Abstract
Recently, longitudinal coronary stent deformation has been highlighted as a possible cause of drug-eluting stent failure. Although bench tests and in vivo studies have demonstrated difference in longitudinal stent strength among the stents with different platforms, its clinical impact is still unknown. Furthermore, it is unknown if modified stent platform favorably affect the incidence of stent deformation. The aim of this study was to investigate the longitudinal deformation of the everolimus-eluting stents (EES) with different stent platforms by using frequency domain optical coherence tomography (FD-OCT). Seventy-eight lesions treated with EES (Xience Prime: n = 26, Promus element: n = 29, Promus premier: n = 23) were studied. After successful stent implantation, FD-OCT was performed and stent length was measured using three-dimensional reconstruction of the images in vivo. Percent longitudinal stent shortening (%SS) was defined as the in vivo stent length divided by nominal stent length. Longitudinal stent deformation was defined as %SS > 10 %. Patients’ and procedural characteristics were similar among 3 EESs. There was no difference in mean %SS between Xience Prime, Promus Element and Promus Premier (1.0 ± 5.8, 2.9 ± 6.7 and 0.8 ± 3.7 %, p = 0.322). Incidence of the longitudinal stent deformation was significantly higher in Promus Element than the other stents (0, 13.8 and 0 %, p = 0.028). Incidence of longitudinal stent deformation was different between EESs with different stent platforms. Stent material, stent design and/or stent delivery balloon may affect longitudinal stent deformation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2nd generation drug-eluting stent such as everolimus-eluting stents (EES) has become widely used in daily clinical practice with numbers of evidence showing non-inferior or even superior safety and efficacy as compared with 1st generation drug-eluting stents [1–4]. Currently, several different stent platforms and balloon delivery systems are clinically available as EES. Although overall efficacy and safety of the EES have been reported similar among the different stent platforms [5], in vitro as well as in vivo studies have suggested possible concerns related to the differences in stent platform, a longitudinal stent deformation, as a possible cause of drug-eluting stent failure [6–13]. Because of the limited resolution of the angiography or intravascular ultrasound, incidence and clinical impact of the longitudinal stent deformation among EES with different stent platforms have not been well elucidated yet [12, 13]. Moreover, it is also unclear if modified or improved stent platform has been translated into decreased incidence of longitudinal stent deformation is unknown [14].
Recently, frequency domain optical coherence tomography (FD-OCT) has become clinically available as an intravascular imaging modality that could provide detailed cross-sectional images of the coronary arteries as well as accurate vessel or stent length as a result of better resolution and higher pullback imaging speed [15]. Accordingly, the aim of this study was to investigate incidence of the longitudinal deformation of the EES with different stent platforms by using FD-OCT in vivo.
Methods
Seventy-eight de novo lesions from 78 angina patients treated with EES with 3 different stent platforms [Xience Prime (Abbott Vascular, Santa Clara, CA, USA): n = 26, Promus element (Boston Scientific, Natick, MA): n = 29 and Promus premier (Boston Scientific): n = 23] were enrolled. Table 1 summarized differences in stent platform. Xience Prime is an EES cut from cobalt chromium tube. It has sinusoidal hoop linked by 3 bridges (or links) that are aligned with the stent long-axis [8]. On the other hand, Promus element is another EES that has sinusoidal hoops made from platinum chromium linked by 2 straight bridges per hoop that are aligned at an angle of about 45° from the stent long axis [8]. Promus Premier is a “modified” version of the Promus Element. The proximal 3 hoops are linked by 4 bridges, otherwise, stent material and design are similar to those of Promus Element [14]. The patients underwent FD-OCT imaging before and after PCI. Coronary artery bypass graft, in-stent restenosis and unable to cross with FD-OCT catheter cases were excluded. This study was in compliance with the Declaration of Helsinki with regard to investigations in humans, and the study protocol was approved by the Ethics Committee of Kawasaki Medical School Hospital. Written informed consent was obtained from all patients before cardiac catheterization.
