Abstract
Many plant products from medicinal herbs are historically important for the treatment and prevention of diseases. Medicinal plants are showing a wide range of activities like antioxidant, anti-inflammatory, and anti-aging. This study was investigated to evaluate the phenolic content, DPPH, ABTS radical scavenging rate, and anti-inflammatory activity from Codonopsis lanceolata (CL) root extract. Total polyphenol and flavonoid content showed the highest amount in 30% EtOH extract of CL and followed by 90 °C hot water extract. The DPPH, ABTS radical scavenging activity was also found that the increase was proportional to the concentration, the highest amount in 30% EtOH extract. Cell viability of RAW 264.7 cell for CL extracts was not significantly changed by extracts up to a concentration of 200 µg/mL, and it was confirmed that the macrophage cell showed little toxicity. LPS-treated experimental group significantly inhibited NO production. The effect of extracts of CL against inflammatory mediators (TNF-α, IL-6, and IL-1β) production showed that the anti-inflammatory activity was most excellent in 30% EtOH. These results suggested that CL extracts tested here have potential anti-inflammatory and antioxidant activity, and these effects are differences depending on the extraction solvent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Codonopsis lanceolata (CL) is well known to affect various pharmacological effects on human health, and its consumption is increasing. The roots of CL have been used as an herbal drug for the treatment of bronchitis, cough, spasm, and inflammation in China, and as a tonic crude drug and an edible plant in Korea (Jiangsu New Medical College 1977), and mainly contain triterpenoid saponins including codonolaside, codonolaside I-V, lancemaside A–G. Their saponins have shown anti-inflammatory effects such as bronchitis and cough, insomnia, and hypomnesia. Lancemaside A, which is a primary constituent of CL was reported to potently inhibit LPS-stimulated, TLR-4-linked NF-jB activation of 293-hTLR4-hemagglutinin (HA) cells (Joh et al. 2010). As an herb, CL is widely used in food preparation, but its therapeutic application has not been explored yet in Korea (Wang et al. 2011). Codonoposide, a triterpenoid saponin, was reported as a characteristic constituent of CL roots and considered to be partially responsible for the therapeutic effects of this crude drug (Lee et al. 2002). Recently, plant and plant-derived products have treated a part of the healthcare system by applying the bioactive phytochemicals. Screening of antioxidant and anti-inflammatory activity from medicinal plants has received much attention throughout the world. Antioxidant compounds in food play an important role as a health-protecting factor. Most of the antioxidant compounds in a typical diet are derived from plant sources and belong to various classes of compounds with a wide variety of physical and chemical properties. The main characteristic of an antioxidant is its ability to eliminate free radicals. Highly reactive free radicals and oxygen species are present in biological systems from a wide variety of sources. Most of the antioxidant compounds in a typical diet are derived from plant sources and belong to various classes of compounds with a wide variety of physical and chemical properties. The main characteristic of an antioxidant is its ability to eliminate free radicals. Highly reactive free radicals and oxygen species are present in biological systems from a wide variety of sources. Medicinal plants including CL are excellent sources of anti-inflammatory agents. Many infectious diseases have been known to be treated with herbal extracts. The evaluation of antioxidant activity and anti-inflammatory property of the CL root is of great interest and importance. The suppressive effect of various plant extracts on the production of proinflammatory cytokines such as TNF-α, IL-6, and IL-1β has been extensively studied in vitro (Choi et al. 2016; Ju et al. 2010; Kim et al. 2018). Recent interests in the study of functional plants have focused on their potential benefits to human health. The functional plants have been used as traditional medicine, but scientific evaluation is still lacking in this regard. To develop functional products using the physiological functionality of CL, specific proof data based on scientific experiments are needed. Therefore, this study was focused on evaluating of phenolic compound content, anti-inflammatory and antioxidant activity of CL in the different extract solvent for biological search on plant-based anti-inflammatory and antioxidant agents.
