Abstract
Objectives
Mindfulness meditation has been shown to improve working memory (WM). However, brain activity underpinning these improvements is underexplored. In meditation-naïve individuals, increased fronto-midline theta and parieto-occipital alpha oscillations, and steeper 1/f aperiodic activity during WM correlate with better WM performance. Resting theta and alpha oscillations have been found to differ in meditators, but WM-related oscillations and 1/f aperiodic activity have not been examined. Additionally, WM-related event-related-potentials (ERPs) are modulated by attention, which is enhanced by mindfulness meditation, so these neural measures are candidate explanations for WM improvement in mindfulness meditators.
Methods
We recorded electroencephalography (EEG) from 29 meditation-naïve controls and 29 experienced mindfulness meditators during a Sternberg WM task and compared theta, alpha and 1/f aperiodic activity during the WM delay, and ERPs time-locked to the WM probe.
Results
Compared to controls, meditators demonstrated greater WM accuracy (p = 0.008, Cohen’s d = 0.688), earlier left-temporal ERP responses and a more frontal distribution of activity (FDR-p = 0.0186, η2 = 0.0903), as well as a reduction in overall neural response strength (FDR-p = 0.0098, η2 = 0.1251). A higher proportion of meditators showed theta oscillations during the WM delay, but no other differences in theta, alpha or 1/f aperiodic activity were present.
Conclusions
Results suggest that increased WM performance in mindfulness meditators might not result from higher amplitudes of typical WM activity, but instead from an alternative pattern of brain region engagement during WM decision making, allowing more accurate responses with less neural activation (perhaps reflecting increased neural efficiency).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mindfulness meditation has been shown to improve attention and alter neural activity related to attention (Lutz et al., 2008; MacLean et al., 2010; Tang et al., 2007). Attentional processes are essential for working memory (WM) performance, which is a capacity limited system that enables behavioural responses in the present to be informed by stimuli presented recently but currently not available for sensory processing (Baddeley, 2012; Christophel et al., 2017; Ma et al., 2014; Wolpaw, 2002). In addition to the effect of mindfulness meditation on attention, research has shown that meditation has a positive impact on WM (Jha et al., 2010; Mrazek et al., 2013; Quach et al., 2016; Van Vugt & Jha, 2011; Zeidan et al., 2010). Currently, very little research has examined neural activity underpinning this improved WM performance in mindfulness meditators.
There are a number of reasons to be interested in exploring the neural changes responsible for these mindfulness meditation-related WM improvements. Firstly, better understanding of WM-related neural activity in mindfulness meditators could be informative regarding whether mindfulness meditation simply leads to an enhancement in attention, and the attention enhancement is solely responsible for all other cognitive enhancements, or whether there are domain-specific enhancements as well (Buttle, 2011; Jha et al., 2010) (see supplementary materials for a detailed discussion of this point). Expanding from this point, the research may be informative of how mindfulness meditation works at a neural level, and as a result, WM research in mindfulness meditation might lead to a biomarker to measure improvements as a result of the practice. This might enable improved interventions that more directly target the mechanism of action, and potential predictors of which clinical groups or individuals might benefit most from mindfulness meditation (Britton et al., 2018). Thirdly, given that mindfulness meditators do show enhanced WM performance, understanding neural activity underpinning WM performance in meditators could be informative regarding neural activity underlying good WM function.
In order to explore the effects of mindfulness meditation on WM, neural activity related to WM can be examined using a modified Sternberg task (Sternberg, 1966), which presents a WM set (referred to henceforth as the ‘WM set presentation period’, which in the case of the current study consisted of eight simultaneously presented visual letters), followed by a ‘WM delay period’ (which was a blank screen in current study), followed by a WM probe period (a single probe letter, which may or may not have been in the memory set, see supplementary materials for a detailed explanation of the selection of WM period labels). To complete the modified Sternberg task, participants are instructed to push one button if the probe letter was in the WM set, and another button if it was not. Separating the different periods of WM in this way allows for separate analysis of neural processes related to specific WM relevant functions, useful for fully characterising the effects of mindfulness on WM.
In research on non-meditators, brain regions associated with WM processing are prefrontal, parietal and medial temporal regions, with other regions recruited for sensory modality specific WM functions (Christophel et al., 2017; Gu et al., 2015). Oscillatory activity is modulated in these brain regions during performance of WM functions. In particular, neural activity measured with EEG during the WM delay period of the Sternberg task shows increased theta activity (4–8 Hz) in fronto-midline electrodes (Brookes et al., 2011; Kottlow et al., 2015; Payne & Kounios, 2009). This neural signal has been suggested to be generated by the anterior cingulate cortex (ACC), and research suggests that it reflects an attentional mechanism enabling selection and activation of the specific task relevant neural processes required to achieve a person’s current goals for enhancement out of an array of multiple competing neural processes (Cavanagh & Frank, 2014; Sauseng et al., 2010; Sauseng et al., 2007). Increased theta power has been associated with better performance both between individuals (Maurer et al., 2015) and within individuals across trials (Scheeringa et al., 2009) as well as being associated with increased WM loads (Jensen & Tesche, 2002). Previous research has also shown that theta activity modulations are associated with meditation (Tang et al., 2009). While this research examined the effect of meditation on theta activity while at rest (rather than during a task), the result suggests the potential for meditators to show increased ability to modulate theta activity. Given the association between theta activity and improved WM, the improved modulation of theta could be a potential candidate mechanism underpinning improved WM in mindfulness meditators.
