Abstract
Induced mutation is valuable for creating genetic variability for use in crop improvement programs. However, there are limited information on the mutagenicity of the African Yam bean (AYB). This study investigated the mitotic chromosomes, pollen fertility and morphological response of African yam bean to gamma radiation. Five accessions of AYB (TSs10, TSs30A, 104B, TSs363 and TSs 364) were planted and evaluated in the screen house using a complete randomized design with three replicates. The accessions were exposed to 0 Gy, 10 Gy, 25 Gy, 100 Gy, 250 Gy and 500 Gy of 60Co (Cobalt 60) gamma source (GAMMA BEAM X 200). In all accessions studied, non-irradiated seeds (0 Gy) showed normal mitotic metaphase; seeds of 25 Gy had sticky metaphase, while those of 10 Gy, 50 Gy, 100 Gy, 250 Gy and 500 Gy gamma doses had varying degrees of scattered arrangement. For all accessions, a decrease in pollen fertility was observed at 250 Gy and 500 Gy radiation doses. The growth characteristics of irradiated seeds differed significantly (p ≤ 0.05) from the control except for plant height and number of branches. Dosage 500 Gy showed a lethal effect having 0% survival rate in all accessions. Similar lethal effect (0% survival rate at 21 DAP) was observed in TSs10, TSs30A and 104B when exposed to 250 Gy dose. Shoot length is positively correlated with radicle length. Also, terminal leaf length is positive and strongly correlated with terminal leaf width and terminal leaf area. The TSs 30A performed best in seed germination and growth characteristics. Therefore, it could be recommended in mutation breeding for AYB improvement.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
The importance of plants in addressing the hunger and malnutrition challenges facing developing and developed nations cannot be over-emphasized. Human population today, however, derives 95% of its food energy from 30 species, indicating that a large proportion of plant food resources are underutilized (Galluzzi and Lopez 2014). One such underutilized plant with great potential for enhancing food security in the tropics is the African yam bean (AOCC 2019).
African yam bean (Sphenostylis stenocarpa (Hochst, Ex A. Rich.) Harms) is one of the neglected and underutilized crops (NUCS) with great potential for addressing the issue of food insecurity and poverty alleviation (Klu et al. 2001). African Yam Bean (AYB) is an indigenous crop of tropical African origin and is the most culturally and economically important of the seven species in the genus Sphenostylis due to its valuable source of plant protein (amino acids–lysine and methionine) in the diets of consumers (Chinedu and Nwiniyi 2012; Adewale and Aremu 2013) and stable yields across a wide range of environments. The tuber which is found beneath the ground has a resemblance to sweet potatoes but tastes more like Irish potatoes. Above ground, it produces good yields of edible seeds. It is usually intercropped with yam, cassava, maize and sorghum and usually, the other crops (cassava, maize and sorghum) or the same stake for yam, serve as a support for the crop (Togun and Egunjobi 1997). The duration of cooking and lack of improved varieties in terms of early maturity traits, dwarf erect architecture and low seed yield, have adversely discouraged its production, large-scale commercialization and its utilization (Klu et al. 2001; Saka et al. 2004; Fasoyiro et al. 2006). The crop has not received adequate research attention compared to other legumes such as cowpea, groundnut and soybean. The AYB has been evaluated for improved farmer preferred traits like disease resistance, drought tolerance and early flowering to reduce the length of the crop cycle; as well as consumer-preferred traits like reduced seed coat hardness or increased permeability for easy cooking and increased nutritional value and/or reduced anti-nutritional factors which are reported to cause discomfort and flatulence when consumed (Onyeike and Omubo–Dede 2002). There is a need to assess the mutagenic variability of African yam bean to gamma radiation.
Mutation is one of nature’s ways of creating variation in living organisms. It is known to enhance the genetic variability of crop plants and facilitate the development of improved varieties in crops (Maluszynski et al. 2000). According to FAO/IAEA (2001), induced mutations have been used in many crops to generate variations in heritable characters such as yield, maturity time and nutritional quality. Mutation breeding offers the possibility of inducing desired attributes that cannot be found either in nature or have been lost during evolution (Roy Chowdhury and Tah 2013). The use of physical and chemical mutagens had also been employed in many legumes and other crops to alter the genetic architecture of plants and isolate the possible mutants with desired plant characteristics such as plant height, number of pods per plant, number of grain per pod, 1000-grain weight, grain yield, oil content and disease resistance in major crops such as wheat, barley, rice, maize and peanuts (Javed et al. 2000; Chopra 2005; Olawuyi and Okoli 2017).
