Abstract
We conducted a lesion-based retrospective sub-analyses of diabetes mellitus (DM), diffuse long lesions (stented segment ≥40 mm; LLs), and small vessels (SVs; reference diameter ≤2.6 mm) in patients who received sirolimus- (SESs) or paclitaxel-eluting stents (PESs) for nonrandom treatment of de novo native coronary stenosis in a clinical practice setting. During the period from May 2007 to February 2009, 490 of 682 PES-treated and 293 of 386 SES-treated lesions were angiographically followed up within 1500 days of PCI, and the retrospective investigation was conducted in April 2013. The frequencies of target lesion revascularization (TLR; any recurrent PCI including both marginal stent restenosis) and binary in-stent restenosis (percentage diameter of in-stent stenosis >50 %) upon follow-up angiography, evaluated by adjusting 25 baseline variables using propensity score matching analysis, after placement of SESs and PESs were the following: DM (n = 124 per arm), 14.5 vs. 15.3 % (p = 0.842), and 14.5 vs. 16.1 % (0.856); LLs (n = 81), 16.0 vs. 21.0 % (0.433), and 12.3 vs. 22.2 % (0.117); SVs (n = 107), 11.2 vs. 29.9 % (<0.001), and 11.2 vs. 30.8 % (<0.001), respectively. The p values of log-rank tests for the cumulative TLR-free ratios after SES and PES placement were 0.504 in DM, 0.625 in LLs, and <0.001 in SVs group, respectively. Thus, compared to PES, SES showed the equivalent efficacy for DM, the tendency to be superior for LLs due to approximately 24–45 % reductions in TLR and binary restenosis rates, and the promising superiority for SVs on the angiographic outcomes during a long-term observational interval.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
More than a decade has passed since the approval of the Cypher Bx Velocity sirolimus-eluting stent (SES; Cordis, Miami, FL, USA) for use in Japan. Although SES dramatically reduced the frequencies of target lesion revascularization (TLR) and binary in-stent restenosis (binary restenosis) compared to those of bare-metal stent (BMS) [1–3], a careful observation after SES placement needed to be continued over a long interval owing to the late adverse angiographic outcomes [1, 2]. The risk factors for late (≥1 year) TLR of SES were generally common to those for early (within the first year) TLR [4].
TAXUS Express paclitaxel-eluting stent (PES; Boston Scientific, Natick, MA, USA), the other major first-generation drug-eluting stents (DES), was also widely used in Japan. However, there were only a few reports comparing the mid- to long-term outcomes after SES and PES placements in Japan [5–7]. Therefore, the superiority of SES to PES with regard to the long-term angiographic outcomes including the frequencies of TLR and binary restenosis in a clinical setting should be further evaluated, particularly concerning about the known risk factors of late TLR of SES [4].
Therefore, we conducted 3 sub-analyses of a long-term angiographic follow-up data from the previous report [5], to determine which stent type produced better angiographic outcomes during a long-term observational interval in patients with (1) diabetes mellitus (DM), (2) diffuse long lesions (LLs; stented segment more than 40 mm long), and (3) stenosis in small vessels (SVs; reference diameter ≤2.60 mm). Although debates about the superiority of PES to SES for DM had heated, there was none of the reports examining the long-term efficacy after PESs and SESs placement in Japanese patients with DM. In addition, LLs and SVs were highlighted, because those were the predictors of late adverse angiographic outcomes after SES placement [4]. It has been unclear whether the short-term angiographic outcomes after SESs placement were superior to those of PESs in LLs (stented segment lesions ≥40 mm long) [8–10]. Thus, the long-term angiographic outcomes after SESs and PESs placement should be examined in LLs. On the other hand, in SVs, the superiority of the short-term angiographic outcomes after SESs placement compared to PESs was consistent [11–14], needed to examine the SES’s long-term superiority compared to PES. For these purposes, the frequencies with which TLR and binary restenosis in relation to the mean magnitude of late luminal loss observed on angiographic follow-up within 1500 days of stent placement were compared between SESs and PESs after baseline adjustment using propensity score matching analysis [15].
