Abstract
Fusarium head blight (FHB) is one of the destructive diseases in wheat-producing areas of the world. Field experiments were conducted during the 2019 main cropping season to determine an economically feasible time of application that consisted of fungicides and spray regimes to reduce FHB pressure and grain yield loss under field conditions at five locations in southern Ethiopia. The study was comprised of two fungicides and spray regimes. Totally seven treatment combinations were designed, including the unsprayed control plots. The field was designed by a randomized complete block design with four replications. Analysis of variance revealed interaction of fungicides and spray regimes significantly (P < 0.001) affected rates of disease progress, disease severity (DS), area under disease progress curve (AUDPC), and yield-related parameters. Lowest (0.0159 units day−1) and highest (0.0694 units day−1) disease progression rates were estimated from the application of Tebuconazole before disease onset and unsprayed control plots, respectively. Application of Tebuconazole before disease onset significantly reduced DS (67.68%), followed by application of Propiconazole + Tebuconazole before disease onset (67.16%) and Tebuconazole after disease onset (65.77%). Moreover, Propiconazole + Tebuconazole and Tebuconazole applications before disease onset considerably reduced AUDPC by 61.38%-days and 58.60%-days, respectively. The highest grain yield (5.02 t ha−1) was recorded from the application of Tebuconazole before disease onset. The grain yield loss of 44.22% was computed on an unsprayed plot compared to the plot protected with the application of Tebuconazole before disease onset. Overall, the application of fungicides' appropriate spray regimes reduces FHB pressure, and consequently, increases grain yields. However, the application of Tebuconazole before disease gave a better monitory advantage over the others. This could be suggested for the producers to manage FHB and enhance wheat production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat (Triticum aestivum L.) is the world’s frontmost cereal crop where more than 1/3rd of the population of the world utilize it as a basic food (FAO et al. 2018; USDA 2018). The world’s total production of wheat has reached more than 809 million tons of grain yields, which was cultivated on a total of more than 214 million hectares of land during 2018. The world-leading wheat-producing country was China (145 million tons), followed by India (110 million tons), Russia (79.5 million tons), United State (56.5 million tons) and France (39.5 million tons) (FAOSTAT 2018). In this regard, Ethiopia is the major wheat producer next to Egypt and Morocco in Africa, which provides an annual grain production of 4,838,074.05 tons (CSA 2018; FAOSTAT 2018). Cultivation of wheat comprises a substantial part of the human diet, commercial and animal feed in Ethiopia (CSA 2018; Anteneh and Asrat 2020). Its worldwide production also approves high commercial and dietary values (FAOSTAT 2018; USDA 2018). In Ethiopia, the crop is of great significance and value cereal crop next to Tef (Eragrostis tef (Zucc.) Trotter) in terms of production and distribution. Wheat production holds 18.23 and 19.80% in terms of area coverage and production related to the total produced areas and production of cereals in Ethiopia (CSA 2018).
In Ethiopia, the crop is potentially grown in the highland and some lowland areas of the country. During the 2018 cropping season, wheat was cultivated on 1,747,939.31 hectares of land with the production of 4,838,074.05 tons of grain yields and productivity of 2.77 t ha−1. In southern Ethiopia, the crop is produced on 151,583.58 hectares of land and contributes 402,857.46 tons of grain yields with a productivity of 2.66 t ha−1 (CSA 2018). However, the average productivity of wheat is low both in the region and national levels compared with the world (3.11 t ha−1) (CSA 2018; FAOSTAT 2018). In this regard, the reports presented by MoANR and EATA (2018) showed that the productivity of wheat crop is more than 7 t ha−1 under research farm and more than 4 t ha−1 under farmers’ field conditions. Even though the availability of wheat cultivars with high yield potential and favorable environmental conditions for cultivation, both the national and regional average productivity is very low in the country. As reported by Zegeye et al. (2001), Ayele et al. (2008), and Shude et al. (2020), the low productivity is accredited due to different and incomprehensible constraints, which includes abiotic, biotic, and socioeconomic and those related to crop management in wheat-producing areas of the world, including Ethiopia. Of the biotic factors, several plant pathogens took part in a significant role in limiting the production and productivity of wheat across the world and Ethiopia as well (Bekele et al. 2001; Ayele et al. 2008; Dean et al. 2012; Zewdie and Paul 2013; Tewodros et al., 2016). Among fungal diseases, Fusarium head blight (FHB) (Fusarium graminearum Schwabe (Teleomorph: Gibberella zeae (Schwein.) Petch) is the fourth most destructive disease of wheat worldwide (Dean et al. 2012; Lenc et al. 2015; Shude et al. 2020). However, the distribution, importance, and characterization of FHB have not been studied under field conditions as other major diseases of wheat in Ethiopia.
Fusarium head blight is an important disease of wheat, barley, and other small cereals (Hoover 2011). The pathogen overwinters and survives between crops in infected chaff, grass stubble, grains, and stem stalk residues left on the ground and the soil. The disease frequently occurs in humid wheat-producing areas of the world (Sutton 1982; Trail et al. 2002; Karasi et al. 2016). The damages are greatly connected with a decrease in grain production and quality of seed, which are provoked by contamination of grains with mycotoxins production, especially deoxynivalenol (Muthomi et al. 2008; Andersen et al. 2014). The occurrence and intensity of FHB epidemics are driven by the amount of airborne inoculum, which may arise from both within and outside of the field, amalgamate with humid air conditions during and post-anthesis period that favors infection of the crop (McMullen et al. 2012; Karasi et al. 2016). In wheat, yield loss due to FHB was estimated to be 50–70% of the total production. However, under favorable conditions, yield losses of 100% have been recorded on the highly susceptible wheat genotypes (Windels 2000; Pirgozliev et al. 2003). Globally, the disease is a significant and causes 1.3 billion dollars in direct losses from 1991 to 1996 across the United States (McMullen et al. 1997, 2012). During 1980, FHB has resulted in significant yield and quality losses in the Prairies and Eastern Canada (Gilbert et al. 2001). Also, losses in Canada have been ranged from 50 million dollars annually since the early 1990s (AFAC 2012). According to the report of the Southern Regional Bureau of Agriculture and respective districts of the office of Agriculture within the region, an exceedingly destructive outbreak has occurred during the 2017 and 2018 cropping seasons in Ethiopia. The damage had been important in the south and southwest of the region (Adiyo, Bench, and North Ari districts); in some fields of wheat, nearly 100% yield loss has been recorded during the growing seasons. To reduce yield losses and sources of infection, preventing the spread of the disease is of great importance in controlling FHB.
Many kinds of researches have been carried out so far for FHB management in different parts of the world. Accordingly, many management alternatives have been reported, including removal of crop residues, deep ploughing, intercropping with legume crops, crop rotations, cultivation of moderately resistant crop varieties, seed treatment and foliar sprays of fungicides, and integrated management approaches (Karasi et al. 2016; Shude et al. 2020; Getachew et al. 2021). Cultural approaches could merely manage FHB to a limited extent (Shude et al. 2020). However, the tendency of many cultivators and the reports of past research on the disease management strategy of FHB had mainly towards the use of fungicides in the world (Gilbert and Haber 2013; Shude et al. 2020). As recently became important and threatening to the farming community, empirical research works on FHB management has not been done under Ethiopian conditions. In addition, no fungicide have been registered for FHB of wheat, although there have been some varieties released with various levels of resistance to major diseases of wheat. According to the reports of the Regional Bureau of Agriculture and respective districts of the office of Agriculture within the region for the last three cropping years (2017–2019), the observation made in the study areas pointed out the disease caused significant yield loss than rust diseases. Management option has not been forwarded for the farmers to manage FHB in the study areas and the country as well. As a result, growers in the study areas need alternative management options along with cultural approaches.