There is limited information on the mutagenic effect of gamma radiation on African yam beans. Gamma ray is a physical mutagen employed for mutation studies due to its shorter wavelength; it possesses ion of more energy per photon than X-rays for higher penetration into the tissue (Khin 2006; Zhu et al. 2006). The biological effect of ionizing radiation like gamma rays depends primarily on the amount of energy absorbed by the biological system of which chromosomes are the most important target (Van Harten 1998). Gamma rays may induce cytological, biochemical, physiological, morphological and genetic changes in cells and tissues (Kiong et al. 2008). There is a need to provide information on the mutagenicity of gamma radiation on AYB improvement. Therefore, this study investigated mitotic chromosomes, pollen fertility and morphological response of AYB to gamma radiation.
Materials and methods
Germplasm collection
Five accessions (TSs10, TSs30A, 104B, TSs363 and TSs 364) were collected from the Genetic Resource Centre of the International Institute for Tropical Agriculture (IITA), Ibadan, Oyo State, Nigeria.
Source of mutagen and seed treatment
The irradiation was done in ambient conditions at the National Institute of Radiation Protection and Research (NIRP), University of Ibadan. The five accessions were exposed to five dosages (10 Gy, 25 Gy, 100 Gy, 250 Gy and 500 Gy) of 60Co (Cobalt 60) gamma source (GAMMA BEAM X 200), while 0 Gy served as control. Twenty seeds were treated per irradiation dose. The dose rate was generated using a cylindrical ionization chamber with serial number 2545. The radiation field size was 25 cm × 25 cm at source to surface of a defector distance of 80 cm. The established dose rate as at the time of irradiation was found to be 0.012 Gy/s.
Germination test
Germination test was carried out on Whatman No.1 filter paper in sterilized 5 cm Petri dishes at room temperature. For each treatment in each accession, five seeds were sown in labeled Petri dishes moistened with distilled water. The seeds were observed daily and those that have protrusions at the radicle were considered to have germinated as described by Ikhajiagbe et al. (2009). For each Petri dish, three to four sprouted seeds out of the five seeds planted were taken as an indication of viability. Seeds observed to have sprouted were recorded and used to determine the germination percentage.
Planting and cultural practices
The polythene bags were watered in the screen house before sowing three seeds of AYB at a depth of 3 cm in the bags spaced at 1 m × 1 m apart. After three weeks of sowing, plant stand per bag was thinned to one. Stakes 3 m high were provided for each plant stand after three weeks of seedling emergence. The plants were staked individually to avoid mixing accessional yields. The polythene was watered regularly after planting till harvest.
Data collection
The plants were observed for both vegetative and reproductive characteristics in five accessions of African yam bean at different treatments. Scoring of qualitative characters was carried out based on visual evaluation, while the quantitative characters were scored by counts and measurements using the metre rule. All qualitative and quantitative characterization was carried out according to the Standard African yam descriptor (Adewale and Dumet 2011) and Methuen handbook of colours chart (Kornerup and Wanscher 1978). Data were collected for the following characters as follows:
Germination percentage (GP)
A count of germinated seeds per accession in the laboratory was made 2 weeks after sowing and expressed in percentage.
Survival percentage (GP)
A count of sprouted seeds with vigorous growth on the field was made 2 and 3 weeks after planting and expressed in percentage.
Number of branches/plants (NB)
The primary branches borne on the main shoot of three randomly sampled plants were counted and recorded.
Plant height (PH)
The plant heights were determined by measuring the vine length in m of the sampled plants per treatments from the soil surface of the polythene bag to the tip of the apex using a metre rule. This measurement was taken at 11 weeks after planting (WAP); the average height was calculated and recorded.
Root length (RL)
The mean length of ten radicles was measured from the base to the root tips. The average length was calculated and recorded.