Methods
Study design and population
The present study was a sub-analysis of data from coronary stenosis patients who had DM, LLs, or SV stenosis who took part in our recent nonrandomized, lesion-based retrospective study [5] performed at Saitama Cardiovascular and Respiratory Center. The rationale was approved by the local ethics committee. The retrospective examination was performed in April 2013. As reported previously, patients were deemed eligible for inclusion if they had de novo stenosis in native coronary arteries successfully treated electively and exclusively with a Cypher Bx Velocity SES or TAXUS Express PES and had no history of coronary artery bypass grafting (CABG) or intra-aortic balloon pump therapy. Percutaneous coronary intervention (PCI) was considered successful when no periprocedural complications (e.g., death, Q-wave myocardial infarction, emergency CABG) occurred; further, patients were enrolled only if postprocedural antegrade coronary flow was grade 3 on the Thrombolysis in Myocardial Infarction (TIMI) scale and stent expansion considered acceptable on angiographic and intravascular ultrasound (IVUS) assessment. The composite exclusion criteria were bailout stenting, hybrid stenting, preprocedural reference diameter (RD) more than 5.0 mm, and postprocedural percentage diameter of stenosis (%DS) more than 33 % (see “Quantitative coronary angiography” below). The study was carried out from May 2007 (when the Express PES was approved in Japan) to February 2009 (before the second-generation Taxus Liberté PES was approved in Japan) and recorded data for 1134 lesions in 798 patients, all successfully treated: 682 with a PES, 386 with an SES, and 66 with a bare-metal stent [5]. Thus, a DES (PES or SES) was used in 94.2 % of lesions, and 63.9 % of the lesions treated with a DES were treated with a PES [2]. The angiographic outcome was determined by follow-up coronary angiography (CAG), which was planned for approximately a year after the procedure; this study includes data from all follow-up CAG examinations performed within 1500 days of the index procedure (SES: 293 lesions; PES: 490 lesions). Within this 1500-day period, severe clinical events such as cardiac death, nonfatal recurrent myocardial infarction, and definite stent thrombosis were observed in 12 patients (1.8 %) with 18 lesions (1.7 %) treated with an SES or PES, with a mean interval of 1397 ± 309 days between clinical observations. Since the frequency of severe clinical cardiac events in the cohort was very low, and most of the patients underwent their elective PCI without complications and were hemodynamically stable afterward, the present study focuses on the corresponding 1500-day angiographic outcomes at follow-up.
DM was defined as (1) previous clinical diagnosis with or current therapy for DM, (2) serum fasting plasma glucose level (FGP) ≥126 mg/dL, or (3) serum hemoglobin A1c level ≥6.5 % according to the report of the committee of Japan Diabetes Society (JDS) on the classification and diagnostic criteria of DM. Among patients with DM who underwent follow-up CAG within 1500 days of the index procedure, 41 % (with 135 lesions) received an SES (SES DM group) and 43 % (with 202 lesions) a PES (PES DM group).
Length of the stented segment was calculated by adding the lengths of each stent, regardless of any overlap; 84 SES-treated lesions and 147 PES-treated lesions in our analysis were LLs (SES LL and PES LL groups, respectively). SVs were defined by a preprocedural RD of less than 2.60 mm. In lesions with full occlusion, percentage diameter of stenosis (%DS) was defined as 100 % and minimal lumen diameter (MLD) as 0. For such lesions, postprocedural RD was substituted for preprocedural RD in the analysis (details of the analysis are described below). Among the SV lesions analyzed in this study, 116 were treated with an SES (SES SV group) and 185 with a PES (PES SV group).
Stenting and antiplatelet therapy
All patients were informed of the rationale for PCI and stenting, and consent to treatment was obtained. Whether to use a device such as a rotablator to ensure successful stenting of the lesion was subject to the doctor’s discretion. Stents were implanted, largely under IVUS guidance, to cover the entire baseline lesion as determined by visual angiography. When further stent dilation was needed, high-pressure balloon inflation was generally performed, using a noncompliant balloon.
Periprocedural antiplatelet therapy was conducted as previously reported [5]. Aspirin (81–100 mg) and ticlopidine (200 mg) were administered orally beginning approximately 10 days before the index procedure; aspirin was continued as long as possible. After the procedure, ticlopidine (200 mg/day) was prescribed for a minimum of 12 months; these prescriptions were not prospectively randomized.
