head by the fluoroscopy. After checking maximum arterial pulse, duplex ultrasound-guided puncture were performed. The ultrasound-guided puncture was performed using 12 MHz linear transducer (NEMIO MS, Toshiba, Tochigi, Japan). The transducer was covered with a sterile pouch containing gel (Fig. 1a). The bifurcation of CFA to SFA, DFA and the location of venous were visualized by ultrasound with short and long axis views (Fig. 1a i–iii). After identification of planned puncture site, local skin anesthesia with 20 cc of Lidocaine 2 % was infiltrated. Also, the needle was punctured using ultrasound with short axis view aiming for the top of the anterior wall of the CFA avoiding calcification and atherosclerotic plaque (Fig. 1b i–iii).
Method of ultrasound-guided access for CFA. Firstly echo scanning covered with a sterile pouch containing gel was made from upper CFA to proximal SFA (a). Either long axis (a i) and short axis (a ii, iii) can be obtained. The bifurcation of CFA to SFA, DFA and the location of venous were visualized ultrasound (a i–iii). Arterial access was obtained with 18-G needle (b). The needle was inserted at an angle of about 45° from the skin just below the level of the center of the femoral head. In viewing short axis, try to aim the top of the vessel (b i, ii). During flash back of blood (b iii), a gentle wire insertion must be made
EVT and hemostasis procedure
An angiogram and EVT were performed in standard fashion. Intra-arterial heparin (5000 IU) was administered after 6 Fr sheath insertion. After EVT, hemostasis was achieved using the VCDs by the manufacturers’ instructions. Two physicians experienced in the use of the two VCDs participated in this study. The choice of the two VCDs, EXOSEAL (Cordis, Bridgewater, NJ, USA) or PROGLIDE (Abbott Vascular, Abbott Park, IL, USA), was left to each physician’s discretion. We did not check the routine activating clotting time and the reverse heparinization. Three minutes of manual compression was applied to the puncture site, and the patient was required to remain in supine position in bed for 4 h in the EXOSEAL arm of the study and 3 h in the PROGLIDE arm. After the prescribed time, the operator confirmed the patient’s hemostasis, after that the patients could be ambulatory.
Definitions of primary and secondary endpoints
The primary endpoint was successful hemostasis achieved using the VCDs with no post-procedural access site complications within 30 days. Unsuccessful hemostasis was defined as persistent bleeding that required subsequent manual compression until hemostasis. Also, patients requiring more than 4 h supine bed rest in the EXOSEAL or more than 3 h in the PROGLIDE group were considered unsuccessful. Major access site-related complications were defined as those requiring intervention or surgical correction, bleeding that required transfusion, a massive hematoma (>5 cm), pseudoaneurysm, vessel occlusion, or arteriovenous fistula. Any other access site-related complication was categorized as minor.
The secondary endpoint criteria were as follows: (1) time to ambulation (hr); (2) time to discharge (days) and (3) predictive factors for unsuccessful hemostasis by multivariate analysis. The clinically prespecified predictive factors used in this analysis were: age ≥ 75 years, hypertension, diabetes, obesity, hemodialysis, dual antiplatelet therapy (DAPT), bilateral approach, antegrade approach, severe calcification, and closely sited stenting which is a nitinol stent implanted within 5 cm of the puncture site. A severely calcified common femoral artery was defined as obvious densities noted within the apparent vascular wall in the angiogram [15].
Clinical follow-up
Clinical evaluations were made at the time of discharge and 30 days. These included confirmation of hemostasis by ultrasound and testing for the presence of infection with a serum test.
Statistical analysis
Statistical analysis was performed with the JMP statistical software version 10.0 (SAS Institute, Cary, North Carolina, USA). Results for categorical variables are presented as frequencies (percentages) and results for continuous variables as the mean ± standard deviation. Where appropriate categorical variables were evaluated by Fisher’s exact test and continuous variables were assessed by the t test. When data before and after procedures were available, paired t tests were applied to compare repeated measures for continuous variables. Clinically prespecified potential predictive factors for unsuccessful hemostasis were entered into the multivariate Cox proportional hazards model, with the results presented as hazard ratios (HR) with 95 % confidence intervals (CI). p values of <0.05 were considered significant.
Results
Baseline demographics, clinical characteristics, and procedural variables
The main characteristics of the included studies are reported in Table 1. EXOSEAL was applied in 406 patients and PROGLIDE in 107 patients. There were no significant differences in patient characteristics and medications between those arms. A total of 205 patients (39.9 %) had severe calcification. 58 patients (11.3 %) required a bilateral approach. There were 251 cases (49.7 %) of AI artery disease and 301 cases (59.0 %) of SFA disease. In AI lesions, the use of PROGLIDE was significantly higher and in SFA lesions, EXOSEAL was more frequently employed.