Angiography and interventional procedures
After intravenous or intraarterial bolus injection of the heparin (100 U/kg) and intracoronary nitroglycerin (200 μg) or isosorbide dinitrate (1 mg), coronary angiography and percutaneous coronary intervention using EES were performed by either radial or femoral approach using 6 or 7 French guiding catheter. After successful coronary intervention, intracoronary FD-OCT imaging and final coronary angiography were performed.
FD-OCT image acquisition and analysis
FD-OCT was performed using the ILUMIEN™ OCT imaging system (St. Jude Medical, Minneapolis, MN, USA) and C7 Dragonfly™ intravascular imaging catheter (St. Jude Medical). The C7 Dragonfly catheter was inserted distal to the stent. And imaging of the FD-OCT was performed using an automatic pullback system (10 or 20 mm/s) during contrast injection at a rate of 3–4 ml/s for 3–4 s FD-OCT images were digitally stored for later analysis.
Quantitative, off-line analysis of the OCT images was performed using proprietary computer software (St. Jude Medical). Automatic stent strut detection and enhancement for three-dimensional reconstruction of the FD-OCT images was performed by the software. Before stenting, arc of calcium and area of calcium were measured. After stenting, stent length and minimum stent area were measured [15]. In vivo, actual stent length was measured using longitudinally reconstructed FD-OCT images [16, 17]. The length of the stent according to the FD-OCT analysis was calculated as the distance between the first and last three-quarters circumferential appearance of stent struts.
Percent longitudinal stent shortening (%SS) was calculated as 100 × [(nominal stent length) − (actual stent length measured by FD-OCT)]/(nominal stent length). Longitudinal stent deformation was defined as %SS > 10 % [13]. Incomplete stent apposition, stent edge dissection and tissue protrusion were evaluated by an experienced analyst. Incomplete stent apposition was defined as a clear separation between at least one stent strut and the vessel wall and was defined as a distance between the center reflection of the strut and the vessel wall of greater than the actual stent thickness +20 μm on OCT image [15, 18]. Stent edge dissection was defined as a disruption of the vessel luminal surface in the stent edges (5 mm proximal and distal) with visible flap. Protrusion was defined as a tissue between stent struts extending inside a circular arc connecting adjacent struts [15, 18].
Statistics
Statistical analysis was performed using StatView 5.0 (SAS Institute, Cary, NC). Categorical variables are presented as percentages, and continuous variables are reported as mean ± SD. Differences in the patients and procedural characteristics between the three different stents were compared with one-way ANOVA. A p value of <0.05 was considered statistically significant. Reproducibility of stent length by IVUS, intra-observer variability is 2.6 ± 2.5 %, and inter-observer variability is 4.3 ± 3.4 %.
Results
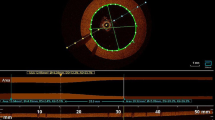
Patient characteristics were similar among 3 EESs (Table 2). Procedural characteristics were also similar among 3 EESs (Table 3). Mean %SS showed a trend toward higher in Promus Element (2.9 ± 6.7 %) than the other 2 EESs (Xience Prime: 1.0 ± 5.8 %, Promus Premier: 0.8 ± 3.7 %), though the difference did not reach statistical significance (p = 0.322). Incidence of longitudinal stent deformation (%SS > 10 %) was significantly higher in Promus Element (13.8 %) than the other 2 EESs (both 0 %, p = 0.028) (Table 4). Representative image of longitudinal stent shortening is shown on Fig. 1. The left panel shows 3-dimensional reconstruction FD-OCT image of the Xience Prime stent. Stent deformation was not documented and %SS was 3.6 %. Right panel shows 3-dimensional reconstruction image of the Promus Element stent. Stent deformation as a result of proximal stent edge shortening was clearly visualized and %SS was 13.6 %.
Representative images. The left panel shows 3-dimensional reconstruction FD-OCT image of the Xience Prime stent. Stent deformation was not documented and %SS was 3.6 %. Right panel shows 3-dimensional reconstruction image of the Promus Element stent. Stent deformation as a result of proximal stent edge shortening was visualized and %SS was 13.6 %
Discussion
The principal findings of this study are that incidence of longitudinal stent deformation was significantly higher in Promus Element, the first version of the EES with platinum chromium stent platform, than Xience Prime, the EES with cobalt chromium stent platform and Promus Premier, the second version of the EES with platinum chromium stent platform. These results suggest that stent platform, especially numbers and their alignment of links between hoops of the stent may be responsible for longitudinal stent deformation. Importantly, increased numbers of the links between hoops at the proximal part of the stent could improve longitudinal strength and decrease the chance of stent deformation.