Materials and methods
Growth condition of CL and sampling
The roots of CL plant were collected from Jeju region, Korea and 3-year-aged roots were used to carry out the present study. The roots of CL plant were freeze-dried and then ground to a fine powder. Each sample powder was stored at − 20 °C for experiments. To ensure safety as a food material, the sample was extracted with low concentration EtOH (alcohol for food) and hot water. The freeze-dried extract powder was immersed in 30% EtOH, distilled water (DW) and the filtrate was collected thrice with constant stirring of the mixture at every 24 h interval for 72 h. The 70 °C, 90 °C hot water extraction was carried out two times repeatedly for 6 h using a hot water extractor with a vertical reflux condenser (Custom-made equipment). The filtrate was then concentrated under reduced pressure at 45 °C using a vacuum rotary evaporator (IKA® RV 10 Basic Digital, IKA Co., Munich, Germany). The concentrated extract was stored at − 20 °C until further analysis.
Total polyphenol determination
Total phenols were determined by the modified method the Folin-Ciocalteu assay (Singleton and Rossi 1965). Freeze-dried samples were extracted with methanol, the extract was concentrated under reduced pressure, and freeze-dried in powder. Freeze-dried powder (1 mg) dissolved in 95% methanol and 500 µL of Folin-Ciocalteu reagent were added to a 25 mL volumetric flask and were mixed for 5 min at 30 °C in a water bath. 500 µL saturated solution of 7.5% Na2CO3 was added to the mixture, and then was incubated for 1 h at room temperature, and the absorbance was read at 725 nm using a spectrophotometer (Libra S22, Biochrom Co., Cambridge, England). Total phenolic of the sample was expressed as mg chlorogenic acid equivalent in 1 g dry weight of the sample extract.
Total flavonoid determination
Total flavonoid was measured using the modified method that previously described (Zhishen et al. 1999). Briefly, freeze-dried samples (1 mg) dissolved in 95% methanol, and 1 mL of extract solution, 10 mL diethylene glycol and 0.1 mL 1 N NaOH were added to a 25 mL volumetric flask. The mixture was incubated for 1 h at 37 °C in a water bath. The absorbance was measured at 420 nm using a spectrophotometer (Libra S22, Biochrom Co., Cambridge, England). Total flavonoid of the samples was expressed as mg narincin equivalent in 1 g dry weight of the sample extract.
Assay of DPPH radical scavenging rate
One hundred µL of various concentrations (0.5, 1, 2.5, 5 and 10 mg/mL) of extracts in CL were added to 900 µL of 100% methanol containing 100 µM DPPH, and the reaction mixture was shaken for 5 min in the slight vortex. Leaving room temperature for 30 min under darkness, the absorbance of DPPH was determined by spectrophotometer at 517 nm. The DPPH radical scavenging activity was calculated according to the following equation:
where A is the absorbance at 517 nm without pigment compositions and B is the change in absorbance at 517 nm with pigment compositions incubation (Brand-Williams et al. 1995).
Assay of ABTS radical scavenging rate
The spectrophotometric analysis of ABTS (2,2′-azinbis-(3-ethyl-benzothiazoline-6-sulfonic acid) radical cation (ABTS•+) scavenging activity of C. lanceolata was determined according to the method described previously (Re et al. 1999). ABTS solution (7 mM) was mixed with potassium persulfate (2.45 mM), and the mixture was incubated in the dark at room temperature for 15 h, and then diluted to the absorbance of 0.7 at 734 nm. Fifty µL of each sample prepared in different concentrations with 950 µL diluted solution was added and was shaken for 10 s by vortex mixer, and then was reacted for 5 min at room temperature, and the absorbance was read at 734 nm using a spectrophotometer (Biochrom Co., England). The ABTS•+ scavenging activity showed as RAEAC (relative ascorbic acid equivalent antioxidant capacity), was calculated by the following equation:
ΔAaa is the change of the absorbance after the addition of ascorbic acid, Caa is the concentration of ascorbic acid, ΔAs is the change of the absorbance after addition of sample solution, Cs is the concentration of the sample.