Shoot length (SL)
The mean length of ten shoots measured from the base to the root tips. The average length was calculated and recorded.
Days to peduncle initiations (DP):
Days from seedling emergence until 50% of the plant stands within a plot begin to initiate peduncles; taking 5 plants as sampling units.
Hypocotyl length (HL)
Mean length of 10 hypocotyl seedlings was measured from the base to the tip when the first primary leaves have fully expanded.
Terminal leaf length (TLL)
The average metric distance from the pulvinus to the apical tip of 10 fully developed terminal leaflets taken from 5 different plants at the peak of flowering.
Terminal leaf width (TLW)
The average metric distance measured along the widest part of 10 fully developed terminal leaflets taken from 5 different plants at the peak of flowering.
Petiole length (PL)
Mean length of the 10 petioles from 5 sample plants, measured from the base to the point where the three leaflets join.
Mitotic studies
To determine the effect of gamma ray on the chromosomes of AYB, mitotic study was conducted according to the method described by Adesoye and Nnadi (2011). Root tips were harvested between the hours of 8 am and 12 noon, pre-treated with 0.4% colchicine solution for 3 h, fixed in Carnoy’s fluid for 24 h before being transferred to 70% ethanol for preservation and stored in the refrigerator at 4 °C until when needed. Slides were prepared using young roots generated from sprouted seeds. The roots were harvested and pre-treated with 0.4% Colchicine solution when their radicles were about 1 cm long for 3 h. Then, they were rinsed in clean tap water and fixed in 1:3 acetic acid/ethanol (v/v) for 24 h. The roots were hydrolyzed in 1 N HCL for five minutes at 60 °C, squashed and stained with aceto-orcein using the squashing techniques described by Adegbite and Olorode (2002).
Pollen slide preparation
According to the protocols described by Jackson (1962) and Olorode and Baquar (1976), pollen grains were collected between 9 am and 11 am from unopened, matured flower buds. Slides were prepared by dusting pollen grains from mature anthers on a clean slide in a drop of cotton blue in lacto-phenol and then covered with a cover slip. Five slides from five different flowers were prepared for each of the accessions and treatment. To estimate the pollen fertility, pollen grains were counted from at least ten fields on each of the slides prepared for each accession and treatment at × 100 magnification. Pollen grains observed to have full cytoplasms were considered fertile while those with half or shrinked cytoplasms were considered sterile. To estimate the percentage of pollen fertility, the number of fertile pollen grains was expressed as a percentage of the total pollen grains counted. Pollen size was determined by measuring the diameter of forty full and deeply stained randomly selected pollen grains on the slides prepared for each accession and treatment at × 400 magnification using an ocular micrometer. The ocular measurements were converted to microns using the stage micrometer.
Statistical analysis
Data obtained were subjected to analysis of variance (ANOVA) using the generalized linear model (GLM) procedure in SPSS version 17.0 statistical software. Means were separated using Duncan’s Multiple Range Test (DMRT) at p ≤ 0.05.
Results
The mean square variance of morphological characters of the African yam bean is presented in Table 1. It shows that gamma treatment of AYB has an effect on some of the morphological characters; however, these effects varied from one accession to the other. For instance, whereas gamma treatment was observed to significantly induce variation in the shoot length and hypocotyls length across all accessions, significant variation in the number of branches was only observed in accession TSs 10. Among all the accession, TSs 30A was observed to have the highest number of significant variation in morphological traits (plant height, hypocotyls length, number of branches, days to peduncle initiation, petiole length, terminal leaf length, terminal leaf width, terminal leaf area, shoot length and root length) resulting from gamma treatment; this is closely followed by 104B (plant height, hypocotyls length, petiole length, terminal leaf length, terminal leaf width, terminal leaf area, shoot length and root length). This suggests that accessions TSs 30A and 104B are more radiosensitive than others.