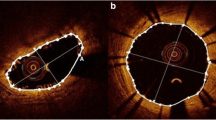
EES has been developed as one of the 2nd generation drug-eluting stent to overcome the limitations and problems of the 1st generation drug-eluting stent. Initially, only a single type of the EES with 2 different brand names, Xience V (Abbott Vascular) or Promus (Boston Scientific), was commercially available and widely used. Two different EESs with different stent platforms and a delivery balloons from 2 different companies has then become available for clinical use. The Xience Prime is a one of the EES made of cobalt chromium that has in-phase sinusoidal hoops linked by 3 bridges that join peaks and troughs and are aligned with the stent long axis [8] (Table 1; Fig. 2) [14]. Each connector has a U-shaped loop to increase flexibility. The Promus Element is another EES made of platinum chromium that has sinusoidal hoops with off-set peaks linked by 2 straight bridges per hoop (Table 1; Fig. 2) [14]. Previous in vitro experimental study of the bare-metal stent and the first generation drug-eluting stents demonstrated that longitudinal stent strength may be different among different drug-eluting stents with different stent platforms [6, 7]. Those differences in longitudinal stent integrity may be responsible for the differences in the incidence of the stent deformation and/or stent fracture in vivo and thus the incidence of stent restenosis or thrombosis [19, 20]. Recently, in vitro studies including the 2 different EES [8, 9] demonstrated that EES with 2 links between hoops, or Promus Element, may have less longitudinal strength and thus has higher incidence of longitudinal stent deformation in vivo [9]. Ormiston et al. demonstrated in an in vitro experimental study that stents with 2 links required significantly less force to be compressed up to 5 mm and elongated by 1 mm [8]. Abdel-Wahab et al. similarly demonstrated that only Promus Element stent irreversibly shortened at 30 g compression [9]. They also demonstrated that stent deformation was documented by fluoroscopy in 0.13 % of the Promus Element stent and none of the Xience Prime stent [9]. On the other hand, another clinical studies using angiography [11] or intravascular ultrasound [13] did not find differences in the incidence of stent deformation between Promus Element and Xience Prime stent. In our study, although overall %SS was similar between the 3 stents, possibly because of small sample size, incidence of the stent deformation was significantly higher in Promus Element stent. One possible reason for the discordant results between their results and our present results is the differences in the imaging modalities used for detecting stent deformation. FD-OCT not only has higher spatial resolution but also is more reliable than angiography or intravascular ultrasound for length measurements. This is due to the fact that spatial resolution technology has a higher pullback speed that reduces the chance of motion artifact during the cardiac cycle [16, 17]. In addition, three-dimensional reconstruction of the FD-OCT data may allow for better visualization of the stent strut with stent deformation [10].
Difference of everolimus-eluting stents designs. The Xience Prime (left panels) is a one of the EES made of cobalt chromium that has in-phase sinusoidal hoops linked by 3 bridges that join peaks and troughs and are aligned with the stent long axis. Each connector has a U-shaped loop to increase flexibility. The Promus Element (center panel) is another EES made of platinum chromium that has sinusoidal hoops with off-set peaks linked by 2 straight bridges per hoop. The Promus Premier (right panel) has the same design as the Element except that the proximal 3 hoops are linked by 4 connectors, in contrast to 2 connectors in the Element. The red arrows indicate the connectors
Interestingly, despite the similar stent platform using the same material (platinum chromium), increased numbers of the links from 2 to 4 at the proximal 3 hoops resulted in decreases incidence of the longitudinal stent deformation in this study. Therefore, numbers of the link between stent hoops rather than stent material (cobalt chromium or platinum chromium) itself might have been associated with differences in the incidence of stent deformation.