The effects of fungicide on FHB of wheat had been recognized by a number of researchers worldwide as resumed by Gilbert and Haber (2013) and Shude et al. (2020). However, there is no report on registered fungicide for FHB, and no entirely resistant wheat variety to FHB in Ethiopia. These reasons lead to the need for better alternative management options using registered fungicides for other wheat diseases by considering the active substance of the fungicide. Many reports on fungicide application showed that the efficacy of fungicides may differ with the active ingredients comprised within the product development and the pathogen itself to withstand the active ingredients. The effectiveness of fungicide also depends not only on the active substances constituted but also on the way applied, application date, and frequency of sprays along with coverage of the fungicide on the spike (Parry et al. 1995; Liggitt et al. 1997; Homdork et al. 2000). Moreover, the efficacy of fungicide relies on the interaction between the pathogen development, weather conditions, the virulence of the strains of the pathogen, and the pathosystems exhibit in the fields considered (Parry et al. 1995; Mesterhazy and Bartok 1996).
Considering the fungicide use and determining the appropriate spray regime and right combinations of them will be preferred over a fungicide that demands no/little fungicidal activity under the different spray regimes. In addition, evaluating fungicides in combination with spray regimes for the management of FHB in areas where the environmental conditions are favorable for epidemic development of the disease across the environments may be a useful way for determining the effectiveness of the intended management approaches. Thus, this paper reports the results of a multi-location field experiment conducted in southern Ethiopia. The objective was to determine an economically feasible time of application that consisted of fungicides and spray regimes to reduce FHB pressure and grain yield loss under field conditions in southern Ethiopia.
Materials and methods
Descriptions of experimental sites
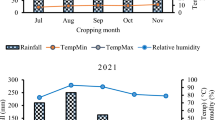
The field experiments were conducted at Adiyo, Bonke, Chencha, Sodo Zuriya, and North Ari districts in southern Ethiopia during the 2019, a main cropping season. These locations constituted in Kafa (Adiyo), Gamo (Bonke and Chencha), South Omo (North Ari), and Wolaita (Sodo Zuriya) administrative zones in Southern Nation, Nationality and Peoples’ Regional state (SNNPRs), Ethiopia. These areas are selected based on the production potential of the crop and the importance of the disease, the areas are hotspots for the epidemic development of FHB. The altitude ranged from 2116 (at Sodo Zuriya) to 2786 (at Bonke) meters above sea level in the study areas. The field experiments were carried out starting from late July (2019) and ending to January (2020) based on the onset and end of precipitation during the growing season in all locations. The details of the areas experimented with and their corresponding geographical positions are depicted in Fig. 1. The study areas have a bimodal rainfall pattern where the short rainy season befalls from March to May and the main rainy season, July to November. Monthly minimum and maximum temperatures (oC), total rainfall (mm), and relative humidity (%) of the study areas during the growing season were incurred from nearby meteorological stations and presented in Fig. 2. Diversified soil physic-chemical properties were observed in the experimental sites. In North Ari and Sodo Zuriya, the soil is distinguished by a moderately acidic pH (5.8–6.2) with moderate organic matter contents (4.96 and 5.75%, respectively) and a textural class of clay-loam and sandy-loam, respectively. In Adiyo, the soil is strongly acidic pH (5.1) with high organic matter contents (11.56%) and clay-loam in textural class. While in Bonke and Chencha, the soil is characterized by a strongly acidic pH (4.9 and 5.3, respectively) with low organic matter contents that ranged from 0.25 to 1.05%, and sandy-loam (MoANR and EATA 2016).
Treatments and experimental design
The study was carried out under natural epiphytotic environments, and natural inoculation was considered to be the source of inoculum. The study was conducted using systemic fungicides spray regimes. The evaluated fungicides were Tebuconazole (Natura 250 EW) and Propiconazole (Tilt 250 EC). The sprayed regimes were comprised application of fungicides before and after disease onset. Susceptible wheat variety (Hidase) was used to receive a more precise cognition of the fungicidal effects against FHB. The variety was currently existed under production and potential yielder but known to susceptible to major diseases of wheat (MoANR and EATA 2018; Getachew 2020). The treatments are composed of two fungicides and spray regimes with the sole (Propiconazole and Tebuconazole) and mixed (Propiconazole + Tebuconazole with 1:1 ratio) application. A total of seven treatment combinations were designed, including the unsprayed control plots. The treatments were arranged in a randomized complete block design with four replications. The treatments were allocated at random to experimental plots within a block.
Experimental procedures
Land preparation was done oxen-driven with four (Bonke and Chencha) to five (North Ari, Adiyo, and Sodo Zuriya) ploughing frequencies. Ploughing frequencies were depending on the characteristic nature of the soil in each location. The trial field was designed with a total of 11.3 m width × 18.60 m length. The total field measurement was 210.18 m2. The unit plot size was designed with 1.8 m width and 2.4 m length. The space between plots and adjacent replications was 1.0 m and 2.0 m, respectively. Seed sowing was done by hand (manually) on the date of 27th (at Sodo Zuriya) July and 4th (at North Ari) of August 2019, which were depending on agro-ecological conditions of the experimental sites during the cropping season. Sowing date of the rest of the locations was found between these dates. The seeds were drilled in an inter-row spacing of 25 cm at the soil depth of 3 cm as per the recommendations advised by MoANR and EATA (2018). The plot was comprised of 10 rows, and eight central rows of the plot were used for data collection, leaving the two border rows.
Based on the manufacturer's recommendation, Propiconazole at the rate of 0.5 L ha−1 with 250 L water and Tebuconazole at the rate of 0.5 L ha−1 with 300 L water was sprayed at 15-day intervals. Application of fungicide was started at the first disease symptom of FHB observed on the cultivar on Zadok growth stage of 61–69 (during anthesis) at Adiyo and North Ari, followed by Sodo, Bonke, and Chencha at Zadok growth stage (ZGS) of 71–73 (post-anthesis) (Zadok et al. 1974). The spraying was continued as per prearranged spray regimes and frequency for the fungicides in all locations. Three consecutive sprayings of fungicide were practiced during the growing period. Fungicide spray was accomplished using a manual knapsack sprayer regulated to deliver 500–700 L of water ha-1. The unsprayed plots were left as a control to allow maximum FHB development.
The seed was obtained from Kulumsa Agricultural Research Center, Ethiopian Institute of Agriculture Research. Recommended NPS blended fertilizer rate (100 kg ha−1) was applied in rows during planting. In addition, N-fertilizer at the rate of 200 kg ha−1 was applied, of which 1/3rd of it during planting and 2/3rd of it on 35 days after planting (DAP). Weeding and regular monitoring of the field were done appropriately and uniformly as per the recommendations to cultivate a successful crop production suggested by MoANR and EATA (2018). During tillering (ZGS of 20–30) to inflorescence (ZGS of 31–45), the entire plots were sprayed with Rex® Duo [Epoxiconazole + Thiophanate-methyl] at the rate of 0.5 L ha−1 with 300 L water to manage wheat rusts (Puccinia spp.) and septoria net blotch (Septoria tritici) in all locations.
Data collection and analysis
Disease parameters
Disease parameters such as incidence, severity, and the area under disease progress curve (AUDPC) were used to find out the efficacy of fungicides and spray regimes against FHB. Disease incidence and severity were scored in 10-day intervals, beginning from the initial visual characteristic symptoms of the FHB showed on the spikelet. Assessment of FHB incidence and severity was begun at ZGS of 61–69 (at Adiyo and North Ari) and 71–73 (Sodo, Bonke, and Chencha). Twenty plants per plot were randomly taken from the middle rows to determine the disease severity. Disease incidence (%) was calculated as the numerator of the number of plants showing disease symptoms and denominator of the total number of plants considered. Disease severity was rated following the 0–100% scale described by Robert and Marcia (2011). A total of five disease scoring was made per location in each plot and ceased with the crop attain physiologically mature, ZGS of 90 (soft dough stage). The average values of disease severity obtained from 20 assessed plants of each plot were used for data analysis.
The area under disease progress curve, which means the progression and buildup of disease on the whole spike or part of the spike during the epidemic periods, was computed from disease severity data recorded at different DAT for each plot following the formula advised by Campbell and Madden (1990).
where n is the total number of disease assessments, ti is the time of the ith assessment in days from the first assessment date and xi is the disease severity of FHB at the ith assessment. AUDPC value was expressed in %-days because severity (x) is expressed in percent and time (t) in days.