In addition to selecting for neural processes involved in WM functioning, non-relevant regions need to be inhibited to enable WM performance. Alpha oscillations in brain regions that perform functions that are not engaged by the task have been suggested by previous research to reflect a process that inhibits the activity of those regions (Klimesch et al. 2007). In the Sternberg task neural activity during the WM delay period (which displays no visual stimuli so does not require visual processing) shows increased upper alpha power (10–12.5 Hz) in parieto-occipital electrodes (which sit above areas of the brain related to visual processing) (Klimesch et al. 2007). This sustained high power alpha activity has been suggested to reflect suppression of cortical gain (in contrast to low power bursting alpha which may enhance gain) (Peterson & Voytek, 2017). As such, high power alpha may reflect top-down inhibition of visual processing brain regions that are not relevant processes required in the WM delay period, as visual information processing is not required during the WM delay period (a blank screen) (Klimesch et al. 2007). Increased alpha activity in these regions is associated with decreased fMRI blood flow measured in the visual cortex, reflecting the suppression of potentially disruptive activity in this region (Scheeringa et al., 2009). This suppression of non-relevant brain regions leads to facilitation of WM performance. On a single trial basis, trials with higher alpha power are more likely to be followed by a correct response (Scheeringa et al., 2009) and larger WM set sizes (higher WM loads with more stimuli objects to remember) are associated with increased alpha power (although higher WM loads with more features of objects to remember are not) (Busch & Herrmann, 2003, Jensen & Tesche, 2002). Previous research has also indicated that mindfulness meditators are better able to modulate alpha activity in brain regions processing (tactile) distractors (Kerr et al., 2013; Lagopoulos et al., 2009; Tang et al., 2009). These findings suggest that an increase in the ability to modulate alpha and theta oscillations during WM delay periods might reflect an increase in attentional mechanisms underpinning task adaptation, leading to increased performance in meditators. Additionally, individuals scoring high in trait mindfulness and low in anxiety (but no meditation experience) have been demonstrated to show higher alpha power during a WM task than individuals scoring low in trait mindfulness and high in anxiety (Jaiswal et al., 2019).
In addition to examining oscillatory activity, recent research has shown that while traditional measures of oscillatory power include aperiodic (non-oscillatory) neural activity, it is important to separate oscillatory power from aperiodic neural activity (which shows a ‘1 divided by the frequency value’ distribution, termed ‘1/f aperiodic activity’) when assessing the functional relevance of neural oscillations (Haller et al., 2018). Without this separation, significant differences in measures of power within a specific oscillation frequency may reflect differences in the 1/f aperiodic activity rather than a difference in the oscillation (Haller et al., 2018). This research has also shown that the slope of the 1/f aperiodic activity reflects neural activity that is not rhythmic or oscillatory but is suggested to be produced by the Poisson distribution of spiking synaptic potential timing (where the average rate of synaptic potentials is constant, but the time between potentials is variable and of similar amplitude to the mean), with steeper 1/f aperiodic activity slopes perhaps reflecting reduced excitation/inhibition ratios (driven by glutamate and GABA respectively) (Gao et al., 2017). The excitation/inhibition balance has been suggested to be vital for information transmission and gating (Gao et al., 2017). Shallower 1/f aperiodic activity slopes are related to WM performance decline in ageing while steeper slopes are better than oscillatory power as a predictor of schizophrenia (Gao et al., 2017; Peterson et al., 2018; Voytek et al., 2015). The 1/f aperiodic activity offset has also been shown to be related to overall neuronal firing rates/spiking activity (Manning et al., 2009; Miller et al., 2012) and the fMRI bold signal (Winawer et al., 2013). As such, measures of 1/f aperiodic activity are likely to be of interest when examining WM related neural activity in meditators.
In addition to neural activity during WM delay periods, a significant amount of research has examined neural activity associated with memory probe presentation. This research has typically focused on neural activity time-locked to the onset of the probe and averaged over many presentations of probe stimuli. This approach eliminates neural activity that is not consistently time locked to the WM probe, thus comparing neural activity only associated with probe stimulus processing, assumed to reflect activity associated with WM decisions (Friedman & Johnson Jr, 2000). The measurement of neural activity resulting from this time-locking to a stimulus and averaging is referred to as an event-related potential (ERP). Previous memory research has shown memory probe related ERPs with positive voltages in central/lateral parietal regions, surrounded by negative voltages (maximal in fronto-central regions) from 300 to 700 ms following stimuli presentation. The ERP has been given different names by different researchers and has been subdivided in different ways depending on task demands and time periods of analysis.
