Abstract
The influence of sputtered Pt40Pd57Al3 thin films of varying thickness, in the as-deposited (AD) and annealed (AN) state, was investigated for the electro-oxidation of aqueous SO2. From physical characterisation (scanning electron microscopy (SEM) and grazing incidence X-ray diffraction (GIXRD)), significant differences were found in the morphology and crystallinity between AD and AN samples. In terms of electrochemical activity, the current output for the AD and AN thin films decreased as the thickness increased, whilst the AN thin films in general resulted in lower current outputs. No specific trend was observed for stability in an acidic environment and a 60 nm Pt40Pd57Al3 thin film proved to be the optimum thickness for aqueous SO2 electro-oxidation. In addition, the 60 nm Pt40Pd57Al3 AN thin film outperformed pure Pt (60 nm, AD and AN) in terms of current density and stability, emphasising the fact that multicomponent electrocatalysts can be superior compared with their pure metal counterparts.

.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen, as a realistic alternative energy option, is promoted by the recent advances made in technologies for producing, storing, and utilising hydrogen [1]. Although hydrogen itself is considered a clean fuel, it is important to realise that the different processes of hydrogen production are not always environmentally accommodating (Fig. 1, red being unfavourable). From the various hydrogen production technologies (Fig. 1), electrolysers can be seen as one of the most promising technologies for the clean production of hydrogen when integrating renewable sources such as solar or wind energy. Some of the main types of electrolysers, and their operating potentials for hydrogen production, are given in Fig. 1. From the overall net reaction potentials for water electrolysis (alkaline water electrolysis (AWE), proton exchange membrane electrolysis (PEM), and solid oxide electrolysis (SOE)), in comparison with SO2 depolarised electrolysis (SDE), it is evident that the SO2-assisted water electrolysis is almost 1 V lower with regard to its energy requirement compared with conventional electrolysers. Two attractive cycles involving SDE are the hybrid sulphur cycle (HyS, closed cycle for large-scale H2 production) and the Outotec® open cycle [4] (H2 and H2SO4 production). The HyS cycle is a two-step cycle involving a high-temperature thermochemical step and a low-temperature electrochemical step. During the thermochemical step, H2SO4 is decomposed to form SO2 which is fed to the SDE. In the SDE, SO2 is oxidised to form H2 and H2SO4 (recycled back to thermochemical step). The Outotec® cycle on the other hand is a one-step process, not requiring the thermal decomposition of H2SO4. SO2 can be obtained from flue gas emissions (e.g. effluent (SO2, SO3, N2, and O2) from a sulphuric acid production plant [5]), which results in the production of a useable energy carrier and simultaneously addresses an environmental pollutant, SO2 [5]. Irrespective of the cycle, both cycles utilise SDE; however, the overall SDE performance is limited due to poor anode material kinetics [6]. Extensive studies have been conducted on platinum (Pt) [7,8,9,10] as an anode material; however, it is not an economically viable option for long-term and large-scale production of hydrogen as Pt is a rare and expensive metal. Hence, various attempts to reduce or eliminate the Pt content, whilst not compromising performance, have been made. A recent study has shown that a sputtered Pt40Pd57Al3 thin film (60 nm) electrocatalyst annealed at 900 °C proved to be competitive in comparison with its pure Pt competitor [11]. However, the effect that rapid thermal annealing (RTA) has on varying film thickness (a structure effect), and the subsequent effect on the electro-oxidation of SO2, was not investigated. In this work, the electrochemical activity of a Pt40Pd57Al3 thin film electrocatalyst towards oxidising SO2 was investigated as a function of both film thickness and RTA. The electrochemical SO2 oxidation trends (activity and stability) were investigated by linear polarisation (LP) and cyclic voltammetry (CV). Physical characterisation of the thin films included GIXRD and SEM analysis.
Experimental
Preparation of Thin Films
Polished glassy carbon electrode inserts (GC’s, SIGRADUR G, HTW Germany) with a geometric area of 0.196 cm2 were employed as the substrate for the sputtered thin films. A detailed description of the polishing procedure is available in a previous publication [12]. The Pt40Pd57Al3 thin films with varying thickness (40–200 nm) were prepared by means of physical vapour deposition (PVD). The power of the magnetron guns containing the metal targets (ACI alloys) was kept constant (Pt 80 W, Pd 72 W, Al 25 W), and the deposition time regulated to produce varying thickness. Calibration of the sputtering rate can be found in an earlier publication [13]. In addition to varying film thickness, the effect of annealing temperature was also considered; henceforth, the thin films were investigated in the AD state as well as the AN state. Annealing was conducted ex situ, under an argon atmosphere [12], employing a RTA oven (AccuThermo AW610). The temperature regime [12] entailed rapid ramping up to 900 °C, holding the temperature for 40 s, followed by rapid cooling.