Evaluation of in vitro anti-inflammatory activity
Cell culture
RAW 264.7 cell line is a mouse monocyte-macrophage cell line established from the ascites of a tumor induced in the male mouse. The RAW264.7 cells were purchased from Korean Cell Line Bank (KCLB) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 1% antibiotic–antimycotic. The cells were maintained in a 5% CO2 incubator at 37 ºC.
Assay of cell viability
The cytotoxicity against RAW 264.7 cell of CL extracts was experimented using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) conversion assay. MTT method involves the conversion of MTT to colored formazan via intact mitochondria of the cells. The RAW 264.7 cells were plated at a density of 1 × 104 cells/well in a 96-well plate for 24 h and followed by the addition with various concentrations (50, 100, 200 and 400 µg/mL) of CL extract. After 16 h incubation, 50 µg/mL of MTT solution (5 mg/mL) was added to each well and allowed to stand for 4 h at 37 ºC. The medium was carefully removed so that the formazan produced by reduction of MTT did not fall off the medium, and then 300 µg/mL DMSO allowed the formazan crystals to dissolve. Absorbance was measured at 540 nm using UV–visible microplate reader (Bio-Rad xMark, USA). The cell viability was calculated as sample O.D./blank O.D. × 100(%).
Measurement of LPS-induced NO (nitric oxide) production
RAW 264.7 cells were cultured in a 96-well plate and incubated for 18 h at 37 ℃ in 5% CO2. The cells were then incubated for 18 h after treatment of 0.5 µg/mL LPS (Sigma, St. Louis. MO. USA) at each sample. The NO content was determined by Griess reagent system. The RAW 264.7 cells were seeded at a density of 5 × 104 cells/well in 6 well plate for 24 h, and then the cells were pre-treated with 0.5 µg/mL of LPS and added to various concentrations (50, 100 and 200 µg/mL) of the CL extract. After the cells were incubated for 16 h, the next process about measuring NO levels was led by the Griess reagent system’s protocol using supernatant fluid. Absorbance was determined at 540 nm using UV–visible microplate reader (Bio-Rad xMark, USA).
Assay for cytokines
Murine RAW264.7 peritoneal macrophage cells were cultured at a density of 1 × 104 cells/well in 6-well plates for 24 h. They were then washed with phosphate buffered saline (PBS) and treated with various concentrations (50, 100 and 200 µg/mL) of CL extract with 0.5 µg/mL LPS for 24 h. Supernatants were collected, and levels of IL-1β, IL-6, and TNF-α released into the culture supernatants were measured using a commercially available cytokine ELISA test kits (all from Bender R&D Systems, Inc.), according to the manufacturer’s instructions.
Data analysis
The statistical analysis was performed using the procedures of the Statistical Analysis System (SAS version 9.1, SAS Institute Inc., Cary, NC, USA). The analysis of variance (ANOVA), followed by Duncan’s multiple range tests was used to determine the significant difference (p < 0.05) between the treatment means.
Results and discussion
Total polyphenol and flavonoid contents
Polyphenols are widely distributed in plants, and phenolic antioxidants have been found to act as free radical scavengers as well as metal chelators (Sanchez-Moreno et al. 1999; Shahidi and Wanasundara 1992). It has been recognized that the compounds such as polyphenol, flavonoid show antioxidant activity and their effects on human nutrition and health are considerable. The results for total polyphenol and total flavonoid content in the CL extracts are presented in Figs. 1 and 2. Total polyphenol content by various solvents decreased in 30% EtOH > 90 °C hot water > 70 °C hot water > DW extract order. The total flavonoid content also showed the highest amount in 30% EtOH extract of CL and followed by 90 °C hot water extract, DW extract, and 70 °C hot water extract. These results were considerably consistent with the finding of antioxidant activity. Several studies have focused on the biological activities of phenolic compounds, which are potent antioxidants and free radical scavengers (Marja et al. 1999; Rice-Evans et al. 1995; Sugihara et al. 1999). Zhou and Yu (2006) also reported that total phenolic content of the tested vegetable extracts was correlated with the DPPH radical scavenging activity, suggesting that total phenolics can play a major role in the antioxidant activity of plant materials. The high phenol and flavonoids contents of CL root extract may cause high antioxidant activity of this plant.