The variation in morphological characters of African yam bean by accession is presented in Table 2. It shows that plant height ranged from 2.75 m in TSs 364 to 2.88 m in TSs 30A. Similarly, the number of branches and root length ranged from 3.73 (TSs 104B) to 5.33 (TSs 30A) and 2.21 cm (TSs 363) to 2.66 cm (TSs 364), respectively. These variations in plant height, number of branches and root length were observed to be statistically insignificant. The hypocotyl length in TSs 30A (15.01 cm) is significantly (p ≤ 0.05) higher than other accessions except TSs 10 (13.01 cm). Leaf length, leaf width and leaf area were observed to be highest in TSs10 (9.70 cm, 3.4 cm and 33.68 cm2) but lowest in TSs 363 (9.04 cm, 3.21 cm and 29.21 cm2). TSs 10 also initiated peduncle earlier (70.60 days) than any other accession. Generally, TSs 30A had the best mean performance for plant height (2.88 m), hypocotyls length (15.01 cm), number of branches (5.33 cm), petiole length (6.02 cm) and days to peduncle initiation (78.47 days) while TSs 10 had the best mean performance for leaf length (9.70 cm), leaf width (3.4 cm) and leaf area (33.68 cm2).
Presented in Table 3 is the mutagenic effect of different doses of gamma ray on the morphological and growth characteristics of the African yam bean. The result shows that there is no significant variation in plant height, days to peduncle initiation and petiole length across all treatments and accessions except in TSs 30A where a significant variation was observed for plant height. Gamma doses of 25 Gy, 50 Gy and 100 Gy were observed to significantly decrease the hypocotyls length in TSs 10 but significantly increase the same in TSs 363. In TSs 10, TSs 30A and TSs 363, gamma doses of 25 Gy 100 Gy and 100 Gy were observed to induce a significant increase in the number of branches. Gamma dose of 25 Gy was observed to result in a significant reduction in terminal leaf area in TSs 30A and TSs 364 but a significant increase in the same character in 104B.
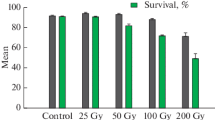
The effect of gamma radiation on the percentage survival of African yam bean accessions is presented in Table 4. Observations on seedling survival rate at 14 and 21 days after planting showed that a high dose of gamma radiation has a lethal effect on seedling survival. Seedlings from 500 Gy had the lowest survival rate of 0%. A survival percentage of 0% was recorded in the 250 Gy doses of TSs 10, TSs 30A and 104B on 21 days after planting as opposed to the survival percentage of 100% in both TSs 363 and TSs 364 at the same dose (Table 4). Percentage seed germination was observed in all treatments in all accessions (Table 5). The result showed that the effect of exposure to different doses of gamma radiation varied from one accession to the other. Generally, the germination percentage varied from 11.11% in 500 Gy to 77.78% in 25 Gy. Non-radiated seeds (control) in all accessions have a germination percentage of 33.33% except accession 104B with 0%. Although a gamma dose of 10 Gy induced an increase in the germination percentage of accessions TSs 30A, 104B and TSs 364, it caused a decrease in the germination of TSs 10 and had no effect on TSs 363. Similarly, a dose rate of 25 Gy had no effect on the germination rate of TSs 30A but evidently raised the germination rate in 104B and TSs 363 when compared to the control. Dose rates of 50 Gy and 100 Gy were observed to consistently increase the percentage of germination in all accessions except TSs 10. However, no dose produced a consistent reduction in percentage germination in all the accessions.
Presented on Table 6 is the result of the correlation analysis of the growth and morphological traits of the African Yam Bean. Generally, it shows that the morphological traits are more strongly related to each other in TSs 30A and 10B than in any other accession. It also reveals that the location and mode of inheritance of these traits—except terminal leaf length, terminal leaf width and terminal leaf area, vary from one accession to another. In all accessions, there exist a strong relationship between the terminal leaf length, terminal leaf width and terminal leaf area. This indicates that the traits are co-located on the same locus, and are jointly segregated and inherited. There is a highly significant positive relationship between the root and shoot lengths in TSs 30A, 104B, TSs 363 and TSs364. Similarly, in TSs 30A and 104B, there is a strong correlation between the hypocotyls length, plant height, days to peduncle initiation, petiole length, terminal leaf length, terminal leaf width and terminal leaf area. In TSs 30A, 104B and TSs 364, days to peduncle initiation, terminal leaf length, terminal leaf width and terminal leaf area strongly correlates with each other but not in TSs 10 and TSs 363 (Table 6). The result of the principal component analysis in Table 7 delineated the growth characteristics of the African yam bean into nine components. The first four components with Eigen value above 1 explain 72.32% of the total variation. Component 1 with Eigen value of 3.22 accounts for 29.06% of the total variation is a measure of the leaf character which shows that the leaf length, leaf width and leaf area are related. This indicates that an increase or decrease in any of these characters induced by a dose of gamma radiation will result in a corresponding increase or decrease in others. Prin 2 accounted for 18.51% of the total variation and shows that the root length is associated with the shoot length, while the number of branches and days to peduncle initiation in prin 3 are positively related but negatively related with the plant height (Table 7). Phylogenetic relationship of qualitative morphological characters of the African yam bean is presented in Fig. 1.