Yield parameters and relative yield loss assessment
Thousand seed weight (TSW) and grain yield (GY) were recorded from the middle eight rows of each plot. These parameters were significantly associated with the effect of FHB (Gilbert and Haber 2013; Karasi et al. 2016; Shude et al. 2020). Grain harvesting was carried on 135 and 159 DAP (ZGS of 100) at Sodo Zuriya and Bonke, respectively. Similarly, harvesting dates of the rest of the locations were found between these dates. The harvested grain yield of wheat was measured in kg and transformed into t ha−1. Thousand seed weight was also assessed in gram (g) for each treatment on a plot basis. During harvesting, a moisture content tester was used to determine the seed moisture content of the seed. Subsequently, the harvested grain yield was corrected at 12.5% based on the moisture content of the seed by following the procedure of Taran et al. (1998). Thousand seed weight was measured from randomly sampled grains obtained from the total harvested grains of each plot and corrected them to 12.50% moisture content. On the other hand, the relative yield loss for each treatment was determined following the formula suggested by Robert and James (1991) as followed.
where Ybt = mean yield of the best treatment in the experiment (maximum protected plot) and Ylt = mean yield of the other treatments (low to medium protected plots). Likewise, the relative yield for each treatment was determined as the ratio of the yield obtained from individual treatment compared with the maximum yield obtained under treatment considered and multiplied by 100%.
Data analysis
Disease and yield-related parameters data were subjected to the analysis of variance (ANOVA) following the general linear model procedure of SAS version 9.2 (SAS 2009) to determine the treatment effects. Monomolecular, ln (1/1 – y), (Van der Plank, 1963) epidemiological model was applied for the estimation of rates of disease progression (RDP). The slope of the regression line, when transformed disease severity data were regressed over time, was used to estimate the disease progression rate. The five locations are believed as different environments, and consequently, Bartlett’s chi-square test (Gomez and Gomez 1984) was employed to test for heterogeneous error variance. Bartlett’s chi-square test of the error variances for the parameters studied exhibited most of the parameters were heterogeneous of the data (Pr < χ2) across the locations. Although Bartlett’s chi-square test showed heterogeneous data, combined data analyses were performed across the locations. Mean separations between and among the treatments were achieved using Fisher’s protected least significant difference (LSD) at a 5% probability level (Gomez and Gomez 1984). The relationship between disease development and grain yield was examined through correlation and linear regression analysis. The linear regression analysis was assessed using Minitab® (Release 15.0 for windows® 2007).
Economic feasibility study
Economic feasibility analysis for FHB of wheat management option was computed by following the procedures described by CIMMYT (1988). During economic feasibility analysis, the total input cost of production, gross benefit, net benefit, and benefit–cost ratio were considered. The total input cost of production refers to the sum of all costs of variable and fixed input costs. The fixed cost included expenses of land rent, fertilization, and weeding and harvesting wages since these costs are the same for all plots. The expense of fungicides, knapsack sprayer, and labor for fungicide application was considered under the variable cost of production. The gross benefit was determined as the products of unit market price and grain yield. The net benefit was computed as the difference between the gross benefit and the total cost. The benefit–cost ratio is an index displaying the relationship between the relative costs and benefit obtained and was incurred as the ratio of gross benefit and total cost of production. Before applying economic feasibility analysis, a statistically significant test was made for the collected data to relate the mean grain yield obtained between treatments. And then, as the differences between treatment means were detected, the economic feasibility analysis was performed for the tested management option of FHB.
The price of land rent per one growing period was $78.49, 127.19, 127.19, 143.08, and 174.88 (at the exchange rates of United State $1 = Ethiopian birr 31.45) on a hectare basis in North Ari, Adiyo, Bonke, Chencha, and Sodo Zuriya in 2019, respectively. The purchasing prices of NPS blended and N fertilizers were $42.43 and 39.11 per 100 kg of the bundle during sowing time, respectively. The costs of Propiconazole and Tebuconazole were $31.79 and 38.79 per liter, respectively. The cost price of the Knapsack sprayer was $38.16 as collected from the central market, Addis Ababa, Ethiopia. Based on the dominant wage rates in the areas, costs of labor per man per day were $1.11, 1.58, 1.58, 1.58, and 1.90 around North Ari, Adiyo, Bonke, Sodo Zuriya, and Chencha, respectively. The unit cost of the grain at Adiyo, Sodo Zuriya, North Ari, Bonke, and Chencha was $0.51, 0.64, 0.67, 0.76, and 0.79 kg−1, respectively, at the time of selling in the market. All costs, expense cost and benefit obtained, were changed into a hectare basis for determining the economic feasibility of the additional costs in the management of FHB. The current grain yield was corrected by 10% downward to assess the grain yield difference between the experiential research as well as the farmers’ practice could anticipate from similar treatment.
Results
Analysis of variance
The combined ANOVA of disease and yield-related parameters data exhibited significant variation between experimental treatments and locations (Table 1). Combined ANOVA revealed significant effects on incidence, severity, AUDPC, and yield-related parameters due to fungicides and spray regimes across the locations. Disease and yield-related parameters were significantly (P < 0.0001) affected by the locations and fungicides, and a significant effect of location by fungicide interactions. However, the variations due to spray regimes on these parameters showed various levels of significance at P > 0.05 (disease incidence) to < 0.0001 (grain yield) across the locations (Table 1). Except for incidence, all parameters were highly affected by the interaction of fungicides and spray regimes with various levels of significant (P < 0.01 to < 0.0001) under crosswise evaluations. Interestingly, the combined ANOVA exhibited significant (P < 0.001) interaction effects due to locations x fungicides x spray regimes on disease and yield-related (except, thousand seed weight) parameters under crosswise assessment (Table 1). The higher or lower difference of the mean square values of disease and yield-related parameters might be due to the effects of evaluated treatments and the locations. That is a fungicide, spray regimes, location, and the interactions of location x fungicide x spray regimes responded differently for FHB development, and consequently, affecting yield-related traits during the growing periods.
Rate of fusarium head blight progression
Rates of disease progression, parameter estimates, and significant (P < 0.0001) levels for FHB development are exhibited in Tables 1 and 2. Disease progression was increased regardless of treatment combinations to its higher level at the final disease assessment date in all locations during the growing season, 2019 (Table 2). However, various levels of the mean RDP were observed among the locations and treatment combinations, fungicides x spray regimes. Crosswise assessment showed that the highest mean RDP (0.1051 units day–1, R2 = 96.90%) was recorded at North Ari, followed by Sodo Zuriya (0.0434 units day–1, R2 = 91.50%) and Adiyo (0.0374 units day–1, R2 = 96.20%) compared with Bonke (0.0120 units day–1, R2 = 85.20%) and Chencha (0.0142 units day–1, R2 = 86.10%) in all interaction effects, fungicides x spray regimes. The overall RDP was relatively higher in North Ari than other locations during the growing season (Table 2).
Regarding treatment combinations, the highest mean (0.0694 units day–1, R2 = 84.50%) RDP was recorded on unsprayed control plot, followed by before (0.0664 units day–1, R2 = 84.20%) and after (0.0657 units day–1, R2 = 95.00%) application of Propiconazole as compared to before (0.0159 units day–1, R2 = 96.60%) and after (0.0186 units day–1, R2 = 93.70%) application of Tebuconazole (Table 2). Rates of disease progression were found slower on plots sprayed with the application of different fungicides and spray regimes than unsprayed control plots across the locations. Overall, results showed that the RDP at which FHB progression slowed when the different fungicides were applied before disease onset as compared to after disease onset and unsprayed plots across the locations. However, before and after application of Tebuconazole resulted in maximum protection, thereby retarded FHB epidemic development (Table 2). In this regard, before and after application of Tebuconazole reduced mean RDP by 77.09 and 73.63%, respectively, as compared to unsprayed control plots.