Quantitative coronary angiography
Quantitative coronary angiographic (QCA) parameters were measured with a TCS cardiovascular imaging system (CAAS-2 or -5, Pie Medical, Maastricht, The Netherlands) as described previously [5] at 3 time points: before PCI (preprocedural), immediately after successful PCI (postprocedural), and long-term (follow-up). The in-stent MLD, %DS, and RD were measured, and the acute luminal gain (postprocedural MLD minus preprocedural MLD) and late luminal loss (postprocedural MLD minus follow-up MLD) were calculated.
Binary in-stent restenosis (ISR) was defined as %DS >50 % on follow-up CAG. Since the mean length of stent in our institute became long under the guidance of IVUS, binary restenosis of the present study was defined as binary in-stent restenosis, but not binary in-segment restenosis. Mehran et al. [16] divided ISR cases into focal (lesion length ≤10 mm at long-term follow-up; type 1) and diffuse (lesion length >10 mm; types 2–4). The prevalence of ISR types 2–4 among lesions with binary restenosis was compared between the SES and PES groups. The frequency with which target lesion revascularization (TLR) was performed after follow-up CAG because of in-stent stenosis (including definite stent thrombosis [17]) or edge restenosis was compared between the SES and PES groups. Thus, if several edge restenosis implicated in TLR, the frequency of TLR might exceed that of binary restenosis. The decision to perform TLR was made if binary restenosis on QCA or edge restenosis was observed and one of the following applied: (1) recurrent angina presumably related to the target vessel; (2) objective signs of ischemia at rest (e.g., electrocardiogram changes) or during exercise test (or equivalent) presumably related to the target vessel; (3) abnormal results on any invasive functional diagnostic test (e.g., fractional flow reserve); (4) %DS greater than 70 %. If criterion (4) was present, TLR was performed even in the absence of other signs and symptoms of ischemia.
Outcome measure
The outcome measure of primary efficacy was the percentage of TLR within 1500 days of PCI as described above. In addition, the presence or absence of binary restenosis (defined above) on follow-up CAG within 1500 days of PCI was also estimated as we have previously described [5].
Statistical analyses
Variables measured at baseline were expressed as mean ± standard deviation (SD). Baseline variables and outcomes in the SES group were compared with those in the PES group using the unpaired t test for continuous values and χ2 or Fisher’s test for categorical values. Because the study was retrospective and nonrandomized, propensity score matching was performed in both groups to adjust the baseline values for covariates [15]. Maximum pressure was excluded from adjustment because the rated burst pressure of the stents usually used in our institute differed between SESs (20 atm) and PESs (16 atm). After these adjustments, baseline variables and outcomes in the SES group were compared with those in the PES group using the signed-rank test for continuous values and McNemar’s χ2 test for categorical values. Cumulative TLR-free ratios after SES and PES placement were analyzed by constructing Kaplan–Meier curves and compared using the log-rank test in each sub-analysis. A p value of less than 0.05 was considered to represent statistical significance. The Stata for Windows software program (version 1; StataCorp, College Station, TX, USA) was used for the statistical analyses.
Results
Baseline characteristics and angiographic outcomes in DM groups
Table 1 shows the baseline characteristics and angiographic outcomes of the lesions followed up angiographically in the SES DM (n = 135) and PES DM (n = 202) groups. The percentage of male patients, the percentage of lesions located in the right coronary artery (RCA), and the mean pressure differed significantly between the SES DM and PES DM groups (71.1 vs. 81.7 %, p = 0.032; 20.7 vs. 32.7 %, p = 0.017; and 19.1 ± 3.0 atm vs. 18.0 ± 3.1 atm, p = 0.001, respectively). The mean late luminal loss was significantly less in the SES DM group (0.35 ± 0.73 mm) than in the PES DM group (0.52 ± 0.70 mm) (p = 0.033). The frequencies of binary restenosis and TLR did not differ significantly between the SES DM and PES DM groups.
Adjusted baseline characteristics and angiographic outcomes in DM groups
Table 2 shows the adjusted baseline characteristics of the patients in the SES DM and PES DM groups (n = 124 in each arm). The mean late luminal loss remained significantly less in the SES DM group than in the PES DM group (0.34 ± 0.67 mm vs. 0.54 ± 0.72 mm, p = 0.020). The frequencies of binary restenosis and TLR did not differ significantly between the groups.