Neural activity earlier in the WM probe period ERP window (from 300 to 450 ms following stimuli presentation) has been referred to as the FN400 (FN referring to ‘frontal negativity’ due to the negative voltages in frontal regions that are typically compared, although it is worth noting that positive activity is typically present in parietal regions) (Curran & Cleary, 2003). The FN400 has been associated with familiarity (Curran & Cleary, 2003; Duarte et al., 2004), conceptual processing (Woodruff et al., 2006) and semantic processing (Federmeier & Laszlo, 2009; Yonelinas, 2002). It is thought to be generated in part by left temporal regions, and FN400 activity in those regions is related to recognition (Stróżak et al., 2016). The FN400 is also modulated by instructions about which parts of stimuli to attend to, suggesting that it is related to attentional processes (Rugg & Curran, 2007). Research has shown that larger FN400 amplitudes are associated with increased familiarity and memory strength (Finnigan et al., 2002; Rugg et al., 1998).
Neural activity later in the WM probe period ERP (450 to 700 ms) typically shows more positive voltages in central and lateral parietal regions when participants have previously seen stimuli than when they have not seen the stimuli before, so the ERP has been referred to as the parietal old/new effect (Stróżak et al., 2016). This activity has been suggested to be associated with conscious recollection (Duarte et al., 2004; Rugg & Curran, 2007; Woodruff et al., 2006; Yonelinas, 2002). The amplitude of the parietal old/new effect has been suggested to reflect attention orientation to recollection (Rugg & Henson, 2002; Wagner et al., 2005) and is associated with accuracy, or confidence in the accuracy of the response (Finnigan et al., 2002). Research has suggested that the earlier processes reflected by the FN400 are less effortful than the later processes reflected by the parietal old/new effect (Rugg & Curran, 2007).
When WM ERPs are measured specifically in the Sternberg WM task, activity across this WM probe period ERP window has been referred to as the P3 (which shows a similar time window and distribution to both the FN400 and the parietal old/new effect) (Chang et al., 2013; Ergen et al., 2012). The WM P3 has been suggested to reflect a process that inhibits widespread cortical regions, suppressing irrelevant neural activity from interrupting the WM relevant processes (Klimesch et al. 2000). In general, P3 amplitudes are thought to indicate attentional resource allocation to stimulus processing while P3 latency is thought to reflect processing speed (Pontifex et al., 2009). Researchers have suggested the P3 indexes WM retrieval, and that it is related to memory scanning and decision making (Ergen et al., 2012). Lastly, it is worth noting that most of the WM ERP research has measured activity from single or small clusters of electrodes. In contrast to this approach, measuring the scalp distribution using all electrodes is informative regarding the engagement of different brain regions, indicating different functional engagement underlying cognition (Friedman & Johnson Jr, 2000). Research by our lab using other cognitive tasks has shown that mindfulness meditators showed altered distributions of neural activity, typically with more frontal distributions (Bailey et al. 2019, Wang et al. 2019). Our research has also demonstrated reduced overall neural response strength concurrent with increased behavioural performance in meditators, including during a WM task (Bailey et al. 2019, Wang et al. 2019).
The aim of the current research was to examine both the distribution and overall strength of neural activity related to both WM delay and WM probe periods in experienced mindfulness meditators compared to healthy (demographically matched) non-meditators. It was hypothesised a priori that (1) meditators would show increased parieto-occipital alpha and fronto-midline theta power during the WM delay period, reflecting increased attentional modulation of top-down inhibitory and executive control functions, (2) meditators would show reduced ERP activity when measured across all electrodes, and (3) meditators would show a more frontal distribution of the WM ERPs. Exploratory post hoc comparisons were made of the 1/f aperiodic activity slope and offset parameters, as well as of alpha and theta oscillations separated from the 1/f aperiodic activity. Lastly, in replication of previous research, we had a confirmation hypothesis that the meditation group would show higher WM accuracy than the control group.
Methods
Participants
Thirty-four mindfulness meditators and 36 demographically matched meditation-naïve controls were recruited through community advertising. Inclusion criteria for meditators consisted of having a current mindfulness meditation practice involving at least 2 h per week of practice, with at least 6 months of meditation experience (all meditators except three had more than 2 years of meditation experience). Phone screening and in-person interviews were administered by experienced mindfulness researchers (GF, KR, NWB) to ensure meditation practices were mindfulness-based, using Kabat-Zinn’s definition ‘paying attention in a particular way: on purpose, in the present moment, and nonjudgmentally’ (Kabat-Zinn, 2009). Further screening ensured meditation practices were consistent with either focused attention on the breath or body-scan. Uncertainties were resolved by consensus between two researchers including the principal researcher (NWB). Control group participants were screened to ensure they had less than 2 h of lifetime experience with any kind of meditation.