Disease incidence, severity and area under disease progress curve
The mean results obtained from the combined ANOVA for disease incidence, severity, and AUDPC across the locations were presented in Table 3. Analysis of variance revealed significant (P < 0.001) interaction effects were observed on disease severity and AUDPC (Tables 1 and 3). Disease symptom was first observed at North Ari during the growing period. Typical distinctive symptoms of FHB were observed on spikes, and those consisted of water-soaked lesions on spikelets, which later appeared as whitened or bleached, and infected kernels look-alike premature, shriveled and shrunken, production of black spherical structures, and discolored with a whitish-brown appearance of the grain, while healthy heads were still green (Fig. 3). Under crosswise evaluation, the highest mean incidence (72.22%), severity (51.62%), and AUDPC (621.10%-days) were recorded at North Ari, while the lowest incidence (23.98%), severity (12.21%), and AUDPC (72.19%-days) were recorded at Bonke. However, no statistically significant differences were observed in the values of disease incidence, severity, and AUDPC recorded at Bonke and Chencha (Table 3). Generally, disease pressure was relatively higher in North Ari than in other locations. Accordingly, at Sodo Zuriya, Adiyo, Chencha, and Bonke the disease severity and AUDPC of FHB were less suffered by 34.97, 43.90, 70.19, and 76.35%, and 29.73, 56.26, 80.28, and 88.38% compared with the disease severity and AUDPC recorded at North Ari, respectively.
Concerning treatment combinations, the interaction of fungicides and spray regimes significantly altered FHB development as compared to the unsprayed plots (Table 3). The highest mean incidence (55.76%), severity (51.62%), and AUDPC (498.11%-days) indices were recorded from unsprayed plots. Incidence, severity, and AUDPC values recorded on unsprayed plots were not statistically different from the values recorded from plots sprayed with Propiconazole in both before and after disease onset applications under crosswise assessment (Table 3). Contrariwise, the lowest mean incidence (29.13%) was recorded from a mixed application (Propiconazole and Tebuconazole) before disease onset. Nonetheless, the magnitude of incidence obtained from the mixed application before disease onset was statistically in part with the values of incidence recorded from the application of Tebuconazole before and after disease onset and mixed application after disease onset (Table 3).
The lowest mean severity (16.69%) and AUDPC (192.36%-days) were recorded from plots sprayed with Tebuconazole and mixed application of fungicides before disease onset, respectively. No statistically significant differences were observed on the mean severity values obtained among the plots sprayed with Tebuconazole before disease onset and mixed application before and after disease onset. In addition, statistically similar results were observed among the values obtained from the mixed application of fungicides before and after disease onset and Tebuconazole before and after disease onset (Table 3). Application of Tebuconazole after disease onset, mixed application of fungicides before disease onset, and Tebuconazole before disease onset reduced mean disease severity by 65.77, 67.16, and 67.67%, respectively, compared with unsprayed control plots. Also, the mean AUDPC was reduced by 19.30, 26.56, 47.41, 58.44, 58.60, and 61.38% compared with the unsprayed one due to the application of Tebuconazole after disease onset, Tebuconazole before disease onset, and mixed application of fungicides before disease onset, respectively (Table 3).
Yield-related parameters
The combined ANOVA for TSW and GY of wheat results are presented in Table 3. Interaction effects of fungicides x spray regimes significantly (P < 0.001) influenced TSW and GY across the locations (Tables 1 and 3). Different weights of TSW and GY of wheat were noted among treatments evaluated across the locations. Across the locations, the highest mean TSW (41.40 g) and GY (5.50 t ha−1) were noticed at Bonke, while the lowest mean TSW (36.25 g) and GY (2.47 t ha−1) were recorded at Sodo Zuriya and North Ari, respectively. Nevertheless, no statistically significant differences were observed for TSW among and between Bonke and the other locations. Grain yield productivity of wheat at North Ari suffered from the FHB damage and some environmental factors and was reduced by 13.94, 27.57, 48.76, and 55.09% compared with wheat production at Adiyo, Chencha, Sodo Zuriya, and Bonke, respectively (Table 3).
On the other hand, the highest mean TSW of wheat were obtained from plots treated with application Tebuconazole before disease onset (42.75 g), which was not significantly different from the mean TSW obtained from plots treated with the application of Tebuconazole after disease onset (39.42 g), and mixed application of fungicides before disease onset (41.60 g). The lowest mean TSW was obtained from plots of unsprayed and application of Propiconazole after disease onset, which were not significantly different from the mean TSW obtained from plots treated with the application of Propiconazole before disease onset (36.93 g), and mixed application of fungicides after disease onset (36.47 g) (Table 3). The highest mean GY was obtained from plots treated with the application of Tebuconazole before disease onset (5.02 t ha−1), which was significantly similar with the mean TSW obtained from plots treated with the mixed application of fungicides before disease onset (4.81 t ha−1), and Tebuconazole after disease onset (4.19 t ha−1). The lowest GY was noted from the unsprayed plots (2.80 t ha−1), which was significantly affected, and GY was reduced among the evaluated treatments across the locations. However, no statistically significant variations were observed on GY among and between the plots unsprayed and sprayed with Propiconazole before and after disease onset and mixed application of fungicides after disease onset (Table 3).
Estimation of relative yield loss
The estimated relative yield advantage and loss for the locations and treatment combinations are presented in Table 4. The mean relative yield advantage and loss were computed for each location and treatment combination. Bonke and maximum protected plot (application of Tebuconazole before disease onset) were used as a reference to calculate relative yield advantage and loss. However, grain yield advantages and losses were varied between and among the locations and well-performing and less performing treatment combinations. Maximum mean relative yield loss (55.09%) was computed at North Ari, followed by Adiyo (47.82%) and Chencha (38.00%) compared with Bonke. Interaction of fungicides and spray regimes showed various levels of relative yield advantage over unsprayed control plots. Among the treatments, the application of Tebuconazole before disease onset attained the highest (100%) yield advantage over the other treatments. On the other hand, the magnitude of grain yield loss was reduced as compared to the unsprayed control plots because of the interaction of fungicides and spray regimes. In this regard, the highest mean relative yield loss (44.22%) was calculated on unsprayed plots as compared to the maximum protected plot. Overall, the interaction of fungicides and spray regimes reduced FHB pressure, and consequently, the magnitude of yield losses across the locations (Table 4).
Relationship between fusarium head blight epidemics and grain yields of wheat
The associations between epidemiological parameters (severity and AUDPC) and GY were analyzed using correlation analysis. Linear regression analyses were also performed to recognize the relationship between epidemiological parameters and GY in relation to yield loss. Epidemiological parameters were used as a predictor variable, whereas GY was represented by the dependent variable in the regression analysis. Significant (P < 0.0001) relationships (correlation and regression analysis) were observed between the epidemiological parameters and GY. The correlation analysis exhibited negative correlations were observed between disease severity and GY (r = − 0.58) and AUDPC and GY (r = − 0.52) (Fig. 4).
Analysis of linear regression for these parameters was achieved under the plot-wise condition for each treatment, as shown in Fig. 4. About 33.70% of R-square (coefficient of determination) was estimated between disease severity and GY relationships. The value of the R-square for the relationship between the AUDPC and GY was 27.20%. The relationship diagram displayed as the effect of disease severity and AUDPC becoming higher the GY getting lower and vice versa. This indicated that the higher the disease severity and AUDPC, the less the effect of treatments evaluated in reducing FHB pressure. The gaps between the line and the points on the graphs deduced whether the regression analysis was captured a relationship as weak or strong. The closer the dots on the graph are to the line, the better the association and vice versa. In addition, the regression analysis equation attempted to find out the yield losses in every unit of disease severity and AUDPC progressions. The regression analysis equation revealed that in every unit progression of disease severity and AUDPC up to 0.0372 and 0.0032, respectively, losses of GY were crashed from wheat production (Fig. 4).
Economic feasibility analysis
Results obtained from the economic feasibility study for the tested treatments were presented in Table 5. Variation in net benefit (NB) and benefit–cost ratio (BCR) among the evaluated treatments across the locations. The crosswise economic feasibility study revealed that the highest NB ($3227.34 ha−1) and BCR (5.87) was observed at Bonke, while the lowest NB ($767.25 ha−1) and BCR (1.41) was observed at Adiyo. Results obtained from the combined analysis showed that application of Tebuconazole before disease onset exhibited the highest NB ($2513.56 ha−1) and BCR (4.73), followed by mixed application of fungicides before disease onset (NB = $2388.71 ha−1 and BCR = 4.51). The lowest NB ($1253.55 ha−1) and BCR (2.82) were computed from the unsprayed plot of the treatment (Table 5).