Physical Characterisation
The morphology of the surfaces and film compositions was investigated employing a SEM (FEI Quanta FEG 250) analysis with an integrated X-Max 20 EDS system (Oxford Instruments), before and after annealing. GIXRD measurements were taken using a Bruker D8 DISCOVER with a Cu source in an asymmetric 2θ geometry at 1° incidence. The sample height displacements and offsets in ω (the angle between the detector and sample) were optimised for each measurement and verified with rocking curves at under specular reflection conditions. A 0.1-mm slit was used without any additional apertures in the primary optics. Optimised scans for the samples were 15 to 90° using 0.2° steps of 60 s each.
Electrochemical Measurements
Electrochemical measurements were performed in a standard three-electrode setup, employing a Pt spiral wire as the counter electrode and a saturated calomel electrode (SCE, Radiometer Analytical REF 421) as the reference electrode. Preconditioning was conducted in degassed (N2 was bubbled for 20 min) 0.1 mol.L−1 HClO4 (Merck). A 1 mol.L−1 H2SO4 acid solution (SAAR5885124LP, Merck) was degassed with N2 for 15 min prior to conducting linear polarisation (LP) scans (Elow = 0.2–1.441 V (SHE), (Fig. S1)) at a scan rate of 10 mV.s−1). A stock solution of 1 mol.L−1 Na2SO3 (Sigma-Aldrich) was used as an in situ SO2 source. Current was taken at the highest point on the SO2 oxidation peak (illustration in [12]) and converted to mass-specific current density by employing the geometrical area and the associated Pt mass. CV was employed as a relatively simple means of obtaining information with regard to stability of the AD and AN samples in a 1 mol.L−1 H2SO4 (pH = 0.55) electrolytic environment. CVs were conducted at a scan rate of 100 mV.s−1 and within a potential window of − 0.041 V (SHE) and 1.441 V (SHE) at 25 °C (Table S1).
Results and Discussion
The following discussion will be divided into three main categories, i.e. (i) microstructure characteristics (appearance of the material on a nm-cm length scale), (ii) crystal structure characteristics (appearance of the material on atomic (or Å) length scale), and (iii) electrochemical performance in terms of current output and stability.
Physical Characterisation
SEM
Two physical parameters are considered, i.e. varying film thickness and RTA (which directly influences film thickness) and the effect that they have on SO2 electro-oxidation. Clear differences between the AD and AN samples for the various film thicknesses can be observed (Fig. 2). Overall, the AD samples appear to be compact and smooth and exhibiting minor lines (grooves) as a direct result of polishing (“Physical characterisation”), whilst the AN samples, on the other hand, exhibit a totally different appearance. The thin films break into disordered arrangements of clusters, with the degree of agglomeration (de-wetting) decreasing with increasing film thickness. This phenomenon may be due to the thinner films (40 and 60 nm) gaining sufficient activation energy from the thermal treatment that uncovers the support surface as a means of reducing the free energy of the film layer [14]. The thicker samples either require higher temperatures and/or longer treatment times to undergo the same phenomenon. The 40 and 60 nm samples may now be referred to not as thin films, but rather micro-sized ternary metal particles [15]. This phenomenon was first described by Mullins [16] in 1958 and can occur at temperatures well below the melting point of the metals present in the thin film [17, 18]. In the case of this ternary electrocatalyst, it is expected that the Al will be affected the most by the annealing temperature of 900 °C, which is above the melting point of Al (660.3 °C). Pt (1768 °C) and Pd (1555 °C) are expected to be the least influenced by the annealing temperature of 900 °C, which is well below the melting point of these two metals. However, as part of a previous investigation into this metal combination, an XPS analysis revealed that a degree of alloy formation occurs between Pt and Al and that contraction of the thin film also occurs [11]. This occurrence of contraction (exposure of the GC) is supported by the different degrees of agglomeration of the thin films as observed by the SEM analysis (Fig. 2). Elemental mapping of the annealed 40-nm thin film (Fig. 3) was employed to investigate the possibility of agglomeration not occurring homogeneously. This entails that the clusters/particles are not ternary as initially sputtered, but rather that the clusters form selectively for a dominant metal. From Fig. 3, it is clear that the metals (even in the agglomerated state) are still homogeneously dispersed, indicating that the ratio of metals across the surface is still distributed in the Pt40Pd57Al3 ratio and that specific areas of the total surface are not predominantly consisting of one or two of the three metals.