Exclusion criteria involved self-reported current or historical mental or neurological illness, or current psychoactive medication or recreational drug use. Participants were interviewed with the Mini International Neuropsychiatric Interview for DSM-IV (Sheehan et al., 1998) and excluded if they met criteria for any DSM-IV illness. Participants were also excluded if they scored in the mild or above range in the Beck Anxiety Inventory (BAI) (Steer & Beck, 1997) or Beck Depression Inventory (Beck et al., 1996). All participants were between 19 and 62 years of age and had normal or corrected to normal vision.
All participants provided informed consent prior to participation. The study was approved by the Ethics Committee of the Alfred Hospital and Monash University (approval number 194/14) and conducted in accordance with the Declaration of Helsinki. At the beginning of the testing session participants completed demographic and self-report forms including their age, gender, years of education, handedness, and estimated how many years they had been practicing meditation for, and the average number of minutes per week they spent meditating in the last 2 months. Participants completed the Freiburg Mindfulness Inventory (FMI) (Walach et al., 2006), Five Facet Mindfulness Questionnaire (FFMQ) (Baer et al., 2006), BAI and BDI-II (see Table 1 for a summary of the self-report data). Prior to the modified Sternberg WM task, participants completed a Go/Nogo task (Wang et al., 2019), colour Stroop task, emotional Stroop task (Marcu et al. in preparation), and Nback task (Wang et al., 2019), which took approximately 1 h to complete in total including breaks within and between tasks.
Data from four control participants were excluded after scoring in the mild depression range on the BDI-II. Data from three control participants were excluded from EEG analysis due to poor EEG signal quality. WM EEG data were not collected from five meditators due to time constraints. Accuracy data from two control and three meditation participants were excluded due to an intermittent button fault during those sessions resulting in unreliable accuracy measurement (while EEG and reaction time data were included as a sufficient number of correctly responded to epochs were still provided). Following exclusions, 29 control and 29 meditation participants were included in the EEG and reaction time analyses, and 27 control and 26 meditation participants were included in accuracy analysis.
Procedure
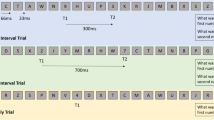
Participants performed a modified Sternberg WM task with eight simultaneously presented letters as stimuli while 64-channel EEG was recorded (see Fig. 1) (Bailey et al., 2019). Letters were selected from a set of 15 potential consonants (B, C, D, F, H, J, K, L, N, R, S, T, Y, W, and Z), and all stimuli were presented with Neuroscan STIM2 software (Compumedics, Melbourne, Australia). Trials began with a fixation cross (800 ms) followed by a blank screen (1000 ms). The WM stimuli set was then presented (WM set presentation period, 4000 ms) followed by a blank screen for the WM delay period (3000 ms). A single probe letter was then presented (WM probe presentation period, 2000 ms), to which participants had to respond with one button if the probe had been present in the memory set, and another if the probe had not been present in the memory set. There was a 50% probability in each trial that the probe had been present in the memory set. Following the offset of the probe, a brief visual mask was presented (166.67 ms), then a blank screen (1883.33 ms), before the fixation cross was once again presented to begin the next trial. The total task consisted of two blocks with 26 trials per block. Participants performed a brief practice version of the task prior to the recording.
Measures
A Neuroscan 64-channel Ag/AgCl Quick-Cap was used to acquire EEG data through Neuroscan Acquire software and a SynAmps 2 amplifier (Compumedics, Melbourne, Australia). Electrodes were referenced to an electrode between Cz and CPz. Eye movements were recorded with a supraorbital electrode above the left eye. Electrode impedances were kept below 5 kΩ. EEG was recorded at 1000 Hz with an online bandpass filter of 0.05 to 200 Hz. Data were pre-processed offline in MATLAB (The Mathworks, Natick, MA, 2018a) using EEGLAB for pre-processing (sccn.ucsd.edu/eeglab) (Delorme & Makeig, 2004). Data were filtered, epoched to correct trials only, artefacts automatically then manually rejected, submitted to independent component analysis (ICA) and cleaned of eye movements and remaining artefacts, then referenced to an average reference. A minimum of 20 accepted epochs were required for inclusion. Full details of analysis steps are specified in the supplementary materials.
In order to compute power measures the EEG data from each accepted epoch for each participant were submitted to a Morlet wavelet transform (with 5 oscillation cycles required to derive power estimates at each timepoint) (Tallon-Baudry et al., 1997). Power was calculated in the upper alpha band (10–12.5 Hz) and theta band (4–8 Hz). Power was baseline corrected to the middle 600 ms of the blank screen that appeared early in the trial (1000 to 1600 ms following the beginning of the trial). Modulation of power was calculated relative to the baseline using the formula: Baseline corrected power (BLC power) = (WM delay period activity − blank screen reference activity)/blank screen reference activity. This formula provides positive values when power is higher in the WM delay period than the blank screen reference period and negative values when power is lower in the WM delay period. Average BLC power was calculated across the entire WM delay period (5800 to 8800 ms after the start of the trial) within both frequency bands, then averaged over trials for each participant.