The accessions were grouped into six (6) main clusters. Group 1 consists of 104B 50 Gy, TSs 363 250 Gy, TSs 363 50 Gy and TSs 364 50 Gy while Group 2 composed of 104B 100 Gy, TSs 363 100 Gy, TSs 363 CTR, TSs 10 10 Gy and TSs 10 25 Gy. Group 3 is a monolifolious group of TSs 364 10 Gy. Group 4 comprises TSs 363 25 Gy, TSs 364 CTR, TSs 364 25 Gy, TSs 363 10 Gy, TSs 364 100 Gy and TSs 364 250 Gy. Group 5 contains TSs 30A 50 Gy, TSs 10 CTR, TSs 30A 10 Gy, TSs 30A 100 Gy, TSs 10 50 Gy, TSs 30A CTR, TSs 30A 25 Gy and the last group is composed of 104B 10 Gy, 104B 25 Gy and TSs 10 100 Gy. It was observed that all the treatments in TSs 30A were closely related to the control and TSs 364 25 Gy, 100 Gy and 250 Gy were more related to the control than 10 Gy and 50 Gy. On the other hand, 104B is less related except 10 Gy and 25 Gy which are in the same group (Fig. 1). Gamma radiation produced abnormalities in the cell division of African yam bean accessions shown in Figs. 2 and 3. The 0 Gy (control) showed a normal metaphase chromosome. However, a gamma dose of 25 Gy had sticky metaphase, while 10 Gy, 50 Gy, 100 Gy, 250 Gy and 500 Gy had scattered metaphase arrangement.
Discussion
Pollen fertility was observed to decrease with an increase in radiation dose. This is in accordance with the report of Priyanka (2006), who observed a decrease in pollen fertility of irradiated soya bean. The absence of visible chromosomal damage despite variations in growth character could imply that chromosomal damage caused by the radiation was only at the base level (deletion, inversion and translocation). The study showed that gamma radiation brought about a reduction in the survival rate indicating that the African yam bean accessions were sensitive to gamma radiation. This conforms to the findings of Kavera (2008) on the survival of gamma-radiated crops. The lower survival rate observed at 250 Gy of TSs 10, TSs 30A and 104B show that they were more sensitive to gamma radiation than TSs 363 and TSs 364. Mutagenic sensitivity of African yam bean accessions could be due to the level of differentiation of rudimentary plant parts at the time of treatment and on the other hand, to the extent of damage to the growth components like rate of cell division, cell elongation and various hormones and biosynthetic pathways related to growth and development (Burghate et al. 2013). Mensah and Obadoni (2007) also attributed decrease in the survival percentage of irradiated plants to the physiological disturbance or chromosomal damage caused to the cells of the plant by the mutagen. This could be the case for the total loss of 500 Gy in all the African yam bean accessions indicating that African yam bean cannot tolerate a dose level higher than 250 Gy. The variation observed in the response of the accessions to different doses of gamma radiation suggests that the response was genotype specific. This could be attributed to the variation that had been reported in the germplasm of AYB (Ikhajiagbe and Mensah 2012; Ojuederie et al 2014). Tshilenge-Lukanda et al (2012) observed that the response of groundnut cultivars to gamma radiation varied with genotype. Benslimani and Khelifi (2009) also observed varietal differences in the response of four groundnut cultivars to gamma radiation. The reduction in seed germination observed in 250 Gy (irradiated) seeds of AYB accessions and the loss of viability in 500 Gy seeds could be due to the mutagenic damage caused by the effect of gamma irradiation. This agrees with previous observations made by Olawuyi et al. (2016). Aparna et al. (2012) reported that germination percentage in groundnut decreased after irradiation and the effect became stronger with an increase in gamma dose. In general, gamma radiation was observed to induce both upward and downward shifts in the growth characters of the African yam bean accessions studied.