Cumulative TLR-free ratios after SES and PES placement in DM-specific sub-analysis
Cumulative primary endpoint-free ratio in the SES DM group was not significantly different from that in the PES DM group (p = 0.504) (Fig. 1).
Cumulative target lesion revascularization-free ratios after SES and PES placement in the DM groups. The cumulative target lesion revascularization (TLR)-free ratio in the SES DM group (black solid line) was not significantly different from that in the PES DM group (black dot line) by the log-rank test
Baseline characteristics and angiographic outcomes in LL groups
Table 3 shows the baseline characteristics and angiographic outcomes of the patients in the SES LL (n = 84) and PES LL (n = 147) groups. The mean length of stent in the SES LL group (60.8 ± 18.3 mm) was not significantly different from that in the PES LL group (60.9 ± 17.9 mm, p = 0.968). The percentage with low ejection fraction, the percentage of lesions located in the left anterior descending artery (LAD), the percentage of lesions located in the RCA, the mean pressure, the postprocedural MLD, and the postprocedural %DS differed significantly between the SES LL and PES LL groups (9.5 vs. 2.7 %, p = 0.025; 66.7 vs. 42.2 %, p < 0.001; 17.9 vs. 41.5 %, p < 0.001; 19.6 ± 2.8 atm vs. 18.5 ± 2.8 atm, p = 0.004; 2.44 ± 0.41 mm vs. 2.61 ± 0.47 mm, p = 0.004; 13.9 ± 9.4 vs. 11.2 ± 7.9, p = 0.026, respectively). The mean late luminal loss was significantly less in the SES LL group (0.35 ± 0.72 mm) than in the PES LL group (0.65 ± 0.75 mm, p = 0.004).
The frequency of binary restenosis in the SES LL group (11.9 %) was on a smaller trend compared to that of PES LL group (21.8 %) (45.4 % reduction, p = 0.062). The frequency of TLR in the SES LL group (15.5 %) was also on a smaller trend compared to that of PES LL group (21.8 %) (28.9 % reduction, p = 0.245). In the SES group, the number of lesion implicated in binary restenosis and TLR were 10 and 13, respectively. The frequency of TLR exceeded that of binary restenosis owing to 3 cases of stent edge restenosis in the SES LLs group.
Adjusted baseline characteristics and angiographic outcomes in LL groups
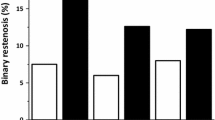
Table 4 shows the adjusted baseline characteristics of the patients in the SES LL and PES LL groups (n = 81 in each arm). The mean late luminal loss in the SES LL group was not significantly different from that in the PES LL group (0.34 ± 0.70 mm vs. 0.48 ± 0.76 mm, 29.2 % reduction, p = 0.310). The frequency of binary restenosis in the SES LL group (12.3 %) was also on a smaller trend compared to that of PES LL group (22.2 %) (44.6 % reduction, p = 0.117). In the SES LL group, the frequency of TLR (16.0 %) exceeded that of binary restenosis (12.3 %), and the frequency of TLR in the SES LL group was not significantly different from that of PES LL group (21.0 %) (24 % reduction, p = 0.433).
Cumulative TLR-free ratios after SES and PES placement in LL-specific sub-analysis
Cumulative primary endpoint-free ratio in the SES LL group was not significantly different from that in the PES LL group (p = 0.625) (Fig. 2).
Baseline characteristics and angiographic outcomes in SV groups
Table 5 shows the baseline characteristics and angiographic outcomes of the lesions followed up angiographically in the SES SV (n = 116) and PES SV (n = 185) groups. Percentage of male patients, mean pressure, preprocedural RD, postprocedural MLD, and postprocedural RD differed significantly between the SES SV and PES SV groups (71.6 vs. 82.7 %, p = 0.022; 18.7 ± 2.7 atm vs. 17.1 ± 3.3 atm, p < 0.001; 2.22 ± 0.41 mm vs. 2.35 ± 0.41 mm, p = 0.007; 2.57 ± 0.53 mm vs. 2.70 ± 0.46 mm, p = 0.029, respectively). The mean late luminal loss was significantly less in the SES SV group (0.28 ± 0.63 mm) than in the PES SV group (0.53 ± 0.70 mm, p = 0.001). The frequencies of binary restenosis and TLR in the SES SV group were on smaller trends compared to those in the PES SV groups (p = 0.059 and 0.075, respectively).