GIXRD Analysis
The multicomponent thin films (Pt, Pd, and Al) correspond to a single FCC phase with a unit cell of a similar size to that of Pd and Pt (3.89 Å). For the AD samples, this phase increased in intensity with scattering volume of the metal layer (thickness); conversely, the observed intensity of the Bragg reflection assigned to the underlying carbon decreased (Fig. 4). This is attributed to more X-rays being absorbed by the thicker metal layers reducing the penetration depth into the GC substrate. A similar although less certain trend was observed with the AN samples with the exception of the 80-nm sample where both the FCC phase and carbon peak near 25° produced higher-intensity reflections than expected. The 80 and 200-nm AN samples showed only slight preferred orientation with the depression of the 200 reflection (Fig. 4, near 47°). With the other samples, no strong changes were observed from annealing.
Using Vegard’s law, in conjunction with lattice constants taken from literature [19] for the individual metals (AMCSD entries: 0011136, 0011155, and 0011157, for Al, Pd, and Pt respectively), the lattice constant of Pd40Pt57Al3 alloy is approximated to 3.9079 Å. The mean experimental lattice parameter was 3.9074 Å (σ 0.0077) estimated using a DIFFRAC.TOPAS V6 for an analytical least squares refinement; this is near identical to what is predicted by Vegard’s law. For a simulated alloy of Pd40Pt57, a lattice constant of 3.9035 Å was calculated, which is within one standard deviation of our experimental value. These differences are too small to either substantiate or refute the presence of alloy formation.
In forming new materials, it is important to differentiate between alloys and multi-phase solids. The Hume-Rothery law suggests that Pd and Pt should be highly or completely miscible considering their very similar electronegativity and unit cell size (< 1% difference in unit cell parameter). Aluminium’s unit cell size is less than 4% larger compared with these precious metals, which is a small difference especially for the low Al content in our system. According to Galinski, the Pt/Al system is almost completely miscible whilst Pd/Al systems have been reported in literature as alloys [20, 21]. This points to the propensity of alloy formation for the PtxPdyAlz system.
Electrochemical Performance
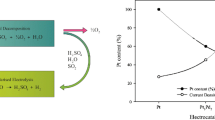
In Fig. 5, the performance of the AD and AN films with varying thickness is shown, taking the Pt content and geometrical area into consideration. Obvious trends in the graph include the following: (a) the current outputs for the AD and AN samples decrease with increasing thickness and (b) the current output for the individual thicknesses (e.g. 40 nm AD vs 40 nm AN) are very similar, with the current of the AD samples being slightly higher overall compared with the AN samples.
Current outputs and stability performance (cycles) for the AD and AN samples with varying thickness. (Standard deviations are negligibly small ranging between 0.326 and 0.136 mA.cm−2. Ptμg−1). Solid circles are indicative of current density and open circles are indicative of the number of cycles (refer to Table S1 for CVs). Data for pure Pt [12] as reference is represented by the colour blue. Inset: EDX analysis of the AD and AN thin films after LP experiments; error bars and associated dashed lines represent an average of the AD thin film compositions
Trend (a) is an indication that the amount of Pt (that increases with film thickness) does not result in an increase of the observed current, which is a clear waste of noble metal content. This is also a possible indication that only the surface Pt is mainly responsible for the current activity and not so much the Pt situated in the bulk layer. Hence, it is suggested that layered sputtering should rather be investigated instead of co-sputtering. Layered sputtering allows for the more costly metal (Pt in this case) to be present only on the surface or near surface and by doing so, less noble metal is employed.