Primary and secondary endpoints
The outcomes are summarized in Table 2. The primary endpoint defined as successful hemostasis was achieved in 91.6 % (470/513) of patients. Comparison of the two study arms showed EXOSEAL to have a higher rate of the primary endpoint (93.6 vs. 84.1 %, p = 0.003, Fig. 2). In the total cohort, major access site-related complications were observed in 5 patients (0.9 %). In the EXOSEAL arm, there was one case of a massive hematoma requiring transfusion. Moreover, two cases of pseudoaneurysm were observed and successfully treated by compression. In the PROGRIDE arm, one case resulted in CFA occlusion after one knot of PROGLIDE, and surgical correction with an artificial graft was required (Fig. 3). Differences between the study arms did not reach statistical significance due to the small number of major complications. Analysis of minor complications defined as device failure, hematoma and prolonged time to ambulation due to other reasons showed in 45 cases (8.7 %). However, hemostasis for all such patients could be achieved by subsequent manual compression. Time to ambulation was 4.1 ± 1.1 h. Time to discharge was 1.3 ± 1.6 days.
Table 3 compares the potential predictors of unsuccessful hemostasis between the EXOSEAL and PROGLIDE arms. With EXOSEAL, multivariate analysis showed that severe calcification (HR, 12.9; 95 % CI −2.8 to −0.8; p = 0.002) and implantation of a stent close to the puncture site (HR, 3.9; 95 % CI −2.0 to −0.1; p = 0.04) were independent predictors of unsuccessful hemostasis. With PROGLIDE, administration of DAPT was an independent predictor (HR, 7.4; 95 % CI −5.8 to 0.7; p = 0.03). The other elements included concomitant hypertension, systolic blood pressure, aging, obesity and also hemodialysis were not independent predictive factors.
Discussion
As EVT procedures increase both in number and specialties involved, the safety and efficacy of access site hemostasis are important issues [16]. Complications at the puncture site are reported to be the most common but unavoidable risks in transluminal procedures. First, the key is accurate femoral access. In recent years, some studies showed the efficacy and safety of ultrasound guidance access [4, 5]. Ultrasound guidance improves safety outcomes of the common femoral artery puncture when compared to palpation and fluoroscopy-guided puncture. In our institution, routine ultrasound-guided access strategy began in January 2013. After that, access site complication was reduced in daily practice included diagnostic catheterization, coronary intervention, and EVT.
By manual compression, major access site bleeding occurred in 10–15 % of patients at the femoral access site [6–8]. Compared with percutaneous coronary intervention (PCI), for EVT procedures, there has been no prospective study of the incidence of access site complications. Most EVT procedures were for peripheral artery disease with extensive atherosclerotic and/or calcification changes even at the puncture site [17]. The effectiveness and safety of VCDs have already been demonstrated in numerous studies. However, most of these trials were for PCI and diagnostic procedures [8–13].
This study demonstrated that the rates of successfully achieving hemostasis with the VCDs in patients with peripheral artery disease after ultrasound-guided vascular access. In this study, the periprocedural or 30-day incidence of major or minor access site-related complications was low for EVT patients (8.4 %). Most of the unsuccessful cases in our study had minor complications. Major complications were seen in only 0.9 % of patients. Despite the enrollment of complex patients with complex or chronic medical conditions, these results were acceptably low compared to past data. Complexities included patients who were elderly, diabetic, obese, on hemodialysis or DAPT, or suffering from critical limb ischemia.
There are several types of VCDs whose safety and efficacy has been demonstrated. EXOSEAL deploys a completely absorbable plug and does not leave behind foreign bodies [12]. The Mynx (AccessClosure, Inc., Mountain View, CA, USA) and VASCADE (Cardiva Medical, Sunnyvale, CA, USA) devices follow the same system [18, 19]. Other VCDs leave behind foreign bodies such as sutures (e.g., PROGLIDE), an anchor (ANGIOSEAL, St. Jude Medical, St. Paul, Minnesota, USA) [8, 9, 20], or a nitinol clip (STARCLOSE, Redwood City, California, USA, and FemoSeal, St Jude Medical Systems, Uppsala, Sweden) [11, 21].
We used EXOSEAL and PROGLIDE in this study. These two VCDs were chosen because of the two physicians’ familiarity and experience with the devices.