Adjusted baseline characteristics and angiographic outcomes in SV groups
Table 6 shows the adjusted baseline characteristics of the patients in the SES DM and PES SV groups (n = 107 in each arm). The mean MLD and late luminal loss on follow-up in the SES SV group significantly differed from those in the PES SV group (1.95 ± 0.63 mm vs. 1.75 ± 0.74 mm, p = 0.033; 0.29 ± 0.63 mm vs. 0.49 ± 0.75 mm, p = 0.024). The frequencies of binary restenosis and TLR in the SES SV group were significantly smaller than those in the PES SV group (11.2 vs. 30.8 %, p < 0.001; 11.2 vs. 29.9 %, p < 0.001).
Cumulative TLR-free ratios after SES and PES placement in SV-specific sub-analysis
Cumulative primary endpoint-free ratio in the SES SV group was significantly higher than that in the PES SV group (p < 0.001) (Fig. 3).
Discussion
Long-term angiographic outcomes of SES and PES placement in DM
The present DM-specific sub-analysis was conducted for the following several reasons: (1) long-term angiographic outcomes after placement of SESs and PESs in Japanese patients with DM were not fully understood, although the J-DEsERT study [18] reported that SESs and PESs had equivalent efficacy at 1 year; (2) a laboratory study in which paclitaxel exerted different effects from sirolimus (referred to as rapamycin in the study) in experimental models of hyperglycemia and insulin resistance [19] needed to be confirmed by long-term angiographic follow-up; (3) a study in which the TAXUS Liberté (the second-generation TAXUS PES) showed a statistically equivalent frequencies of binary restenosis and TLR with significantly greater late luminal loss compared with SESs [20] needed to be confirmed by SES and Express PES by long-term follow-up.
The present study, analyzing 337 angiographically followed up de novo coronary stenosis lesions treated in a clinical practice setting, provides the first evidence that PESs and SESs have equivalent mid- to long-term efficacy in Japanese patients with DM, as measured by the frequencies of binary restenosis and TLR (Table 2). Mean in-stent late luminal loss was consistently greater after PES placement than after SES placement (Tables 1, 2), as in previous reports comparing short-term in-stent late luminal loss between these devices (routine angiographic follow-up approximately 8–12 months after index procedure) [21]. However, because the threshold value at which mean late luminal loss is associated with a significantly increased incidence of TLR is 0.65 mm [22], the greater late luminal loss after PES placement did not translate into higher rates of binary restenosis or TLR relative to SES placement (Table 2), a result consistent with previous reports [5, 20, 21]. The frequency of diffuse ISR among lesions with binary restenosis after DES placement did not differ significantly between the PES DM and SES DM groups (Tables 1, 2). This is attributable to the adjustment of the data using propensity score matching, as discussed above, which was reflected in greater in-stent late luminal loss (>0.30 mm) after SES placement (Tables 1, 2, 3, 4) than observed in previous reports, where it was in the range of 0.19 mm [21]. On the other hand, the mean in-stent late luminal loss in the PES group after baseline adjustment ranging from 0.48 to 0.54 mm was similar to that (1) in a DM cohort (0.46 mm) [14], (2) in a all-comer study of patients with de novo coronary stenosis (0.50 mm) [5], and (3) of lesions with complex lesions defined as the consistent predictors of TLR after SES placement (0.48 mm) [6]. Over all three sub-analyses in the present study, the percentage change after adjustment in mean late luminal loss in the SES group was 27.6 % (from 0.29 to 0.37 mm) and that in the PES group was 12.5 % (from 0.48 to 0.54 mm); this difference may result from the different anti-restenotic properties of SESs and PESs. Therefore, although differences between SESs and PESs with regard to the impact of late restenosis could not be clearly determined in the present group of DM patients, this study can report statistically equivalent long-term angiographic outcomes (mean follow-up intervals of approximately 430 to 450 days) after SES and PES placement for treatment of de novo native coronary lesions in a clinical practice setting in Japanese patients with DM (Table 2; Fig. 1).