Discussion
A big proportion of the wheat production areas in Ethiopia in general and southern region in particular are sown with farm-saved seed as a source of seed (CSA 2018; Anteneh and Asrat 2020). Recurrent use of farm-saved seed may lead to developing the inocula load of several pathogens and dissemination of these pathogens to where the pathogens were not known previously in the areas. In Ethiopia, the newly emerging and even the existing diseases that were previously known as less important are from time to time becoming important on major cereal crops, including maize, wheat and faba bean, produced in the country. According to the reports of 2018 presented by the Ministry of Agriculture and Natural resource and the Regional Bureau of Agriculture as well, the production of wheat had been constrained by the aggressiveness of the existing pathogens and previously unknown or less known pathogens to the country, and particularly in the southern region. Amongst the pathogens, fungi are considered as one of the main limiting factors in wheat production worldwide, including Ethiopia, causing substantial yield losses, poor quality, and reducing the cost-effectiveness for growers (De Villiers 2009; MoANR and EATA 2018). Of the fungal diseases, FHB is now a day ranked the 4th most economically significant plant fungal disease globally (Dean et al. 2012).
Currently, FHB has become of great importance and threatens wheat production in the country. The disease is not new for the country as well as the southern region of the country, but from time to time it becomes a greatly important and major destructive disease next to rust diseases. Previously, the disease had known by no/little effect in wheat production. Previous research confirmed that FHB is a devastating fungal disease that affects small grains cereal crops, wheat, barley, rice, and oat (Martin and Johnston 1982; Wilcoxson et al. 1988; Shude et al. 2020). According to the Bureau of Agriculture in the southern region, Ethiopia, the FHB caused substantial yield losses both in terms of quality and quantity of the grain and price discounting in the market. As reported by Nielsen et al. (2014), Matny (2015), and Shude et al. (2020), the disease had participated directly in yield reduction through damaged kernels, and the associated mycotoxin production (deoxynivalenol), and by price discounting due to the losses of quality and quantity of the grain. Fusarium head blight causes significant yield losses in all small cereal grain crops growing areas of the world (Windels 2000; Pirgozliev et al. 2003; Hoover 2011).
In the current study, considering the importance of the disease and lack of research work, a study on the management of FHB was conducted in the study areas. The study was carried out through fungicides and spray regimes. As reported by McMullen et al. (1997) and Wilcoxson (1996), effective and consistent fungicide control of FHB had required the application of fungicides directly to small cereal crops, wheat and barley. During the study, a symptom of FHB was looked at the spike similar to research findings reported by Murray et al. (2009), Mills et al. (2016), and Ghimire et al. (2020) on symptoms of infected spike due to FHB. Disease onset was relatively late at Bonke and Chencha than other locations. Results of the spatial analysis revealed that the highest mean RDP, incidence, severity, and AUDPC were recorded at North Ari, while the lowest mean results for these parameters were noted at Bonke. The variation in RDP, incidence, severity and AUDPC might be due to the weather conditions (Fig. 2), the susceptibility of the cultivar, fungicide application, spray regimes, earliness of the disease onset, and the inocula load within the environment in the areas for FHB development. Previous research reports indicated that the occurrence of favorable environmental conditions and the abundance of inoculum before, during, and after anthesis of the crop resulted in severe epidemic development of FHB worldwide (Shaner 2003; Brown et al. 2011; Lenc 2015; Reis et al. 2016).
Although the presence of favorable weather conditions and susceptible cultivar to cause FHB epidemic, the RDP, incidence, severity, and AUDPC were lower at Bonke and Chencha. This might be due to the low abundance of inocula within the environment at high altitude in these locations. In fact, due to differences in climatic demands, and genetic and environmental adaptations within the species of FHB complex, Fusarium graminearum is capable of causing disease in a diversity of conditions in wheat-producing areas of the world (Parry et al. 1995; Lenc 2015). Earlier researchers reported that the favorable conditions for infection of FHB had prolonged periods (48–72 h) of high moisture, frequent precipitation, moderately warm temperature (15 to 30 °C) and the occurrence of high air currents. Such conditions were reliable in the study areas (Fig. 2), southern Ethiopia, during the study. However, the disease severity in any situation is related to inoculum pressure, weather conditions, and varietal susceptibility in the pathosystems, so the incidence of individual diseases varies from year to year and from site to site (Campbell and Madden 1990; Agrios 2005). On the other hand, RDP, incidence, severity, and AUDPC were significantly affected by interaction effects of fungicides and spray regimes under crosswise assessment. Among the treatments, application of Tebuconazole before disease onset was exhibited the most consistent ability to reduce FHB progression rates, incidence, severity, and AUDPC, followed by mixed application of fungicides before disease onset across the locations. Rates of disease progression, incidence, severity, and AUDPC were found to be profoundly higher on unsprayed control plots than all other sprayed plots.
The efficacy of fungicides in a different way of application to manage FHB in small grain cereals had been examined in several research reports dating back to four decades (Hoover 2011; Shude et al. 2020). In most parts of the world, several fungicides, including Azoxystrobin, Benomyl, Carbendazim, Metconazole, Propiconazole, Tebuconazole, Thiabendazole, Triadimefon, and other fungicides have been evaluated for their efficacy in reducing the severity of FHB on wheat (Galich 1989; Boyacioglu et al. 1992; Homdork et al. 2000; Jones 2001; Caldwell et al. 2017). In this regard, the fungicides Propeconazole and Tebuconazole applied as sole and mixed with different spray regimes were conducted in the present study and showed significant reductions FHB pressures compared with unsprayed control plots. However, the magnitude of disease reductions were varied between the two fungicides under sole and mixed application before and after disease onset. Tebuconazole exhibited higher disease control than Propiconazole. Similarly, mixed application of fungicides before disease onset showed higher disease control than the mixed application of fungicides after disease onset, next to sole application of Tebuconazole before disease onset.
The present finding on sole fungicide application against FHB was in agreement with the work of Homdork et al. (2000), Jones (2001), Pirgozliev et al. (2008), and Éverson et al. (2019) that confirmed the epidemics of FHB had significantly reduced using a single application of Metconazole, Pyraclostrobin, and Tebuconazole at an early stage of wheat under field conditions. In contrast to the present study, Caldwell et al. (2017) and Shude et al. (2020) reported that a single strategy had not enough to achieve significantly lower FHB pressure. The work had been done by Pirgozliev et al. (2008), and Caldwell et al. (2017) showed that mixed application of Prothioconazole + Tebuconazole at Feekes growth stage of 10.1 had significant effects on the epidemic development of FHB. Many research reports indicated that application of fungicides before FHB onset, during heading and early flower anthesis (Feeks growth stage of 10.1 to 10.5) had lower disease pressure as compared to right after disease onset during the growing periods (Hutcheon and Jordan 1992; Agrios 2005; Megumi et al. 2008; Pirgozliev et al. 2008; Giraud et al. 2011; Caldwell et al. 2017). According to the report of Haidukowski et al. (2004), the application of some fungicides before disease resulted in 77% reductions in FHB severity. Reduced rates of progression and subsequent FHB development in the present study could be due to the role and spray regime of the fungicide through inhibition of lesion progression, infective inoculum production, and establishment of further infection in the field.
Analysis of variance of the tested treatments also exhibited variations for yield-related parameters across the locations. Under crosswise assessment, Bonke received the highest TSW and GY than the other locations. Comparably, North Ari showed the lowest TSW and GY than the other locations. The lowest TSW and GY could be due to the high pressure of FHB and other factors in the study areas. Campbell and Madden (1990) and Agrios (2005) confirmed the presence of a favorable pathosystem among the studied environs significantly favors the epidemic development of the pathogen, and consequently, affected the metabolic process of the crop and reduced the growth and yield-related traits of the genotype, and vice versa. In the current study, the highest TSW and GY were obtained from the application of Tebuconazole before disease onset, although which was not significantly different from plots sprayed with sole application of Tebuconazole after disease onset, and the mixed application of fungicides before disease onset. The probable reason might be the fungicide could improve the wellbeing of the crop that carrying out the normal metabolic process, which might increase the probabilities to defy the pathogen outbreak and support the defense systems of the crop. In addition, variation in TSW and GY among the treatments evaluated might be due to varying levels of FHB severity and AUDPC due to the application of the fungicides with different spray regimes, environmental conditions, and other factors in the study areas. Various previous studies also confirmed fungicide application (Pyraclostrobin, Tebuconazole, Thiophanate-methyl, Pyraclostrobin + Metconazole, and Prothioconazole + Tebuconazole) had consistently reduced FHB pressure and provided the highest crop yield (Jones 2001; Megumi et al. 2008; Caldwell et al. 2017; Bonfada et al. 2019; Shude et al. 2020).