In this study, PROGLIDE showed a little-limited benefit due to several drawbacks. First, PROGLIDE is a large device in itself, approximately 8.5 Fr in size to seal a 6 Fr sheath. One patient required surgical correction for severe dissection after PLOGLIDE deployment. Second, PROGLIDE requires skillful operation of complicated procedures compared to EXOSEAL. In the PROGLIDE arm, unsuccessful hemostasis caused by inaccurate suture was found in 5.6 % of patients. Third, there is a risk of infection. Both VCDs carry potential risks, but a suture or plug left in the vessel wall are more prone to infection. In one PROGLIDE case, we experienced a severe infection with a false aneurysm that required long-term use of antibiotics and extended hospitalization. In the EXOSEAL arm, no cases of infections were seen. The PROGLIDE system also carries the risk of infection as it is necessary to leave behind a monofilament polypropylene suture [22–24]. A randomized controlled trial comparing percutaneous endovascular aneurysm repair and open surgery showed that the use of PROGLIDE carried a 2.0 % risk of infection [13]. From our outcomes, the time to the discharge of the PROGLIDE group is 1 day longer than that of the EXOSEAL group. The reason was some patients prolonged the hospitalization days, in particular, patients needed surgical repair. Accordingly, the average hospitalization days were longer in PROGLIDE arm.
Each device has its merits and demerits. Success with PROGLIDE means that complete hemostasis and early ambulation are guaranteed. In the PROGLIDE arm, time to ambulation was statistically shorter than in the EXOSEAL arm. To select the most appropriate VCD, we performed additional analysis on predictors of unsuccessful hemostasis. This analysis revealed the weak points for EXOSEAL are related to severe calcification at the puncture site and implantation of the stent close to the puncture site (e.g., hemostasis of a retrograde puncture after implantation of a distal external iliac artery stent). The EXOSEAL indicator wire may become caught on the stent, and/or severe calcification may cause this VCD system to fail. The EXOSEAL device should be used with caution in this situation. However, to select the appropriate VCD, other clinical factors such as lesions or individual patient conditions must also be considered. Actually, this study showed the traditional risk factors included as age, obese, hypertension were not the independent predictive factors. Because of combined ultrasound-guided puncture with accurate VCDs usage are safety and efficacy in these high risk patients.
Study limitations
A major limitation of this study was the lack of randomization. The two study arms were hence not matched in every aspect. Second, the manufacturers’ instructions gave different times to ambulation for the two VCDs (4 h for EXOSEAL and 3 h for PROGLIDE), thus influencing the outcomes.
Conclusion
Combined treatment to VCDs with the routine ultrasound guidance access for patients who underwent the EVT procedure showed high efficacy and safety outcomes. Comparison of two VCDs, EXOSEAL, and PROGLIDE, clearly showed EXOSEAL to have a higher success rate.
References
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–67.
Organisation European Stroke, Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clément D, et al. ESC guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2851–906.
Ishihara T, Iida O, Awata M, Nanto K, Shiraki T, Okamoto S, et al. Extensive arterial repair 1 year after paclitaxel-coated nitinol drug-eluting stent vs. bare-metal stent implantation in the superficial femoral artery. Cardiovasc Interv Ther. 2015;30(1):51–6.
Kalish J, Eslami M, Gillespie D, Schermerhorn M, Rybin D, Doros G, et al. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg. 2015;61:1231–8.
Gedikoglu M, Oguzkurt L, Gur S, Andic C, Sariturk C, Ozkan U. Comparison of ultrasound guidance with the traditional palpation and fluoroscopy method for the common femoral artery puncture. Catheter Cardiovasc Interv. 2013;82(7):1187–92.
Gardiner GA Jr, Meyerovitz MF, Stokes KR, Clouse ME, Harrington DP, et al. Complications of transluminal angioplasty. Radiology. 1986;159:201–8.
Sanborn TA, Ebrahimi R, Manoukian SV, McLaurin BT, Cox DA, et al. Impact of femoral vascular closure devices and antithrombotic therapy on access site bleeding in acute coronary syndromes: the acute catheterization and urgent intervention triage strategy (ACUITY) trial. Circ Cardiovasc Interv. 2010;3:57–62.
Biancari F, D’Andrea V, Di Marco C, Savino G, Tiozzo V, Catania A. Meta-analysis of randomized trials on the efficacy of vascular closure devices after diagnostic angiography and angioplasty. Am Heart J. 2010;159:518–31.
Behan MW, Large JK, Patel NR, Lloyd GW, Sulke AN. A randomised controlled trial comparing the routine use of an Angio-Seal STS device strategy with conventional femoral haemostasis methods in a district general hospital. Int J Clin Pract. 2007;61:367–72.