Long-term angiographic outcomes of SES and PES placement in LL
The present LL-specific sub-analysis needed to be evaluated in the long-term interval because LL was the predictor of late adverse angiographic outcome after SES placement [4] and the short- to mid-term superiority of SES to PES on the angiographic outcomes was inconsistent [8–10]. Target lesions in the RCA usually treated using a PES (Table 3), as the type of SES used was prone to fracture when placed in that location [23], were adjusted. The present mean total length of stented segments was more than 60 mm, with a mean late luminal loss not more than 0.37 mm in the SES LL group after adjustment, expressing the great complexity of the LL cohorts. Whereas the mean late luminal loss in the PES LL group after adjustment did not significantly differ from that of SES (Table 4), closing to 0.50 mm as discussed above. The smaller trends in the magnitudes of late luminal loss and of the mean type 2–4 ISR per binary restenosis ratios in the SES LL group did not translate into the significant change in the frequency of binary restenosis compared to PES LL group. However, according to the tendency of the smaller binary restenosis rate in the SES LL group, we could not deny the superiority of SES, or there might be the possibility of SES’s superiority for LL compared to PES. This was the limitation of the present very small cohort. Similarly, 24 % reduction in the frequency of TLR in the SES LL group compared to PES LL group should be evaluated in a larger cohort. Thus, from the present small number of LL-specific sub-analysis, according to the tendency of better outcomes, particularly, in the binary restenosis rate in the SES LL group, there remained the possibility of the SES’s superiority to PES for LLs.
Long-term angiographic outcomes of SES and PES placement in SV
Since SV is the predictor of late adverse angiographic outcome after SES placement [4], the consistent short-term superiority of SES to PES on the angiographic outcomes in SV [11–14] needed to be evaluated in the long-term interval. In the SV-specific sub-analysis, the greater late luminal loss in the PES SV group consistently translated into significantly higher frequencies of binary restenosis and TLR compared with the SES SV group during a long-term observational interval (Table 6; Fig. 3). The present study first confirmed the superiority of SES treatment for SVs, in terms of all of the late luminal loss, binary restenosis, and TLR after adjustment of baseline variables, over PES treatment in Japanese patients.
Limitations
Several limitations of this study must be recognized. First, the study was a retrospective, nonrandomized single-center analysis. However, the population was consecutively enrolled from a cohort in which DES was used at a very high rate. Although propensity score matching analysis was used to adjust baseline variables for covariates [15], the underlying confounders, such as the stent selection bias against the characteristics of target lesion for RCA (Tables 1, 3) and vessel size (preprocedural RD) (Table 5), could not completely adjusted. Second, the study examined angiographic outcomes at only one long-term follow-up interval, so the occurrence of late restenosis [1, 2] could not be determined. Third, the impact of stent fracture (which may be related to stent thrombosis and binary restenosis) on clinical and angiographic outcomes could not be fully defined. It was difficult to determine whether a Bx Velocity SES was fractured by visual estimation alone, particularly after the use of the 2-stent bifurcation technique [24], and the Express PES was radiopaque, with similar effects. Finally, although minimum stent area predicts the outcome of PCI, IVUS assessment of this parameter was not available.
Conclusions
SESs and PESs showed various angiographic outcomes in DMs, LLs, and SVs in terms of TLR, binary restenosis, and late luminal loss within 1500 days of placement for de novo native coronary lesions in a Japanese clinical practice setting. SES showed the equivalent efficacy for DM, the tendency to be superior for LLs, and the promising superiority for SVs on the angiographic outcomes compared to PES during a long-term observational interval.
References
Kimura T, Morimoto T, Nakagawa Y, Kawai K, Miyazaki S, Muramatsu T, Shiode N, Namura M, Sone T, Oshima S, Nishikawa H, Hiasa Y, Hayashi Y, Nobuyoshi M, Mitsudo K, on behalf of the j-Cypher Registry Investigators. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-Cypher Registry. Circulation. 2012;125:584–91.