These averaged BLC power values were used to make statistical comparisons between groups. ERP analysis was conducted time-locked to the onset of the probe stimulus. Voltage data from each electrode for − 100 to 1000 ms around the probe were baseline corrected to the − 100 to − 10 ms period, and all epochs from each participant were averaged for ERP analyses. Probe present and probe absent epochs were averaged together to ensure enough epochs were available for analysis. Exploratory analyses were performed on data from probe present and probe absent conditions averaged separately to check that results were not confounded by averaging conditions. These comparisons are reported in the supplementary materials. In brief, they showed the same result for the group main effect as the primary comparisons (in both the GFP and TANOVA tests), and no interaction between group and probe present/absent. Although there was a main effect of probe present/absent condition, the overlap between the analysis of these conditions separately and the primary analysis which combined the conditions suggests that combining the probe present and probe absent conditions did not confound the current results. Source analysis was also conducted to characterize potential generators of the ERP differences between groups without statistical comparison (methodology reported in supplementary materials).
Data Analyses
Comparisons of self-report and behavioural data were conducted using SPSS version 23 for behavioural and single electrode statistics, with JASP (JASP Team, 2019) to compute eta squared effect size (η2) values (with both η2 and partial eta squared (ηp2) reported where these values differed) and the randomised graphical user interface (RAGU) for other EEG analyses (Koenig et al., 2011). Independent samples t tests were used to verify groups did not differ in age, years of education, BAI, BDI-II, and to test whether groups differed in FMI and FFMQ scores. Chi-squared tests were used to verify groups did not differ in gender or handedness. Because previous research suggested mindfulness meditators show superior WM performance, a one-sided t test was used to compare d-prime accuracy between groups. A repeated measures ANOVA was used to compare reaction times across the 2 groups × 2 conditions (probe present or absent). No outliers were present for data tested with traditional statistics, and data tested with traditional statistics met assumptions of normality, sphericity, and homogeneity of variance.
Primary Comparisons
Primary statistical comparisons between groups in EEG data were performed using RAGU. RAGU uses reference free global field potential (GFP) measures and randomisation statistics to compare neural response strength and scalp field differences across all electrodes and time points without a priori assumptions about locations or time windows showing significant effects. RAGU controls for multiple comparisons in the spatial dimension by collapsing differences to a single scalp difference map value for distribution comparisons and using the GFP for neural response strength comparisons. It controls for multiple comparisons in the time dimension by ranking the length of significant differences in the real data against the randomised data and ensuring the significant periods in the real data are longer than 95% of the randomised data (global duration control), or that the count of significant timepoints in the real data exceeds the count of significant timepoints in 95% of the randomised data (global count control). Further details about RAGU can be found in the supplementary materials and in Koenig et al. (2011). Differences between groups in overall neural response strength (across all electrodes) were compared using the GFP test. Differences between groups in the distribution of activity across the scalp were compared independently of amplitude using the topographical analysis of variance (TANOVA). GFP and TANOVA tests were used to conduct t-test design comparisons comparing ERPs time-locked to the onset of the probe stimuli from − 100 to 1000 ms. Post hoc GFP and TANOVA tests explored significant time periods averaged across time periods of significant effects that passed multiple comparison controls in the time domain.
To make separate between group comparisons of alpha and theta BLC power during the WM delay period, we used t test designs in RAGU with root mean square (RMS) and TANOVA tests (to separately compare overall neural response strength and distribution of neural activity respectively). It should be noted that when frequency comparisons are computed with RAGU, the average reference is not computed on the frequency transformed data (the average reference was computed prior to the frequency transforms). As such, the test is a comparison of the RMS between groups, a measure which is a valid indicator of neural response strength in the frequency domain. In other respects, the statistic used to compare RMS between groups is identical to the GFP test (T. Koenig 2018, Department of Psychiatric Neurophysiology, University Hospital of Psychiatry, personal communication). η2 effect sizes were computed in RAGU for all comparisons of interest. In order to control for multiple comparisons across all primary hypotheses the Benjamini and Hochberg false discovery rate (FDR) was applied to the global count p value for each primary statistical comparison (WM delay period theta and alpha RMS and TANOVA, and WM probe period ERP GFP and TANOVA comparisons) (Benjamini & Hochberg, 1995). It was also separately applied to p values obtained for the average difference between groups during time periods of significance to control for multiple comparisons when focusing on time windows of interest. To enable comparison with other research, both corrected and uncorrected p values are reported for significant comparisons (labelled ‘FDR-p’ and ‘p’ respectively).