Sole and mixed application of and different spray regimes and subsequent variation in FHB pressure could be responsible for comparative yield advantages losses, which were obtained per treatment and locations, along with other factors. Fusarium head blight pressure was higher on unsprayed plots than the other sprayed plots, and this could be comparably explained by spike damage on plots. Under the severe condition, the spikes are almost empty of grains as well as uneconomical due to shriveled and premature drying of the grain. In this condition, yield losses up to 100% would result due to FHB on wheat crops (Windels 2000; Pirgozliev et al. 2003). While on the sprayed plots, the disease progression was reduced and stabilized towards the end of the epidemic period using a sole application of Tebuconazole and mixed application of fungicides before disease onset. Application of fungicide helped the plants to become healthy and endure the effects of the disease, and undertake normal metabolic functions, and led to a lessening of considerable grain yield losses. This reflection was consistent with the results of Jones (2001), Megumi et al. (2008), Caldwell et al. (2017), Bonfada et al. (2019), Shude et al. (2020) report of the sole or mixed application of fungicides had resulted in the lowest diseases severity towards the end of growing periods on highly protected plots and enhanced yield-related attributes of wheat. Considering the medium intensity of wheat rusts and septoria leaf blotch disease, environmental conditions, and other factors might also be responsible for the grain yield losses in addition to FHB. The effects of these factors were not fully explained by the current study, and their confounding effect cannot be underestimated in the grain yield losses.
The correlation study revealed negative and highly significantly association between epidemiological parameters and grain yield. The negative associations between epidemiological parameters and grain yield pointed out the magnitude to which FHB might bear on the grain yield. Additionally, the correlation analysis showed that FHB exhibited a considerable negative effect on the grain yield. The result found in the current study was in agreement with the reports of Campbell and Madden (1990) and Agrios (2005) who reported that epidemiological parameters had been strongly associated with growth and yield-related traits of the produced crop. On the other hand, a linear regression analysis was performed to observe the relationship of epidemiological parameters and grain yield under plot-wise assessment across the locations. In this study, both disease severity and AUDPC were used in predicting the yield losses. This was because to look which one more expressible in high yield losses prediction than the other one. Comparing to the two epidemiological parameters, disease severity at the final assessment date showed a higher grain yield loss predictor than AUDPC under crosswise assessment. Also, disease severity at the last date of assessment was better expressible for grain yield loss predictor as indicated by a higher (33.70%) coefficient of determination than AUDPC (27.20%). It was observed that as the disease severity and AUDPC getting higher, the grain yield becoming lower, implying that the higher the disease severity and AUDPC the more grain yield losses. Wheeler (1969), Campbell and Madden (1990), and Agrios (2005) mentioned plant diseases had strongly associated with losses of growth and yield-related traits of the crop in their every portion of disease progression.
In the current study, it was observed that the application of fungicide exceedingly reduced disease pressure and increased grain yield over the unsprayed one across the location. The economic feasibility study showed that the highest and lowest NB and BCR were observed at Bonke and Adiyo, respectively. However, variation in NB and BCR might be affected by not only disease pressure and environmental factors but also the total input costs of production in the locality. CIMMYT (1988) mentioned the high benefits incurred from a given commodity production had strongly affected by the total cost of production, the time the crop produced, and the selling price of the product in the locality. An economic feasibility analysis also revealed the sole application of Tebuconazole before disease onset, followed by mixed application fungicides before disease onset exhibited the highest NB and BCR over the unsprayed and other sprayed plots. The high NB and BCR from the production of wheat with fungicide supplementation could be ascribed to high grain yield, and the low NB and BCR ascribed were attributed to the low grain yield due to high FHB pressures. To this, it was apparent that the uses of Tebuconazole before disease onset because since it showed most profitable over the other treatments and could be suggested for the producers. Similar reports on the profitability of crop field management practices were presented by CIMMYT (1988) and Foster et al. (2017).
Conclusion
The study exhibited the tested treatments across the locations showed significantly varying levels of disease incidence, severity, AUDPC, and yield-related parameters due to interaction effects of fungicides and spray regimes. Results obtained from the present study showed that North Ari and Adiyo were significantly suffered by FHB pressure, and consequently, resulted in the lowest gain yield as compared to the other locations. While minimum FHB pressure and the highest grain yield were observed at Bonke and Chencha. Application of Tebuconazole and Propiconazole + Tebuconazole before disease onset and Tebuconazole after disease onset significantly reduce FHB pressure and increasing grain yield of wheat, followed by mixed application of Propiconazole + Tebuconazole before disease onset. Overall, the evidence obtained from the current study showed that the use of fungicides in in combination with appropriate spray regimes had a pronounced effect in minimizing the epidemics of FHB and increasing grain yield of wheat across the locations. An economic evaluation for the tested treatments showed the application of Tebuconazole before disease onset provided the highest net benefit and benefit–cost ratio compared with the other treatments. Thus, application of Tebuconazole before disease onset could be suggested to the study areas and elsewhere with similar agro-ecological conditions to efficient management of FHB along with appropriate crop husbandry practices. However, mycotoxin production was not study in its quantification and yield quality losses. Therefore, further research focusing on mycotoxin production should be carried out for more effective and reliable management strategies development in FHB across the locations.
References
AFAC (Alberta Fusarium Action Committee) (2012) Alberta Fusarium graminearum management plan. Edmonton: Alberta Agriculture and Rural Development. https://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/agdex5210/$file/110_6323.pdf?OpenElement. [Accessed on 03 March 2019].
Agrios GN (2005) Plant pathology, 5th edn. Academic Press-Elsevier, New York, p 922
Andersen KF, Morris L, Derksen RC, Madden LV, Paul PA (2014) Rainfeastness of prothioconazole + tebuconazole for fusarium head blight and deoxinivalenol management in soft red winter wheat. Plant Dis 98(10):1398–1406. https://doi.org/10.1094/PDIS-01-14-0092-RE
Anteneh A, Asrat D (2020) Wheat production and marketing in Ethiopia: review study. Cogent Food Agric 6(1):1778893. https://doi.org/10.1080/23311932.2020.1778893
Ayele B, Eshetu B, Betelehem B, Bekele H, Melaku D, Asnakech T, Melkamu A, Amare A, Kiros M, Fekede A (2008) Review of two decades of research on diseases of small cereal crops. In: Tadesse A (eds) Increasing crop production through improved plant protection vol. I. Proceedings of 14th Annual Conference of Plant Protection Society of Ethiopia held in 19–22 December 2006 (pp. 375–416). Addis Ababa, Ethiopia. https://doi.org/10.1016/j.fertnstert.2007.02.050
Bekele B, Makkouk KM, Yusuf A, Alemayu F, Lencho A (2001) Occurrence and distribution of barley yellow dwarf virus (BYDV) isolates in central Ethiopia. Int J Pest Manag 47(2):115–119. https://doi.org/10.1080/09670870151130570
Bonfada EB, Honnef D, Friedrich MT, Boller W, Deuner CC (2019) Performance of fungicides on the control of fusarium head blight (Triticum aestivum L.) and deoxynivalenol contamination in wheat grains. Summa Phytopathol 45(4): 374–380. https://doi.org/10.1590/0100-5405/191941
Boyacioglu D, Hettiarachchy NS, Stack RW (1992) Effect of three systemic fungicides on deoxynivalenol (vomitoxin) production by Fusarium graminearum in wheat. Can J Plant Sci 72(1):93–101. https://doi.org/10.4141/cjps92-010
Brown NA, Bass C, Baldwin TK, Chen H, Massot F, Carion PWC, et al (2011) Characterisation of the Fusarium graminearum-wheat floral interaction. J Pathog. Article ID 626345, 9. https://doi.org/10.4061/2011/626345
Caldwell CD, MacDonald D, Jiang Y, Cheema MA, Li J (2017) Effect of fungicide combinations for Fusarium head blight control on disease incidence, grain yield, and quality of winter wheat, spring wheat, and barley. Can J Plant Sci 97(6):1036–1045. https://doi.org/10.1139/cjps-2017-0001
Campbell CL, Madden LV (1990) Temporal analysis of epidemics I. Description and comparison of disease progress curves. In Introduction to plant disease epidemiology (pp. 532). ISBN 0471832367. John Wiley and Son.