DPPH radical scavenging activity
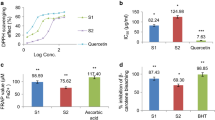
Table 1 shows the DPPH free radical scavenging activities of the extracts in a concentration-dependent manner. The extract obtained by 30% EtOH showed the highest DPPH radical scavenging activity at a concentration ranging from 0.5 to 10 mg/mL. All extracts obtained using 30% EtOH and 90 °C hot water solvent were higher radical scavenging activity than that of the water and 70 °C hot water extract. The investigation of the antioxidant activity of natural substances is based on the measuring of the electron donor capacity of DPPH with the ability to inhibit the oxidation by donating electrons in free radicals causing this lipid peroxidation (Boo et al. 2012), that is, free radical is known to be a significant factor in biological damages, and DPPH has been used to evaluate the free radical-scavenging activity of natural antioxidants (Kim et al. 2017; Yokozawa et al. 1998; Zhu et al. 2001). Active oxygen caused by in vivo metabolism removed by the body's antioxidant system, but excessive free radicals induced stress, causing the lipid peroxidation by combining with unsaturated fatty acids in the cell membrane, and brought intracellular structural and functional damage. The effective source of CL could be employed in all medicinal preparation to combat myriad diseases associated with oxidative stress. Phenolics were the main antioxidant components, and their total contents were directly proportional to their antioxidant activity (Liu et al. 2009). In this study, there is a correlation between total phenolic content and antioxidant activity of CL extracts. That is, 30% EtOH and 90 °C hot water solvent extracts showed high antioxidant activity, and this results also showed that total phenolics level was highly related to the free radical scavenging activity.
ABTS radical scavenging activity
The samples extracted from CL root using four different solvents were tested in this study for ABTS radical scavenging activity. ABTS radical scavenging methods are common spectrophotometric procedures for determining the antioxidant capacities of components (Gülcin et al. 2010). ABTS assay is an excellent tool for assessing the antioxidant activity of hydrogen‐donating antioxidants and chain‐breaking antioxidants (Leong and Shui 2002). The ABTS and DPPH systems provide information on the reactivity of a test sample with a stable free radical. The results of the ABTS radical scavenging activity were shown in Table 2. The ABTS radical scavenging activity was progressively increased in a dose-dependent manner. Our data indicate that the CL extracts were useful scavengers against the ABTS radical species and that it was a similar tendency to the results in DPPH radical scavenging activity. Therefore, the ABTS radical scavenging activity of CL extract indicates its ability to scavenge free radicals, thereby preventing oxidation via a chain‐breaking reaction. Radical scavenging activities are vital due to the deleterious role of free radicals in foods and biological systems. The present investigation demonstrated that the CL extract has potent activity against ABTS.
Cytotoxicity on RAW 264.7 cell
In the RAW 264.7 cell, the cytotoxic effect of CL extracts on cell viability was assessed by the MTT assay. When cells were treated for 2 days with various concentrations (50, 100, 200, 400 and 800 µg/mL) of extracts, the rate of cell survival progressively decreased in a dose-dependent manner (Table 3). Cell viability of CL extract was not significant changed by extracts up to a concentration of 200 µg/mL. However, the cell viability tended to decrease somewhat at concentrations above 400 µg/mL. Thus, a concentration of less than 400 µg/mL of CL extract without cell cytotoxicity was chosen in subsequent anti-inflammatory experiments.