It is, however, still uncertain if stent deformation is relate to unfavorable clinical outcome. Despite the higher incidence of the stent deformation, recent studies demonstrate that Promus Element stent was not associated with unfavorable clinical outcome [21, 22]. On the other hand, another recent study demonstrated that stent deformation of the Promus Element stent could cause higher incidence of in-stent restenosis and target lesion revascularization [23]. According to the concerns about longitudinal stent deformation and possibly unfavorable clinical outcome, a newer generation cobalt chromium EES, Promus Premier, has been developed to overcome these unfavorable mechanical stent problems. The guide catheter compression, secondary imaging catheter or un-infrated balloon catches on mal-apposed proximal stent edge may affect the difference of stent deformation between Promus Element and Promus Premier with the same material. As mentioned above, Promus Premier has same stent material with similar stent design as Promus Element with 4 links rather than 2 links between proximal 3 hoops, which enhances longitudinal strength of the proximal part of the stent and thus might contributed to the decreased incidence of longitudinal stent deformation.
A recent in vitro experimental study demonstrated that increased numbers of the link between proximal hoops in the Promus Premier stent was related to decreased incidence of the stent deformation or distortion [14]. Our present study did confirm that improved stent platform of the Promus Premier stent may be associated with decreased incidence of the stent deformation in vivo.
Currently, Promus Element stent is replaced with Promus Premier stent in the market. Therefore, stent deformation might not be a clinical issue anymore if we are to use any EESs.
Study limitations
Firstly, this is a study with small numbers from a single center experience. Thus the results need to be confirmed by a larger multicenter study. Secondly, the difference of stent delivery system did not take into account in this study. The delivery system may also affect stent deformation. Therefore, there might be some bias in the result. Further study will be needed to compare three different DESs. Finally, Xience Prime and Promus Element were used not only different material, but also different stent design. Therefore, there was no direct comparison to confirm effect of stent material on stent deformation.
Conclusions
Incidence of longitudinal stent deformation was different between EESs with different stent platforms. Stent material, stent design and/or stent delivery balloon may affect longitudinal stent deformation.
References
Kedhi E, Joesoef KS, McFadden E, Wassing J, van Mieghem C, Goedhart D, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375(9710):201–9.
Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010;362(18):1663–74.
Raber L, Juni P, Nuesch E, Kalesan B, Wenaweser P, Moschovitis A, et al. Long-term comparison of everolimus-eluting and sirolimus-eluting stents for coronary revascularization. J Am Coll Cardiol. 2011;57(21):2143–51.
Kimura T, Morimoto T, Natsuaki M, Shiomi H, Igarashi K, Kadota K, et al. Comparison of everolimus-eluting and sirolimus-eluting coronary stents: 1-year outcomes from the Randomized Evaluation of Sirolimus-eluting Versus Everolimus-eluting stent Trial (RESET). Circulation. 2012;126(10):1225–36.
Stone GW, Teirstein PS, Meredith IT, Farah B, Dubois CL, Feldman RL, et al. A prospective, randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM (a Prospective, Randomized, Multicenter Trial to Assess an Everolimus-Eluting Coronary Stent System [PROMUS Element] for the Treatment of Up to Two de Novo Coronary Artery Lesions) trial. J Am Coll Cardiol. 2011;57(16):1700–8.
Ormiston JA, Dixon SR, Webster MW, Ruygrok PN, Stewart JT, Minchington I, et al. Stent longitudinal flexibility: a comparison of 13 stent designs before and after balloon expansion. Catheter Cardiovasc Interv. 2000;50(1):120–4.
Tsunoda T, Hara H, Nakajima K, Shinji H, Ito S, Iijima R, et al. Stent deformation: an experimental study of coronary ostial stenting. Cardiovasc Revasc Med. 2009;10(2):80–7.
Ormiston JA, Webber B, Webster MW. Stent longitudinal integrity bench insights into a clinical problem. JACC Cardiovasc Interv. 2011;4(12):1310–7.
Abdel-Wahab M, Sulimov DS, Kassner G, Geist V, Toelg R, Richardt G. Longitudinal deformation of contemporary coronary stents: an integrated analysis of clinical experience and observations from the bench. J Interv Cardiol. 2012;25(6):576–85.