Henry M, Amor M, Allaoui M, Tricoche O. A new access site management tool: the Angio-Seal hemostatic puncture closure device. J Endovasc Surg. 1995;2:289–96.
Holm NR, Sindberg B, Schou M, Maeng M, Kaltoft A, Bøttcher M, et al. Randomized comparison of manual compression and FemoSeal™ vascular closure device for closure after femoral artery access coronary angiography: the CLOSure dEvices Used in everyday Practice (CLOSE-UP) study. Eurointervention. 2014;10:183–90.
Wong SC, Bachinsky W, Cambier P, Stoler R, Aji J, Rogers JH, et al. A randomized comparison of a novel bioabsorbable vascular closure device versus manual compression in the achievement of hemostasis after percutaneous femoral procedures: the ECLIPSE (Ensure’s Vascular Closure Device Speeds Hemostasis Trial). JACC Cardiovasc Interv. 2009;2:785–93.
Nelson PR, Kracjer Z, Kansal N, Rao V, Bianchi C, Hashemi H, et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). J Vasc Surg. 2014;59:1181–93.
Rutherford RB, Becker GJ. Standards for evaluating and reporting the results of surgical and percutaneous therapy for peripheral arterial disease. J Vasc Interv Radiol. 1991;2:169–74.
Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv. 2014;1(83):E212–20.
Tammam K, Ikari Y, Yoshimachi F, Saito F, Hassan W. Impact of transradial coronary intervention on bleeding complications in octogenarians. Cardiovasc Interv Ther. 2016 [Epub ahead of print].
Tarzamni MK, Eshraghi N, Fouladi RF, Afrasiabi A, Halimi M, Azarvan A. Atherosclerotic changes in common carotid artery, common femoral artery, and ascending aorta/aortic arch in candidates for coronary artery bypass graft surgery. Angiology. 2012;63:622–9.
Fargen KM, Hoh BL, Mocco J. A prospective randomized single-blind trial of patient comfort following vessel closure: extravascular synthetic sealant closure provides less pain than a self-tightening suture vascular compression device. J Neurointerv Surg. 2011;3:219–23.
Hermiller JB, Leimbach W, Gammon R, Karas SP, Whitbourn RJ, Wong SC, et al. A prospective, randomized, pivotal trial of a novel extravascular collagen-based closure device compared to manual compression in diagnostic and interventional patients. J Invasive Cardiol. 2015;27:129–36.
Katzenschlager R, Tischler R, Kalchhauser G, Panny M, Hirschl M. Angio-Seal use in patients with peripheral arterial disease (ASPIRE). Angiology. 2009;60:536–8.
Chu G, Yang W, Zhang G, Zhang Z, Liu S, Sun B, et al. Safety and efficacy of the StarClose vascular closure system following 8-Fr sheath placement for intra-aortic balloon pump: a single-center analysis of 42 consecutive patients. Med Princ Pract. 2014;23:313–7.
Sohail MR, Khan AH, Holmes DR Jr, Wilson WR, Steckelberg JM, Baddour LM. Infectious complications of percutaneous vascular closure devices. Mayo Clin Proc. 2005;80:1011–5.
Franco J, Motaganahalli R, Habeeb M, Wittgen C, Peterson G. Risk factors for infectious complications with angio-seal percutaneous vascular closure devices. Vascular. 2009;17:218–21.
Tiesenhausen K, Tomka M, Allmayer T, Baumann A, Hessinger M, Portugaller H, et al. Femoral artery infection associated with a percutaneous arterial suture device. Vasa. 2004;33:83–5.
Acknowledgments
The authors thank Drs. Keita Odashiro, MD, and Koichi Akashi, MD, Department of Medicine and Biosystemic Science, Kyushu University hospital, who provided data management center; and Dr. Toru Maruyama, MD, Prof, Facility of Arts and Science, Kyushu University, who provided safety committee process.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no commercial, proprietary, or financial interest in any products or companies described in this article.
Human rights statement and informed consent
The study was performed in accordance with the Declaration of Helsinki, and written informed consent was obtained from all patients prior to their enrollment in this study.
Rights and permissions
About this article
Cite this article
Fujihara, M., Haramitsu, Y., Ohshimo, K. et al. Appropriate hemostasis by routine use of ultrasound echo-guided transfemoral access and vascular closure devices after lower extremity percutaneous revascularization. Cardiovasc Interv and Ther 32, 233–240 (2017). https://doi.org/10.1007/s12928-016-0409-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-016-0409-x