Natsuaki M, Morimoto T, Furukawa Y, Nakagawa Y, Kadota K, Yamaji K, Ando K, Shizuta S, Shiomi H, Tada T, Tazaki J, Kato Y, Hayano M, Abe M, Tamura T, Shirotani M, Miki S, Matsuda M, Takahashi M, Ishii K, Tanaka M, Aoyama T, Doi T, Hattori O, Kato M, Suwa S, Takizawa A, Takatsu Y, Shinoda E, Eizawa H, Takeda T, Lee JD, Inoko M, Ogawa H, Hamasaki S, Horie M, Nohara R, Kambara H, Fujiwara H, Mitsudo K, Nobuyoshi M, Kita T, Kimura T, CREDO-Kyoto PCI/CABG registry cohort-2 investigators. Late adverse events after implantation of sirolimus-eluting stent and bare-metal stent: long-term (5-7 years) follow-up of the Coronary Revascularization Demonstrating Outcome study-Kyoto registry Cohort-2. Circ Cardiovasc Interv. 2014;7(2):168–79. doi:10.1161/CIRCINTERVENTIONS.113.000987 Epub 2014 Feb 18.
Kubota T, Ishikawa T, Mutoh M. Retrospective comparison of the clinical and angiographic outcomes of the sirolimus-eluting stent and the bare-metal stent in 2031 nonrandomized consecutive de novo native coronary lesions. Intern Med. 2011;50(21):2463–70 Epub 2011 Nov 1.
Nakagawa Y, Kimura T, Morimoto T, Nomura M, Saku K, Haruta S, Muramatsu T, Nobuyoshi M, Kadota K, Fujita H, Tatami R, Shiode N, Nishikawa H, Shibata Y, Miyazaki S, Murata Y, Honda T, Kawasaki T, Doi O, Hiasa Y, Hayashi Y, Matsuzaki M, Mitsudo K, j-Cypher Registry Investigators. Incidence and risk factors of late target lesion revascularization after sirolimus-eluting stent implantation (3-year follow-up of the j-Cypher Registry). Am J Cardiol. 2010;106:329–36. doi:10.1016/j.amjcard.2010.03.031 Epub 2010 Jun 18.
Nakano Y, Ishikawa T, Hino S, Mutoh M. Propensity score matched lesion-based comparison of long-term clinical and angiographic outcomes after placement of sirolimus (Cypher Bx Velocity) and paclitaxel (TAXUS Express)-eluting stents for de novo native coronary stenosis. Cardiovasc Interv Ther. 2014;29:93–101.
Ishikawa T, Nakano Y, Mutoh M. Retrospective comparison of midterm clinical and angiographic outcomes after the implantation of paclitaxel- and sirolimus-eluting stents for de novo coronary complex lesions in nonrandomized Japanese patients. Intern Med. 2012;51:2695–701.
Tsutsumi J, Ishikawa T, Nakano Y, Yoshimura M, Mutoh M (2014) Long-term clinical and angiographic outcomes after sirolimus- and paclitaxel-eluting stent placement following rotablation for severely calcified lesions: a retrospective nonrandomized study. Cardiovasc Interv Ther (Epub ahead of print).
Kim YH, Park SW, Lee CW, Hong MK, Gwon HC, Jang Y, Lee MM, Koo BK, Oh DJ, Seung KB, Tahk SJ, Yoon J, Park SJ. Comparison of sirolimus-eluting stent, paclitaxel-eluting stent, and bare metal stent in the treatment of long coronary lesions. Catheter Cardiovasc Interv. 2006;67:181–7.
Chu WW, Kuchulakanti PK, Torguson R, Wang B, Clavijo LC, Suddath WO, Pichard AD, Satler LF, Kent KM, Waksman R. Impact of three or more sirolimus-eluting stents versus paclitaxel-eluting stents on clinical outcomes in patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv. 2006;68:62–6.
Kim U, Lee SH, Hong GR, Park JS, Shin DG, Kim YJ, Jang JS, Yang TH, Kim DK, Kim DS, Kim DK, Seol SH, Kim DI, Cho YK, Kim HS, Nam CW, Hur SH, Kim KB. Two-year clinical outcomes of patients with long segments drug-eluting stents: comparison of sirolimus-eluting stent with paclitaxel-eluting stent. J Korean Med Sci. 2011;26:1299–304.
Fukumoto A, Otsuji S, Takiuchi S, Ikushima M, Asano K, Terasoma K, Hasegawa K, Yabuki M, Higashino Y. Comparison of real-world clinical outcomes between Cypher- and Taxus-eluting stents: the GARA-GARA study. Cardiovasc Interv Ther. 2011;26:202–8.