NO production
NO is an inorganic free radical that acts as a signaling molecule with multiple biological functions in vertebrates (Rockel et al. 2002). Macrophages are involved in the innate immune response through phagocytosis or production of a variety of compounds, like cytokines or NO (Dempsey et al. 2003). Figure 3 shows the NO responses to the CL root extract in macrophage cell line. Because of measuring the production of NO in the supernatant after 24 h of pretreatment with CL extracts and stimulating with LPS, the production of NO was remarkably increased in the group treated with LPS alone compared to the group treated with CL extracts. After CL extract pretreatment at 50, 100, and 200 µg/mL, LPS-treated experimental group significantly inhibited NO production in a dose-dependent manner. NO production at 200 µg/mL concentration of CL extract showed the LPS 25.36 μM, 30% EtOH 5.22 μM, DW 7.16 μM, 70 °C hot water 8.98 μM, 70 °C hot water 8.51 μM (Fig. 3). NO has diverse physiological roles and contributes to the immune defense against pathogens (Singh et al. 2000). The root extract of CL significantly inhibited LPS induced NO production in mouse macrophage cells, RAW.264.7, at 100 and 200 µg/mL concentrations. NO is a reactive free radical that can produce the toxic compound peroxynitrite; high NO concentrations exert deleterious effects on lipids, DNA, and proteins, similar to that observed with oxygen-derived species such as hydroxyl radicals. NO is involved in several types of acute and chronic inflammation (Ialenti et al. 1993), including rat carrageenin pleurisy (Sautebin et al. 1998) and zymosan-induced peritonitis in mice (Ajuebor et al. 1998). Large amount of NO produced by iNOS is believed as one of the most critical inflammatory reactions in activated macrophage (Pokharel et al. 2007). Arctigenin blocked LPS-induced various responses of macrophage including the NO overproduction and the release of pro-inflammatory cytokines TNF-α and IL-6 (Zhao et al. 2009). In brief, our study suggests the immunosuppressive potential of CL extract, and in particular, these effects are differences depending on the extraction solvent.
Cytokine production
Because of the growing recognition of cytokine activities, altering cytokine expression and targeting their receptors may offer therapeutic potential. The in vitro and in vivo research demonstrates that the reviewed botanical medicines modulate the secretion of multiple cytokines (Kevin Spelman et al. 2006). We have conducted experiments to verify that the CL extracts according to the extraction solvent have anti-inflammatory activity. We used LPS-stimulated macrophages as a model for testing in vitro root extracts of CL according to extraction solvent for anti-inflammatory activity. The concentrations of TNF-α, IL-6, and IL-1β, in 100 µL of cell supernatant each, were determined by ELISA assay according to the manufacturer’s protocol. Macrophages were treated with LPS, and the concentrations of secreted IL-1β, IL-6, and TNF-a in the supernatant were determined. We investigated the effect of CL extracts on the expression of various pro-inflammatory and inflammatory cytokines induced by LPS in RAW 264.7 cells. After pretreatment with CL extract, the cell supernatants were measured by ELISA after 24 h of stimulation with LPS. As a result, TNF-α production at 200 µg/mL concentration of CL extract showed the LPS 104.25 pg/mL, 30% EtOH 23.72 pg/mL, DW 39.45 pg/mL, 70 °C hot water 42.07 pg/mL, 90 °C hot water 35.15 pg/mL, and it was significantly inhibited in a concentration-dependent manner (Fig. 4). IL-6 production at 200 µg/mL concentration of CL extract showed the LPS 102.38 pg/mL, 30% EtOH 38.42 pg/mL, DW 47.45 pg/mL, 70 °C hot water 50.48 pg/mL, 90 °C hot water 49.21 pg/mL (Fig. 5), and IL-1β production at 200 µg/mL concentration of CL extract showed the LPS 257.06 pg/mL, 30% EtOH 79.43 pg/mL, DW 105.91 pg/mL, 70 °C hot water 97.44 pg/mL, 