Exploratory Analyses
Exploratory analyses were not corrected for multiple comparisons, and as such should be taken as preliminary. To assess a potential relationship between neural activity and behaviour, significant periods from group GFP and TANOVA comparisons were averaged and compared to d-prime scores using the GFP covariance and TANCOVA tests. Methods for separating 1/f aperiodic activity and oscillatory activity are described in the supplementary materials and in Haller et al. (2018). t Test comparisons were conducted using SPSS for the following measures at Fz: theta (4 to 8 Hz) centre frequency, bandwidth, amplitude, 1/f aperiodic slope, 1/f aperiodic offset. Repeated measures ANOVA comparisons were conducted including PO7 and PO8 for alpha (8 to 13 Hz) centre frequency, bandwidth, amplitude, 1/f aperiodic slope and 1/f aperiodic offset. Chi-squared tests were also used to determine whether groups differed in the number of participants showing presence/absence of theta and alpha peaks at electrodes of interest (since not all participants showed peaks in each band once the 1/f aperiodic activity was removed).
In order to estimate the strength of evidence in support of or for rejection of each hypothesis, Bayes factor analyses (with analogous statistical designs to the frequentist analyses) were used to calculate the probability of the alternative hypothesis (BF10) or null hypothesis (BF01) (depending on which hypothesis the frequentist analysis supported) (Rouder et al., 2017). The suggested comparison between models containing a hypothesised effect to equivalent models stripped of the effect was performed for these analyses. Note that Bayesian analyses are not currently possible for comparisons of the distribution of neural activity, which rely on a difference map between the groups rather than a value for each individual enabling comparisons of means (further details of comparisons of the distribution of neural activity are reported in the supplementary materials). Lastly, microstates were also used to explore significant results obtained in the TANOVA analysis and to justify selections of windows of analysis for TANCOVA and source analyses. Microstate analyses are reported in the supplementary materials.
Results
Mindfulness Meditators Showed Enhanced Working Memory Accuracy
No differences between groups were present for age, gender, years of education, BAI scores, BDI-II scores and preferred hand (all p > 0.10). Significant differences were present for FMI and FFMQ scores (p = 0.012 and p = 0.001, respectively). Table 1 reports means, standard deviations and statistics for these comparisons in detail.
D-prime comparisons showed a significant difference, with meditators showing higher performance (t(51) = 2.503, p = 0.008, Cohen’s d = 0.688, BF10 = 3.373, see Fig. 2).
There were no group differences in reaction time (F(1,56) = 0.118, p = 0.732, η2 = 0.002), nor a group by probe condition interaction (F(1,56) = 1.464, p = 0.231, η2 = 0.025), nor was there a difference in probe condition reaction time (F(1,56) = 1.188, p = 0.280, η2 = 0.020, ηp2 = 0.021). The lack of group differences in reaction time suggests that the meditation group did not obtain higher accuracy scores simply due to a speed / accuracy trade off. Means and standard deviations are shown in Table 2. A significant main effect of group in the number of accepted epochs was detected with the meditation group showing a higher number of epochs (F(1,56) = 8.506, p = 0.005, η2 = 0.132). Because differences in the number of epochs included in ERP averages can affect comparisons, post-hoc validation comparisons were conducted between the groups after a random selection of epochs were removed from the meditation participants until each meditation participant had an equal number of epochs to a control participant. This analysis showed the same results as the main comparison and is reported in the supplementary materials.
No Differences in WM Delay Period BLC Alpha, Theta and 1/f Aperiodic Activity Comparisons
No significant group differences were present for either alpha BLC power in the WM delay period in RMS or TANOVA comparisons across the entire epoch using RAGU (all FDR-p and p > 0.05). Similarly, no significant group differences were present for theta BLC power in the WM delay period in RMS or TANOVA comparisons across the entire epoch using RAGU (all FDR-p and p > 0.05). When 1/f aperiodic activity was separated from the oscillatory activity, the meditation group showed a higher proportion of participants with theta peaks at Fz (Chi-squared = 5.156, p = 0.0232, BF10 = 3.660) and a trend towards an interaction between group and electrode for 1/f aperiodic offset at parieto-occipital electrodes (F(1,56) = 4.025, p = 0.050, η2 = 0.057, ηp2 = 0.067, BF10 = 1.579, see Fig. 3), with meditators showing a larger difference between the two electrodes than controls (with lower 1/f aperiodic offset values at PO7). The 1/f aperiodic slope showed no differences between the groups or interactions between group and electrode, and there was no significant main effect of 1/f aperiodic offset at parietal electrodes or at Fz (all p > 0.05, statistics reported in Table 3). No significant differences were detected in alpha centre frequency, bandwidth, or amplitude across PO7 and PO8 (all p > 0.05). Nor were any significant differences detected in theta centre frequency, bandwidth, or amplitude at Fz (all p > 0.05). Values and statistics are reported in Table 3.