Foerst J, Foin N, Hettleman B. Longitudinal stent compression demonstrated by angiographic “wedding band” and 3-dimensional optical coherence tomography. JACC Cardiovasc Interv. 2012;5(12):e39–40.
Kereiakes DJ, Popma JJ, Cannon LA, Kandzari DE, Kimmelstiel CD, Meredith IT, et al. Longitudinal stent deformation: quantitative coronary angiographic analysis from the PERSEUS and PLATINUM randomised controlled clinical trials. EuroIntervention. 2012;8(2):187–95.
Lupi A, Porto I, Rognoni A, Lazzero M, Fattori R, Parisi R, et al. Clinical and biomechanical behavior of a platinum-chromium stent platform in a large all-comer single-center population: insights from the Novara-PROMETEUS registry. J Invasive Cardiol. 2014;26(7):311–7.
Ota H, Kitabata H, Magalhaes MA, Bui A, Kardenas K, Thomas CH, et al. Comparison of frequency and severity of longitudinal stent deformation among various drug-eluting stents: an intravascular ultrasound study. Int J Cardiol. 2014;175(2):261–7.
Ormiston JA, Webber B, Ubod B, White J, Webster MW. Stent longitudinal strength assessed using point compression: insights from a second-generation, clinically related bench test. Circ Cardiovasc Interv. 2014;7(1):62–9.
Kobayashi Y, Okura H, Kume T, Yamada R, Kobayashi Y, Fukuhara K, et al. Impact of target lesion coronary calcification on stent expansion. Circ J. 2014;78(9):2209–14.
Fedele S, Biondi-Zoccai G, Kwiatkowski P, Di Vito L, Occhipinti M, Cremonesi A, et al. Reproducibility of coronary optical coherence tomography for lumen and length measurements in humans (The CLI-VAR [Centro per la Lotta contro l’Infarto-VARiability] study). Am J Cardiol. 2012;110(8):1106–12.
Liu Y, Shimamura K, Kubo T, Tanaka A, Kitabata H, Ino Y, et al. Comparison of longitudinal geometric measurement in human coronary arteries between frequency-domain optical coherence tomography and intravascular ultrasound. Int J Cardiovasc Imaging. 2014;30(2):271–7.
Kume T, Okura H, Miyamoto Y, Yamada R, Saito K, Tamada T, et al. Natural history of stent edge dissection, tissue protrusion and incomplete stent apposition detectable only on optical coherence tomography after stent implantation—preliminary observation. Circ J. 2012;76(3):698–703.
Aoki J, Nakazawa G, Tanabe K, Hoye A, Yamamoto H, Nakayama T, et al. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2007;69(3):380–6.
Imai M, Kadota K, Goto T, Fujii S, Yamamoto H, Fuku Y, et al. Incidence, risk factors, and clinical sequelae of angiographic peri-stent contrast staining after sirolimus-eluting stent implantation. Circulation. 2011;123(21):2382–91.
De la Torre Hernandez JM, Garcia Camarero T, Lerena P, Lee DH, Sainz Laso F, Gorria GM, et al. A real all-comers randomized trial comparing Xience Prime and Promus Element stents. J Invasive Cardiol. 2013;25(4):182–5.
Sarno G, Lagerqvist B, Carlsson J, Olivecrona G, Nilsson J, Calais F, et al. Initial clinical experience with an everolimus eluting platinum chromium stent (Promus Element) in unselected patients from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Int J Cardiol. 2013;167(1):146–50.
Inaba S, Mintz GS, Yun KH, Yakushiji T, Shimizu T, Kang SJ, et al. Mechanical complications of everolimus-eluting stents associated with adverse events: an intravascular ultrasound study. EuroIntervention. 2014;9(11):1301–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interests to disclose in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Yamada, R., Okura, H., Kume, T. et al. Impact of stent platform on longitudinal stent deformation: an in vivo frequency domain optical coherence tomography study. Cardiovasc Interv and Ther 32, 199–205 (2017). https://doi.org/10.1007/s12928-016-0403-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-016-0403-3