Trend (b) is an indication that, in terms of current output, the annealing treatment has no significant effect on increasing the current output of the respective films, which may suggest that no migration of a specific metal to the surface of the thin film occurred [11], i.e. the exposed AN surfaces available for SO2 electro-oxidation have the same surface composition as the AD films. It is expected that the clearly evident morphological changes, as a result of annealing (Fig. 2, AN samples), would result in a differing of the current output, due to the binding sites for SO2 electro-oxidation being different. However, from Fig. 5, this is clearly not the case. Hence, it can be suggested that the observed current output is not so much affected by the film crystallinity and changes in morphology resulting from annealing. This is supported by the lack of observable-induced features noted in the previous section regarding GIXRD on the current output. This may be due to a polaronic effect [22], whereby thinner films have the ability to undergo lattice distortion, which enables the accommodation of accumulated charge through electron transfer, which a thicker film may not exhibit [23].
As a means of evaluating short-term stability, i.e. if any change in the composition of the electrocatalysts occurred as a result of dissolution or degradation, an EDX analysis was conducted before and after the LP experiments (standard deviations are representative for all thicknesses prior to LP experiments). From Fig. 5 (inset), it is clear that there is no significant change in electrocatalyst composition for all thicknesses. Although the EDX analysis is a bulk analysis technique and can therefore not provide any information on the surface composition of the samples, an earlier investigation [11] highlighted some surface enrichment of Al subsequent to RTA (also under an Ar atmosphere) that was ascribed to lattice relaxation and associated atom size differences. This surface enrichment was substantiated by X-ray photoelectron spectroscopy (XPS) depth profiling. If only considering surface composition and not sample morphology, it could therefore be speculated that Al also segregated to the surface during annealing, which is substantiated by the slight decrease in the observed current for the AN thin films (containing an increased Al surface content) compared with the AD thin films. In terms of long-term stability, the electrocatalysts were subjected to continuous cycling in an acidic environment until signs of delamination occurred (Fig. 5) [11]. In general, stability was found to be similar for the different film thicknesses (the black-dashed line in Fig. 5) and would seem to be not affected by film thickness nor annealing (in general). However, there is a single exception with the 60-nm AN thin film exhibiting a clear improvement in stability as well as a higher-than-average current output. An explanation for this will be pure speculation at this stage. Nevertheless, this Pt40Pd57Al3 60-nm thin film was compared with pure Pt (60 nm, AD and AN) and it is evident that the ternary electrocatalyst, especially in the AN state, outperforms pure Pt (AD and AN) that has the same thickness (Fig. 5, data in blue). The ternary composition, containing less Pt metal is therefore more favourable for SO2 oxidation.
Conclusion
Linking electrocatalytic performance to physical characteristics is usually a daunting task as it does not always allow for the observation of direct (and trending) links, complicating the elucidation of results. This emphasises the complex fundamental nature of electrocatalysts and that the identification of superior electrocatalysts do not always rely on forecasted trends. Electrocatalyst development is inextricably linked to the application of the electrocatalyst under investigation and may require a multitude of characterisation techniques. Film thickness as a parameter should not be considered as an initial parameter when investigating thin film electrocatalysts by means of high-throughput testing. Due to the complex and fundamental nature of this parameter, it is suggested that the effect of film thickness should rather be investigated as an application-specific-optimisation parameter, in the event of a potential electrocatalyst being scaled up for a specific application. It is furthermore suggested to initially employ molecular modelling, rather than experimentation, as a means to gain insight into the structural and electronic characteristics. This includes (i) gaining an improved understanding of multi-metallic systems, (ii) obtaining fundamental information on a molecular level of what happens during RTA, and (iii) due to the complex nature of SO2 electro-oxidation, modelling different possible mechanistic pathways to attempt to connect the activity of SO2 electro-oxidation (electronic effects) with the specific surface under investigation.