Mindfulness Meditators Showed Reduced GFP and Altered Distributions of ERPs in WM Probe Period
The TCT showed significant signal indicating consistency of neural activity within all groups across the entire epoch except during the period prior to the stimulus to 80 ms after probe stimulus presentation (see supplementary materials Fig. S1). To compare the strength of neural responses in the WM probe period between groups, we conducted a GFP test. A significant difference that survived duration multiple controls (but not global count multiple controls) was present between groups from 399 to 481 ms, and 489 to 585 ms, with meditators showing reduced GFP values in both these time windows (duration control = 55 ms, global count p = 0.0512, global count FDR-p = 0.1536). When averaged across both significant periods, including the break in the middle, the effect was significant (averaged p = 0.0062, averaged FDR-p = 0.0098, η2 = 0.1251, BF10 = 13.353, see Fig. 4). Across both groups, averaged GFP amplitude during this time period did not covary with d-prime scores (p = 0.9222). Nor did GFP amplitude covary with d-prime scores within just the meditation group (p = 0.1296), nor the control group (p = 0.9110).
Significant between-group difference in GFP from 399 to 585 ms following the WM probe onset. ap Values of the between-group comparison for the real data against 5000 randomly shuffled permutations across the entire epoch (green periods reflect periods that exceed the duration control for multiple comparisons across time = 55 ms, averaged across the time period of significance p = 0.0062, FDR-p = 0.0098, η2 = 0.1251, BF10 = 13.353, global count p = 0.0512, global count FDR-p = 0.1536). b Box and violin plot of the averaged GFP across the entire window of significance (from 399 to 585 ms). Circles reflect scores from each individual and the outer curve reflects the distribution of scores in each group
Because the groups showed differences in the number of accepted epochs included in the analyses, a validation analysis was performed with number of included epochs matched between the groups. Additionally, although probe present and probe absent conditions were averaged to ensure enough epochs for valid ERP comparisons in the main analysis, exploratory analyses were conducted separating the two conditions. These two analyses are reported in the supplementary materials. Both analyses showed similar results to the main analysis, with a significant main effect of group showing the meditation group with smaller GFP values (but no interaction between group and probe present/probe absent). However, the significant time period was shorter in the matched epoch analysis (despite this, averaged across the significant time window from the main analysis, the matched epoch was still significant, p = 0.0218). Both the time window of the GFP difference and the topography of activity in this time window (see the microstate analysis, Fig. S4) indicated that the groups differed in overall amplitude of the parietal old/new effect component.
The TANOVA showed a significant difference between the two groups in the distribution of neural activity, which lasted from 294 to 672 ms following the probe stimuli (see Fig. 5). The significant difference survived all multiple comparison controls (duration control = 49 ms, global count p = 0.0028, global count FDR-p = 0.0168, averaged across significant time period p = 0.002, FDR-p = 0.0186, η2 = 0.0903). The difference appears to be driven by more right frontal positivity in meditators at the beginning of the significant period, which shifted towards more fronto-central positivity towards the end of the significant period. Similar to the GFP comparisons, validation checks were conducted to control for differences in the number of accepted epochs included in analyses (reported in the supplementary materials). Both analyses with matched numbers of epochs across the groups and analyses of probe present/absent conditions separated showed similar results to the main analysis, with a significant main effect of group during the same time period with the same pattern. No interaction was present between group and probe present/probe absent conditions (all p > 0.05). The 294 to 672 ms period of significance was separated into three separate time periods based on distinct microstates for analysis of the relationship between d-prime and neural activity (310–425 ms, 425–600 ms, and 600–672 ms). None of these time periods contained neural activity that showed a relationship to d-prime scores (all p > 0.05, reported in more detail in the supplementary materials).
TANOVA main group effect from 294 to 672 ms. ap Values of the between-group comparison for the real data against 5000 randomly shuffled permutations across the entire epoch (global count p = 0.0028, global count FDR-p = 0.0168, green periods reflect periods that exceed the duration control for multiple comparisons across time, global duration control = 49 ms, averaged across the significant window p = 0.002, η2 = 0.0903). b t-maps for meditators topography minus control topography during the significant time window, split into periods reflecting distinct microstates as reported below (for the average activity over the first time period, η2 = 0.1104, for the average activity over the second time period η2 = 0.0781, and for the average activity over the third time period η2 = 0.0692)
Exploratory Source Analysis of the WM Probe Period Showed earlier Temporal Activation in Meditators
Topographies that differed between the groups in the TANOVA comparisons reflect differences in recruitment of underlying generators of neural activity (Koenig et al., 2011), so source analyses were used to characterise which generators were likely to differ between groups. The microstate analysis identified three separate time windows that depicted distinct topographies during the time window that groups showed significant differences in TANOVA comparisons. These time windows were from 310 to 425 ms (reflecting the FN400, see Fig. 6), 425 to 600 ms, and 600 to 672 ms (both reflecting the parietal old/new effect, see Figs. 7 and 8). The supplementary materials report how decisions for these time periods were made.