Conclusions
These TSs 30A and 104B were the most sensitive to gamma radiation among the studied accessions. Gamma radiation at 500 Gy reduced the fertility of African yam bean pollens. TSs 30A performed best with respect to seed germination and growth characteristics. A dose range between 10 and 50 Gy was recorded as the optimal dose for inducing useful mutations in African yam bean accessions in this study. The TSs 30A performed best and is therefore recommended for mutagenic breeding of AYB.
References
Adegbite AE, Olorode O (2002) Meiotic studies of some populations of three species of Aspilia Thouars (Helianthene-Asteraceae) in Nigeria. Niger J Bot 15:74–53
Adesoye AI, Nnadi NC (2011) Mitotic chromosome studies of some accessions of African yam bean Sphenostylis stenocarpa (Hochst Ex A. Rich) harms. Afr J Plant Sci 5(14):835–841
Adewale BD, Aremu CO (2013) The nutritional potentials and possibilities in African yam bean for Africans. Int J Agric Sci 31:8–19
Adewale BD, Dumet DJ (2011) Descriptors for African yam bean Sphenostylis stenocarpa (Hochst ex. A. Rich.) harms IITA. Res Newsl 1–12. http://old.iita.org/cms/articulefiles/1488-ayb_descriptors.pdf
AOCC (2019) Meet the crops-African orphan crops consortium. http://africanorphancrops.org/meet-the-crops. Accessed 19 Apr 2019
Aparna M, Anurag C, Sreedhar M, Pavan KD, Venu-Babu P, Singhal RK (2012) Impact of gamma rays on the seed germination and seedling parameters of groundnut (Arachis hypogaea L.). Asian J Exp Biol Sci 4:1
Benlismani N, Khelifi L (2009) Induction of dormancy in Spanish groundnut seeds (Arachis hypogaea L.) using cobalt 60 gamma irradiation. In: Shu QY (ed) Induced plant mutations in the genomics era. FAO, Rome, pp 381–384
Burghate SK, Mishra MN, Chikhale NJ, Mahalle AM, Dhole VJ (2013) Impact of mutagens its efficiency and effectiveness in groundnut. J Agric Sci 3(7):284–288
Chinedu SN, Nwinyi CO (2012) Proximate analysis of Sphenostylis stenocarpa and Voadzeia subterranean consumed in South-Eastern Nigeria. J Agric Biotechnol Sustain Dev 4(3):57–62
Chopra VL (2005) Mutagenesis: investigating the process and processing the outcome for crop improvement. Curr Sci 89:353–359
FAO/IAEA (2001) Mutation Breeding Review. IAEA-Vienna, pp 1–42
Fasoyiro SB, Ajibade SR, Omole AJ, Adeniyan ON, Farinde EO (2006) Proximate, minerals and antinutritional factors of some under-utilized grain legumes in southwestern Nigeria. Nutr Food Sci 36(1):18–23
Galluzzi G, Lopez NI (2014) Conservation and use of genetic resources of underutilized crops in the Americas—a continental analysis. Sustainability 6:980–1017. https://doi.org/10.3390/su6020980
Ikhajiagbe B, Mensah JK (2012) Genetic Assessment of Three Colour Variants of African Yam Bean [Sphenostylis Stenocarpa] Commonly Grown in the Midwestern Region of Nigeria. Int J Mod Bot 2(2):13–18
Ikhajiagbe B, Mgbeze GC, Erhenh HA (2009) Growth and yield response of Sphenostylis stenocarpa (Hohst ex A. Rich) harms to phosphate enrichment of soil. Afr J Biotechnol 8(4):641–643
Jackson RC (1962) Interspecific hybridization in Haplopappus and its bearing on chromosome evolution in the Blepharodon section. Am J Bot 49(2):119–132
Javed MA, Khatri IA, Khan MA, Siddiqui MA, Arain AG (2000) Utilization of gamma irradiation for the genetics im-provement of oriental mustard (Brassica juncea Coss). Pak J Bot 32:77–83