90 °C hot water 98.70 pg/mL (Fig. 6). The CL extracts showed a considerable range of influence on cytokine secretion. The treatment results of CL extract by different extraction solvent showed that the anti-inflammatory activity was most excellent in 30% EtOH as a whole. The inflammatory mediators TNF-α, IL-6 and IL-1β, are known to regulate the inflammatory response in both in vivo and in vitro. These cytokines are known to interact with each other and are reported to be induced by inflammatory stimuli such as LPS (Feldmann et al. 1996). LPS is known to induce the secretory activation of inflammatory cytokines by activating NF-κB in RAW 264.7 cells (Karin and Ben-Neriah 2000; Willeaume et al. 1996). TNF-α and IL-1β are proinflammatory molecules whose secretion can be potently induced by lipopolysaccharide (Alexander and Rietschel 2001). Inflammation not only plays a role in the inflammatory diseases but also in the progression of cancer. Several inflammatory stages have been shown to predispose patients to cancer, such as inflammatory bowel disease, predisposing patients to colorectal cancer, H. pylori-induced gastritis to gastric cancer, or prostatitis to prostate cancer (Balkwill et al. 2005). The present study demonstrates improved anti-inflammatory response in an LPS-stimulated macrophage model upon treatment with root extract of CL via reduction of IL-6 or TNF-a production, enhancement of IL-1β production, or reduction of expression of NO. These results indicate that CL extracts tested here may have potential anti-inflammatory activity. However, numerous and in-depth studies should be carried out for this purpose.
References
Ajuebor MN, Virag L, Flower RJ, Perretti M, Szabo C (1998) Role of inducible nitric oxide synthase in the regulation of neutrophil migration in zymosan-induced inflammation. Immunology 95:625–630
Alexander C, Rietschel ET (2001) Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res 7:167–202
Balkwill F, Charles KA, Mantovani A (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7(3):211–217
Boo HO, Shin JH, Shin JS, Choung ES, Bang MA, Choi KM, Song WS (2012) Assessment on antioxidant potential and enzyme activity of some economic resource plants. Korean J Plant Res 25(3):349–356
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Food Sci Technol 28:25–30
Choi EY, Heo SI, Kwon YS, Kim MJ (2016) Anti-oxidant activity and anti-inflammatory effects of Spiraea fritschiana Schneid extract. Korean J Med Crop Sci 24(1):31–37
College JNM (1977) Dictionary of Chinese materia medica. Shanghai Science and Technology Publisher, Shanghai, pp 195–196
Dempsey PW, Vaidya SA, Cheng G (2003) The art of war: innate and adaptive immune response. Cell Mol Life Sci 60:2604–2621
Feldmann M, Brennan FM, Maini RN (1996) Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 14:397–440
Gülcin I, Huyut Z, Elmastas M, Aboul-Enein A (2010) Radical scavenging and antioxidant activity of tannic acid. Arab J Chem 3:43–53
Ialenti A, Moncada S, Di Rosa M (1993) Modulation of adjuvant arthritis by endogenous nitric oxide. Br J Pharmacol 110:701–706
Joh EH, Lee IA, Han SJ, Chae SJ, Kim DH (2010) Lancemaside A ameliorates colitis by inhibiting NF-κB activation in TNBS-induced colitis mice. Int J Colorectal Dis 25:545–551
Ju JH, Kim JS, Kang SS, Son KH, Chang HW, Kim HP (2010) Anti-inflammatory and anti-arthritic activity of total flavonoids of the roots of Sophora flavescens. J Ethnopharmacol 127(3):589–595
Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination: the control of NF-kB activity. Annu Rev J Immunol 18:621–663
Kim ID, Dhungana SK, Kim HR, Shin DH (2017) Quality characteristics and antioxidant potential of seeds of native Korean persimmon genotypes. Korean J Plant Res 30(6):670–678
Kim MS, Kim N, Kwon SJ, Kim HR, Lee DY, Oh MJ, Kim HJ, Lee CH, Oh CH (2018) Anti-inflammatory and immune regulatory effects of Aucklandia lappa Decne 70% ethanol extract. Korean J Med Crop Sci 26(1):8–18