CSA (2018) Agricultural sample survey, 2017/2018 (Report on area and production of crops (Private peasant holdings, main season). Statistical Authority, Addis Ababa, Ethiopia. Statistical Bulletin No. 446. Volume 5. 60 pp.
De Villiers CIP (2009). A comparison of screening techniques for Fusarium head blight of wheat in South Africa Faculty of Natural and Agricultural Sciences: University of the Free State Bloemfontein. http://scholar.ufs.ac.za:8080/xmlui/bitstream/handle/11660/848/DeVilliersCIP.pdf?sequence=1. [Accessed on 03 March 2021].
Dean R, van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Marty D, Regine K, Jeff E, Foster GD (2012) The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13(4):414–430. https://doi.org/10.1111/j.1364-3703.2011.00783.x
Éverson BB, Daniela H, Maria TF, Walter B, Carolina CD (2019) Performance of fungicides on the control of fusarium head blight (Triticum aestivum L.) and deoxynivalenol contamination in wheat grains. Summa Phytopathol 45(4):374–380. https://doi.org/10.1590/0100-5405/191941
FAO, IFAD, UNICEF, WFP, WHO (2018) The state of food security and nutrition in the world 2018. (Building climate resilience for food security and nutrition) (pp. 202). FAO. License: CC BY-NC-SA 3.0 IGO. ISBN 978–92–5–130571–3. https://creativecommons.org/licenses/by-nc-sa/3.0/igo.
FAOSTAT (Food and Agriculture Organization of the United Nation) (2018) Agricultural data: production and indices data crop primary. http://www.fao.org/faostat/en/#data/QC/visualize. Accessed at 15 April 2020.
Foster AJ, Lollato R, Vandeveer M, De Wolf ED (2017) Value of fungicide application in wheat production in southwest kansas. Kansas Agric Exp Stat Res Rep 3(5): 8. https://doi.org/10.4148/2378-5977.7385
Galich MT (1989) Importancia y distribucion de la fusariosis del trigo en Argentina. In: Taller sobre la fusariosis de la espiga en America del Sur. Kohli MM (ed). Mexico, D.F. CIMMYT, pp: 7–26
Getachew GM, Shiferaw MA, Zerhun TL, Misgana MS, Kedir BF, Yosef BT, Asaminew AM, Abate GE, Dizgo CC, Agdew BW (2021) Integration of host resistance, fungicides, and spray frequencies for managing Fusarium head blight of bread wheat under field conditions in southern Ethiopia. Heliyon 7:e07938. https://doi.org/10.1016/j.heliyon.2021.e07938
Getachew GM (2020) Management of yellow rust (Puccinia striiformis f.sp. tritici) and stem rust (Pucciniagraminis f.sp tritici) of bread wheat through host resistance and fungicide application in Southern Ethiopia, Cogent Food Agric 6(1): 1739493. https://doi.org/10.1080/23311932.2020.1739493
Ghimire B, Sapkota S, Bahri BA, Martinez-Espinoza AD, Buck JW, Mergoum M (2020) Fusarium head blight and rust diseases in soft red winter wheat in the southeast United States: state of the art, challenges and future perspective for breeding. Front Plant Sci 11:1080. https://doi.org/10.3389/fpls.2020.01080
Gilbert J, Haber S (2013) Overview of some recent research developments in Fusarium head blight of wheat. Can J Plant Pathol 35(2): 149–174. https://doi.org/10.1080/07060661.2013.772921
Gilbert J, Abramson D, Mccallum B, Clear R (2001) Comparison of Canadian Fusarium graminearum isolates for aggressiveness, vegetative compatibility, and production of ergosterol and mycotoxins. Mycopathologia 153:209–215. https://doi.org/10.1023/A:1014940523921
Giraud F, Pasquali M, El Jarroudi, M, Cocco M, Delfosse P, Hoffmann L, Bohn T (2011) Timely fungicide application: A strategy to minimize Fusarium head blight and associated mycotoxin production in winter wheat. J Plant Pathol 93(1): 15 – 18. https://www.jstor.org/stable/41998924
Gome KA, Gomez AA (1984) Statistical procedures for agricultural research (2nd ed). John Wiley and Sons, Inc.
Haidukowski M, Pascale M, Perrone G, Pancaldi D, Campagna C, Visconti A (2004) Effect of fungicides on the development of Fusarium head blight, yield and deoxynivalenol accumulation in wheat inoculated under field conditions with Fusarium graminearum and Fusarium culmorum. J Sci Food Agric 85(2):191–198. https://doi.org/10.1002/jsfa.1965
Homdork S, Fehrmann H, Beck R (2000) Effects of field application of tebuconazole on yield, yield components and the mycotoxin content of Fusarium-infected wheat grain. J Phytopathol 148(1):1–6. https://doi.org/10.1046/j.1439-0434.2000.00460.x
Hoover J (2011) Strategies for managing fusarium head blight in western Canada: a review of literature. In: 7th Canadian Workshop on Fusarium Head Blight. Winnipeg, MB. November 27–30, 2011. Proceedings available at: http://www.cwfhb.org/programs/7_CWFHB_2011_Winnipeg.pdf
Hutcheon J, Jordan V (1992) Fungicide timing and performance for Fusarium control in wheat. Brighton crop protection conference, pests and diseases-1992. Vol. 2. British Crop Protection Council, Alton, UK. ISBN: 0948404655, pp: 633–638.
International Maize and Wheat Improvement Center (CIMMYT) (1988). Farm agronomic data to farmer recommendations: an economics training manual. Completely Revised Edition. DF: CIMMYT, Mexico. ISSN: 968-6127-18-6, pp 124
Jones RK (2001) Assessments of fusarium head blight of wheat and barley in response to fungicide treatment. Plant Dis 84(9):1021–1030. https://doi.org/10.1094/PDIS.2000.84.9.1021
Karasi M, Jorge DS, Pierce Al (2016) Fusarium head blight or head scab of wheat, barley and other small grain crops. In: Agriculture and Natural Resources, Ohio State University, USA. Online. https://ohioline.osu.edu/factsheet/plpath-cer-06 (Acceessed on 27 February 2021).