Kastrati A, Dibra A, Mehilli J, Mayer S, Pinieck S, Pache J, Dirschinger J, Schömig A. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents. Circulation. 2006;113:2293–300.
Elezi S, Dibra A, Mehilli J, Pache J, Wessely R, Schömig A, Kastrati A. Vessel size and outcome after coronary drug-eluting stent placement: results from a large cohort of patients treated with sirolimus- or paclitaxel-eluting stents. J Am Coll Cardiol. 2006;48:1304–9.
Togni M, Eber S, Widmer J, Billinger M, Wenaweser P, Cook S, Vogel R, Seiler C, Eberli FR, Maier W, Corti R, Roffi M, Lüscher TF, Garachemani A, Hess OM, Wandel S, Meier B, Jüni P, Windecker S. Impact of vessel size on outcome after implantation of sirolimus-eluting and paclitaxel-eluting stents: a subgroup analysis of the SIRTAX trial. J Am Coll Cardiol. 2007;50:1123–31.
D’Agostino RB. Propensity Scores in Cardiovascular Research. Circulation. 2007;115:2340–3.
Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, Pichard AD, Kent KM, Stone GW, Leon MB. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100:1872–8.
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, rel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW. Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51.
Nakamura M, Muramatsu T, Yokoi H, Okada H, Ochiai M, Suwa S, Hozawa H, Kawai K, Awata M, Mukawa H, Fujita H, Shiode N, Asano R, Tsukamoto Y, Yamada T, Yasumura Y, Ohira H, Miyamoto A, Takashima H, Ogawa T, Matsuyama Y, Nanto S (2014) On behalf of the J-DESsERT investigators. Outcomes of the largest multi-center trial stratified by the presence of diabetes mellitus comparing sirolimus-eluting stents (SES) and paclitaxel-eluting stents (PES) in patients with coronary artery disease. The Japan drug-eluting stents evaluation: a randomized trial (J-DESsERT). Cardiovasc Interv Ther (Epub ahead of print).
Patterson C, Mapera S, Li HH, Madamanchi N, Hilliard E, Lineberger R, Herrmann R, Charles P. Comparative effects of paclitaxel and rapamycin on smooth muscle migration and survival: role of AKT-dependent signaling. Arterioscler Thromb Vasc Biol. 2006;26:1473–80.
Suzuki T, Ishikawa T, Nakano Y, Hino S, Mutoh M. Propensity score-matched lesion-based comparison of mid-term angiographic outcomes of TAXUS Liberté with Cypher Bx Velocity stents for de novo native coronary stenosis and in patients with diabetes. Intern Med. 2014;53:1265–73 Epub 2014 Jun 15.
Dibra A, Kastrati A, Mehilli J, Pache J, Schühlen H, von Beckerath N, Ulm K, Wessely R, Dirschinger J, Schömig A. ISAR-DIABETES Study Investigators. Paclitaxel-eluting or sirolimus-eluting stents to prevent restenosis in diabetic patients. N Engl J Med. 2005;353:663–70.
Ellis SG, Popma JJ, Lasala JM, Koglin JJ, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Stone GW. Relationship between angiographic late loss and target lesion revascularization after coronary stent implantation. Analysis from the TAXUS-IV trial. J Am Coll Cardiol. 2005;45:1193–200.
Lee SW, Park DW, Lee CW, Hong MK, Park SW, Ko JK, Park JH, Lee JH, Choi SW, Seong IW, Cho YH, Lee NH, Kim JH, Chun KJ, Park SJ, Long-DES-II study investigators. Incidence and predictors of drug-eluting stent fractures in long coronary disease. Int J Cardiol. 2009;133:354–8.
Endo A, Ishikawa T, Suzuki T, Kashiwagi Y, Mutoh M. Direct microscopic observation of striations in a fractured section of a sirolimus-eluting stent (Cypher Bx Velocity®) indicates induction of stent fracture by continuous shear stress. Int Heart J. 2011;52:248–51.
Conflict of interest
None to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakano, Y., Ishikawa, T. & Mutoh, M. Long-term angiographic outcomes of sirolimus- and paclitaxel-eluting stent placement in diabetes, long lesions, and small vessels. Cardiovasc Interv and Ther 30, 327–337 (2015). https://doi.org/10.1007/s12928-015-0321-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-015-0321-9