References
D.L. Stojić, M.P. Marčeta, S.P. Sovilj, Š.S. Miljanić, Hydrogen generation from water electrolysis—possibilities of energy saving. J. Power Sources 118(1-2), 315–319 (2003)
R. C. Weast, CRC Handbook of Chemistry and Physics, 70th edn. (CRC Press Inc, Boca Raton, 1989–1990)
W.S. Douglas, Dogma-breaking catalysis. Nature. 553, 160–161 (2018)
A. Lokkiluoto, P.A. Taskinen, M. Gasik, I.V. Kojo, H. Peltola, M.H. Barker, K.-H. Kleifges, Novel process concept for the production of H2 and H2SO4 by SO2-depolarized electrolysis. Environ. Dev. Sustain. 14(4), 529–540 (2012)
Z. Meddeb, H. Hajjem, A. Mabrouk, N. Hajjaji, N. Hajji, Idea, process and analyses of hydrogen production from atmospheric pollutant. Int. J. Hydrogen Energ. 42(13), 8602–8610 (2017)
L. Xue, P. Zhang, S. Chen, L. Wang, Pt-based bimetallic catalysts for SO2-depolarized electrolysis reaction in the hybrid sulfur process. Int. J. Hydrogen Energ. 39(26), 14196–14203 (2014)
A.J. Appleby, B. Pinchon, The mechanism of the electrochemical oxidation of sulfur dioxide in sulfuric acid solutions. J. Electroanal. Chem. 95(1), 59–71 (1979)
C. Quijada, A. Rodes, J. Vazquez, J. Perez, A. Aldaz, Electrochemical behaviour of aqueous sulphur dioxide at polycrystalline Pt electrodes in acidic medium. A voltammetric and in-situ FT-IR study Part II. Promoted oxidation of sulphur dioxide. Reduction of sulphur dioxide. J. Electroanal. Chem. 398(1-2), 105–115 (1995)
E.T. Seo, D.T. Sawyer, Electrochemical oxidation of dissolved sulphur dioxide at platinum and gold electrodes. Electrochim. Acta 10(3), 239–252 (1965)
J. O’Brien, J. Hinkley, S. Donne, The electrochemical oxidation of aqueous sulfur dioxide. J. Electrochem. Soc. 157(9), F111 (2010)
A. Falch, V.A. Badets, C. Labrugère, R.J. Kriek, Co-sputtered PtxPdyAlz thin film electrocatalysts for the production of hydrogen via SO2 (aq) electro-oxidation. Electrocatalysis 7(5), 376–390 (2016)
A. Falch, V.A. Lates, H.S. Kotze, R.J. Kriek, The effect of rapid thermal annealing on sputtered Pt and Pt3Pd2 thin film electrocatalysts for aqueous SO2 electro-oxidation. Electrocatalysis 7(1), 33–41 (2016)
A. Falch, V.A. Lates, R.J. Kriek, Combinatorial plasma sputtering of PtxPdy thin film electrocatalysts for aqueous SO2 electro-oxidation. Electrocatalysis 6(3), 322–330 (2015)
C. Boragno, F.B.d. Mongoet, R. Felici, I.K. Robinson, Critical thickness for the agglomeration of thin metal films. Phys. Rev. B 79(15), 155443 (2009)
S. Koutsopoulos, S.B. Rasmussen, K.M. Eriksen, R. Fehrmann, The role of support and promoter on the oxidation of sulfur dioxide using platinum based catalysts. App. Catal. A: Gen. 306, 142–148 (2006)
W.W. Mullins, The effect of thermal grooving on grain boundary motion. Acta Metall. 6(6), 414–427 (1958)
R. Saxena, M. Frederick, G. Ramanath, W. Gill, J. Plawsky, Phys.l Rev. B. 72(115425), 1 (2005)
H. Kim, N. Theodore, T. Alford, Comparison of texture evolution in Ag and Ag(Al) alloy thin films on amorphous SiO2. J. Appl. Phys. 95(9), 5180–5188 (2004)
R.T. Downs, M. Hall-Wallace, Am. Min. 88, 247–250 (2003)
H. Galinski, T. Ryll, L. Schlagenhauf, F. Rechberger, S. Ying, L.J. Gauckler, F.C. Mornaghini, Y. Ries, R. Spolenak, M. Döbeli, Dealloying of platinum-aluminum thin films: dynamics of pattern formation. Phys. Rev. Lett. 107(22), 225503 (2011)
T. Harumoto, Y. Tamura, T. Ishiguro, Ultrafine nanoporous palladium-aluminum film fabricated by citric acid-assisted hot-water-treatment of aluminum-palladium alloy film. AIP Adv. 5(1), 017146 (2015)
A.S. Loselevich, V.V. Sivak, Cotunneling and polaronic effect in granular systems. Phys. Rev. B 95(21), 214205 (2017)
S.V.G. Pacchioni, Oxide Ultrathin Films: Science and Technology (Wiley-VCH, Singapore, 2012), p. 351
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 1219 kb)
Rights and permissions
About this article
Cite this article
Falch, A., Shnier, A., Wamwangi, D. et al. The Effect of Sputtered Pt40Pd57Al3 Thin Film Thickness on SO2(aq) Electro-Oxidation. Electrocatalysis 10, 399–405 (2019). https://doi.org/10.1007/s12678-019-00526-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-019-00526-5