Lenc L (2015) Fusarium head blight (FHB) and Fusarium populations in grain of winter wheat grown in different cultivation systems. J Plant Prot Res 55(1):94–109. https://doi.org/10.1515/jppr-2015-0013
Lenc L, Czecholiński G, Wyczling D, Turów T, Kaźmierczak A (2015) Fusarium head blight (FHB) and Fusarium spp. on grain of spring wheat cultivars grown in Poland. J Plant Prot Res 55(3): 266–277. https://doi.org/10.1515/jppr-2015-0038
Liggitt J, Jenkinson P, Parry DW (1997) The role of saprophytic microflora in the development of Fusarium ear blight of winter wheat caused by Fusarium culmorum. Crop Prot 16(7):679–685. https://doi.org/10.1016/s0261-2194(97)00039-2
Martin RA, Johnston HW (1982) Effects and control of Fusarium diseases of cereal grains in the Atlantic Provinces. Can J Plant Pathol 4(2):210–216. https://doi.org/10.1080/07060668209501327
Matny ON (2015) Fusarium head blight and crown rot on wheat and barley: losses and health risks. Adv Plants Agric Res 2(1): 2–7. https://doi.org/10.15406/apar.2015.02.00039
McMullen M, Jones R, Gallenberg D (1997) Scab of wheat and barley: a reemerging disease of devastating impact. Plant Dis 81(12):1340–1348. https://doi.org/10.1094/PDIS.1997.81.12.1340
McMullen M, Bergstrom G, De Wolf E, Dill-Macky R, Hershman D, Shaner G et al (2012) A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis 96(12):1712–1728. https://doi.org/10.1094/PDIS-03-12-0291-FE
Megumi Y, Takashi N, Michiyoshi A, Fumihiko S, Kenta T (2008) Effect of the timing of fungicide application on fusarium head blight and mycotoxin accumulation in closed-flowering barley. Plant Dis 92(8):1164–1170. https://doi.org/10.1094/PDIS-92-8-1164
Mesterhazy A, Bartok T (1996) Control of Fusarium head blight of wheat by fungicides and its effect on the toxin contamination of the grains. Pflanzenschutz-Nachrichten Bayer 49(2):181–198
Mills K, Salgado J, Paul PA (2016) Fusarium head blight or head scab of wheat, barley and other small grain crops. CFAES Publishing, Ohio State University, Columbus. https://ohioline.osu.edu/factsheet/plpath-cer-06
MoANR (Ministry of Agriculture and Natural Resources) and EATA (Ethiopian Agricultural Transformation Agency) (2016) Soil fertility status and fertilizer recommendation Atlas of the southern nations, nationalities and peoples’ regional state, Ethiopia. ATA.
MoANR (Ministry of Agriculture and Natural Resources) and EATA (Ethiopian Agricultural Transformation Agency) (2018) Crop production and development package. In: Amharic Version (pp 215). Ministry of Agriculture.
Murray TD, Parry DW, Cattlin LD (2009) Diseases of small grain cereal crops: a colour handbook. Manson Publishing Ltd, London, pp: 2–4. https://doi.org/10.1201/b15911
Muthomi JW, Ndungu JK, Gathumbi JK, Mutitu EW, Wagacha JM (2008) The occurrence of Fusarium species and mycotoxins in Kenyan wheat. Crop Prot 27(8):1215–1219. https://doi.org/10.1016/j.cropro.2008.03.001
Nielsen LK, Cook DJ, Edwards SG, Ray RG (2014) The prevalence and impact of Fusarium head blight pathogens and mycotoxins on malting barley quality in UK. Int J Food Microbiol 179(100):38–49. https://doi.org/10.1016/j.ijfoodmicro.2014.03.023
Parry DW, Jenkinson P, McLeod L (1995) Fusarium ear blight (scab) in small grain cereals: a review. Plant Pathol 44(2):207–238. https://doi.org/10.1111/j.1365-3059.1995.tb02773.x
Pirgozliev SR, Edwards SG, Hare MC, Jenkinson P (2003) Strategies for the control of Fusarium head blight in cereals. Eur J Plant Pathol 109:731–742. https://doi.org/10.1023/A:1026034509247
Pirgozliev SR, Ray RV, Edwards SG, Hare MC, Jenkinson P (2008) Effect of timing of fungicide application on the development of fusarium head blight and the accumulation of deoxynivalenol (DON) in winter wheat grain. Cereal Res Commun 36(2):289–299. https://doi.org/10.1556/CRC.36.2008.2.9
Reis EM, Boareto C, Danelli ALD, Zoldan SM (2016) Anthesis, the infectious process and disease progress curves for fusarium head blight in wheat. Summa Phytopathol 42(2):134–139. https://doi.org/10.1590/0100-5405/2075
Robert GD, James HT (1991) A Biomerical approach. Principles of statistics, 2nd ed. McGraw-Hill College. New York, USA. ISBN-10: 0070610282; ISBN-13: 978–0070610286.
Robert WS, Marcia PM (2011) A visual scale to estimate severity of fusarium head blight in wheat. NDSU extension service. North Dakota State University, Fargo, North Dakota, USA, pp 2. https://www.ag.ndsu.edu/ndipm/publications/wheat/documents/pp1095.pdf. (Accessed on 17 March 2018).
SAS (Statistical Analytical Software) Institute Inc (2009) SAS/stat guide for personal computers, version 9.2 edition. SAS Institute Inc., Cary
Shaner G (2003) Epidemiology of Fusarium head blight of small grain cereals in North America. In: Leonard KJ, Bushnell WR (eds) Fusarium head blight of wheat and barley. APS Press, St. Paul, pp 84–119
Shude SPN, Yobo KS, Mbili NC (2020) Progress in the management of Fusarium head blight of wheat: an overview. South Afr J Sci 116(11/12): 7. Art. #7854. https://doi.org/10.17159/sajs.2020/7854
Sutton JC (1982) Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Can J Plant Pathol 4(2):195–209. https://doi.org/10.1080/07060668209501326
Taran SA, Kakar MS, Bugti RA (1998) Performance of maize varieties/hybrids under irrigated conditions of Balochistan. Sarhad J Agric 14(2): 113–116. Avaiable at: http://agris.fao.org/agris-search/search.do?recordID=PK1998000486.
Tewodros TW, Sunil K, Gabriel BS, Abera T, Alemu L (2016) Spatial prediction of wheat septoria leaf blotch (Septoria tritici) disease severity in Central Ethiopia. Ecol Inform 36:15–30. https://doi.org/10.1016/j.ecoinf.2016.09.003
Trail F, Xu H, Loranger R, Gadoury D (2002) Physiological and environmental aspects of ascospore discharge in Gibberella zeae (anamorph Fusarium graminearum). Mycologia 94(2):181–189. https://doi.org/10.1080/15572536.2003.11833223
USDA (United State Department of agriculture) (2018) Foreign agricultural service: World agricultural production global analysis (World agricultural supply and demand report). 31. Circular series WAP 11–18, DC 20250–1051. Foreign Agricultural Service/USDA
van der Plank JE (1963) Plant Diseases: epidemics and control. Academic Press, London, UK, 349 pp
Wheeler BJ (1969) An Introduction to Plant Diseases. John Wiley and Sons, Inc. Mitton, P. J. 2000. 374 pp
Wilcoxson RD (1996) Fungicides for control of Fusarium head blight—a review. Minn Agric Exp Stn. Paper no. 22,507. St. Paul.
Wilcoxson RD, Kommedahl T, Ozmon EA, Windels CE (1988) Occurrence of Fusarium species in scabby wheat from Minnesota and their pathogenicity to wheat. Phytopathology 78(5):586–589. https://doi.org/10.1094/Phyto-78-586
Windels CE (2000) Economic and social impacts of Fusarium head blight: changing farms and rural communities in the Northern Great Plains. Phytopathology 90(1):17–21. https://doi.org/10.1094/PHYTO.2000.90.1.17
Zadoks JC, Chang TT, Kanzak CF (1974) A decimal code for the growth stage of cereals. Weed Res 14(6):415–421. https://doi.org/10.1111/j.1365-3180.1974.tb01084.x
Zegeye T, Taye G, Tanner D, Verkuijl H, Agidie A, Mwangi W (2001) Adoption of improved bread wheat varieties and inorganic fertilizer by small scale farmers in Yelmana Densa and Farta Districts of Northwestern Ethiopia (29 pp). Ethiopian Agricultural Research Ogranization [EARO] and CIMMYT.
Zewdie B, Paul CS (2013) Farmer’s seed sources and seed quality: 2. seed health. Int J Plant Prod 7(4): 637–657. https://www.researchgate.net/publication/289842794
Acknowledgements
The authors are grateful to the Southern Agricultural Research Institute for financial support of the field experiments. The authors are also acknowledged the staff of the Crop Research Work Process and the drivers in each respective Agricultural Research Center under institution for their facilitation and technical assistance during conducting the experiments. Last but not least heartfelt appreciation goes to Tahir Hajdi Mohammed (MSc) for his willingness to drawing the map of the experimental locations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Mengesha, G.G., Abebe, S.M., Fedilu, K.B. et al. Fusarium head blight progression and yield response of bread wheat as affected by fungicides and spray regimes under field condition in southern Ethiopia. J. Crop Sci. Biotechnol. 25, 565–582 (2022). https://doi.org/10.1007/s12892-022-00152-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12892-022-00152-6