Kavera SB (2008) Genetic improvement for oil quality through induced mutagenesis in groundnut
Khin T (2006) Rice mutation breeding for varietals improvement in Myanmar. Pl Mut Rep 1(1):34–36
Kiong AA, Ling SH, Pick GL, Harun AR (2008) Physiological responses of Orthosiphon stamineus plantlets to gamma irradiation. Am Eurasian J Sustain Agric 2:135–149
Kiu GYP, Amoatey HM, Bansa D, Kumaga FK (2001) Cultivation and use of African yam bean (Sphenostylis stenocarpa) in the Volta Region of Ghana. J Food Technol Afr 6:74–77
Kornerup A, Wanscher JH (1961) Methuen Handbook of Colour. London, Fletcher and Son Ltd
Maluszynski KN, Zanten LV, Ahlowalia BS (2000) Officially released mutant varieties. The FAO/IAEA Database. Mut Breed Rev 12:1–12
Mensah JK, Obadoni B (2007) Effects of sodium azide on yield parameters of groundnut (Arachis hypogaea L.). Afr J Biotech 6(6):668–671
Ojuederie OB, Balogun MO, Fawole I, Igwe DO, Olowolae MO (2014) Assessment of the genetic diversity of African yam bean (Spehnostylis sternocarpa Hochst ex. A Rich. Harms) accessions using amplified fragment length polymorphism (AFLP) markers. Afr J Biotech 13(18):1850–1858
Olawuyi OJ, Okoli SO (2017) Genetic variability on tolerance of maize (Zea mays L.) genotypes induced with sodium azide mutagen. Mol Plant Breed 8(3):27–37
Olawuyi OJ, Bello OB, Abioye AO (2016) Mutagenic effects of ultraviolet radiation on growth and agronomic characters in maize cultivars. Mol Plant Breed 7(1):1–10
Olorode O, Baquar SR (1976) The Hyparrhenia involucrate—H. suplumosa (Gramineae) complex in Nigeria: morphological and cytological characterization. Bot J Linn Soc 72:212–222
Onyeike EN, Omubo-Dede TT (2002) Effect of heat treatment on the proximate composition, energy values and levels of some toxicants in African yam bean (Sphenostylis sternocarpa) seed varieties. Plant Foods Human Nutr 57:223–231
Priyanka R (2006) Induced Desynaptic male steril;e lines in soybean. Cytologia 71(4):337–343
Roychowdhury R, Tah J (2013) Crop improvement: new approaches and modern techniques. In: Rehman H, Parvaiz A, Münir Ö (eds). Springer, New York, p 155
Saka JO, Ajibade SR, Adeniyan ON, Olowoyo RB, Ogunbodede BA (2004) Survey of underutilized grain legume production systems in south-west agricultural zone of Nigeria. J Agric Food Inf 6(2/3):93–108
Togun AO, Egunjobi JK (1997) Reproductive development and seed yield in African yam bean. Niger J Sci 2:29–35
Tshilenge-Lukanda L, Funny-Biola C, Tshiyoyi-Mpunga A, Mudibu J, Ngoie-Lubwika M, Mukendi-Tshibingu R, Kalonji-Mbuyi A (2012) Radio-sensitivity of some groundnut (Arachis hypogaea L.) genotypes to gamma irradiation: indices for use as improvement. Brit J Biotechnol 3:169–178
Van-Harten AM (1998) Mutation breeding: theory and practical applications. Cambridge University Press, Cambridge
Zhu XD, Chen HQ, Shan JX (2006) Nuclear techniques for rice improvement and mutant induction in China National Rice Research Institute. Plant Mut Rep 1:25–28
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olawuyi, O.J., Inyang, V.E., Oladele, D.D. et al. Mitotic studies, pollen fertility and morphological response of African yam bean (Sphenostylis stenocarpa (Hochst. ex A. Rich) Harms) to gamma radiation. J. Crop Sci. Biotechnol. 26, 499–510 (2023). https://doi.org/10.1007/s12892-023-00194-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12892-023-00194-4