Lee KT, Choi J, Jun WT, Nam JH, Jung HJ, Park HJ (2002) Structure of a new echinocystic acid bisdesmoside isolated from Codonopsis lanceolata roots and the cytotoxic activity of prosapogenins. J Agri Food Chem 50:4190–4193
Leong LP, Shui G (2002) An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem 76:69–75
Liu SC, Lin JT, Wang CK (2009) Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis sonn.) flowers. Food Chem 114:577–581
Marja PK, Anu IH, Heikki JV, Jussi-Pekka R, Kalevi P, Tytti SK, Marina H (1999) Antioxidant activity of plant extracts containing phenolic compounds. J Agri Food Chem 47:3954–3962
Pokharel YR, Liu QH, Oh JW, Woo R, Kang KW (2007) 4-Hydroxykobusin inhibits the induction of nitric oxide synthase by inhibiting NF-kB and AP-1 activation. Biol Pharm Bull 30:1097–1101
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Evans CR (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med 26(9–10):1231–1237
Rice-Evans C, Miller NJ, Bolwell GP, Bramley PM, Pridham JB (1995) The relative antioxidants activities of plant-derived polyphenolic flavonoids. Free Radic Res 22:375–383
Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53(366):103–110
Sanchez-Moreno C, Larrauri JA, Saura-Calixto F (1999) Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res Int 32:407–412
Sautebin L, Ialenti A, Ianaro A, Di Rosa M (1998) Relationship between nitric oxide and prostaglandins in carrageenin pleurisy. Biochem Pharmacol 55:1113–1117
Shahid F, Wanasundara PKJPD (1992) Phenolic antioxidants. Crit Rev Food Sci Nutr 32:67–103
Singh VK, Mehrotra S, Narayan P, Pandey CM, Agarwal SS (2000) Modulation of autoimmune diseases by nitric oxide. J Immunol Res 22:1–19
Singleton V, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Amer J Enol Viti 16:144–158
Spelman K, Burns JJ, Nichols D, Winters N, Ottersberg S, Tenborg M (2006) Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Altern Med Rev 11(2):128–150
Sugihara N, Arakawa T, Ohnishi M, Furuno K (1999) Anti and pro-oxidative effects of flavonoids on metal induced lipid hydroperoxide-dependent lipid peroxidation in cultured hepatocytes located with α-linolenic acid. Free Radical Biol Med 27:1313–1323
Wang L, Xu ML, Hu JH, Rasmussen SK, Wang MH (2011) Codonopsis lanceolata extract induces G0/G1 arrest and apoptosis in human colon tumor HT-29 cells - Involvement of ROS generation and polyamine depletion. Food Chem Toxicol 49(1):149–154
Willeaume V, Kruys V, Mijatovic T, Huez G (1996) Tumor necrosis factor-alpha production induced by viruses and by lipopolysaccharides in macrophages: similarities and differences. J Inflamm 46:1–12
Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka GI, Nishioka I (1998) Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2-picrylhydrazyl radical. Biochem Phamacol 56:213–222
Zhao F, Wang L, Liu K (2009) In vitro anti-inflammatory effects of arctigenin, a lignan from Arctium lappa L., through inhibition on iNOS pathway. J Ethnopharacol 122:457–462
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Zhou K, Yu L (2006) Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. LWT-Food Sci Technol 39(10):1155–1162
Zhu N, Wang M, Wei GJ, Lin JK, Yang CS, Ho CT (2001) Identification of reaction products of (-)-epigallocatechin, (-)-epigallocatechin gallate and pyrogallol with 2,2-diphenyl-1- picrylhydrazyl radical. Food Chem 73:345–349
Acknowledgements
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through High Value-added Food Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (Grant No. 114036-04-4-SB010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Boo, HO., Park, JH., Kim, HH. et al. Effect of extraction solvent on in vitro anti-inflammatory, antioxidant activity, total phenol and flavonoid contents in Codonopsis lanceolata. J. Crop Sci. Biotechnol. 24, 127–136 (2021). https://doi.org/10.1007/s12892-020-00062-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12892-020-00062-5