Source reconstruction during the 310 to 425 ms window using sLORETA and minimum norm imaging, unconstrained to cortex (to minimise assumptions). Activity during this window is likely to reflect the FN400. Note that the source modelling method used depicts only absolute activation so that more positive values in source analysis reflect stronger values in either positive or negative directions. Difference maps reflect meditator minus control activity (red reflecting more activity in meditators compared to controls, blue reflecting less activity in meditators)
Source reconstruction during the 425 to 600 ms window using sLORETA and minimum norm imaging, unconstrained to cortex (to minimise assumptions). Activity during this window is likely to reflect the parietal old/new effect. Note that the source modelling method used depicts only absolute activation so that more positive values in source analysis reflect stronger values in either positive or negative directions. Difference maps reflect meditator minus control activity (red reflecting more activity in meditators compared to controls, blue reflecting less activity in meditators)
Source reconstruction during the 600 to 672 ms window using sLORETA and minimum norm imaging, unconstrained to cortex (to minimise assumptions). Activity during this window is likely to reflect the parietal old/new effect. Note that the source modelling method used depicts only absolute activation so that more positive values in source analysis reflect stronger values in either positive or negative directions. Difference maps reflect meditator minus control activity (red reflecting more activity in meditators compared to controls, blue reflecting less activity in meditators)
Source analysis indicated that from 310 to 425 ms poststimulus, both groups showed broad parietal activation, and the meditation group additionally showed left temporal activation (see Fig. 6). Absolute activations are depicted on the cortex from source analyses in Figs. 6, 7 and 8. Note that the source modelling method used depicts absolute activation, so that more positive values in source analysis reflect stronger values in either positive or negative voltages. As such, the increased left temporal activation in meditators in the 310 to 425 ms period is likely to reflect more negative activity in the left temporal regions at the scalp level (as can be seen in the TANOVA comparisons). From 425 to 600 ms, both groups showed centro-parietal activation, although the meditation group showed a much smaller region of activation that exceeded the threshold (see Fig. 7). This is likely to reflect the reduced overall neural response strength shown in the GFP test, as the source analysis does not separate differences in the distribution of activity from differences in amplitude (in contrast to analyses performed with RAGU). Lastly in the 600 to 672 ms window, meditators showed less left temporal and fronto-central activation, and more occipital activation than the control group, probably reflecting a combination of differences in overall neural response strength and in the distribution of activity (see Fig. 8). Taken together, source analysis of these three time windows suggests that meditators activate their left temporal region earlier than controls, and that this activity is completed earlier, with less left temporal activity and less overall activity later in the epoch.
Discussion
This study cross-sectionally compared neural activity related to working memory in experienced mindfulness meditators and demographically matched meditation-naïve controls. In agreement with two of our primary hypotheses, the meditation group showed an altered distribution of both the FN400 and parietal old/new effect neural activity during the WM probe period, as well as a probable reduction of overall neural response strength during parietal old/new effect (with medium effect sizes). Scalp analysis of the EEG data suggested that the meditation group showed more left temporal negative voltages and frontal right positive voltages during the FN400. During the parietal old/new effect the meditation group showed more fronto-central positive voltages and less positive parietal voltages. However, in contrast to one of our primary hypotheses, no overall differences were detected in the WM delay period BLC theta or alpha power. These results were concurrent with higher accuracy in the WM task in the meditation group in agreement with our confirmation hypothesis and similar to previous research. Exploratory source analysis suggested the differences were the result of more neural activity in the left temporal lobe in the meditation group during the FN400, followed by less neural activity in central-parietal regions during the parietal old/new effect. Exploratory analyses also showed the meditation group had a higher proportion of participants with fronto-midline theta peaks above the 1/f aperiodic activity during the WM delay period. However, in alignment with the null result of our primary analysis focused on the WM delay period, no differences were detected in exploratory analyses of WM delay period neural activity, including measures of theta and alpha activity following the removal of 1/f aperiodic activity, and measures of 1/f aperiodic slope and offset (although there was a trend towards interaction between group and electrode for 1/f aperiodic offset at parieto-occipital electrodes, with the meditation group showing lower values at PO7 only).
Mindfulness Meditators Showed Altered Distribution and Probable Reduced Amplitude of WM Probe Period ERP
The most conservative interpretation of the WM probe period ERP differences is that mindfulness meditators show a different scalp measured pattern of neural activity, which can be inferred to be generated by the engagement of different brain areas and reduced neural response strengths during the recall period. Further interpretation involves engaging in reverse inference—a term used to describe the process of inferring differences in hypothetical cognitive processes between groups after detecting differences in brain activity, without directly measuring those cognitive functions. Conclusions drawn from reverse inference are not deductively valid (particularly when activated regions are broad as per EEG source analyses) as each brain region (and each ERP component) has multiple functional associations (Poldrack, 2006). As such, increases or decreases in activity within specific brain regions (or ERP components) could reflect differences in a range of possible functions (with no way to be certain that the functions proposed by the authors are included in the list of functions that actually differ). Interpretation of the current results is also complicated by the complexity of the results (reflecting differences in multiple WM ERP components as well as both distribution and overall neural response strength differences). However, with these caveats in mind, reverse inference can still provide information (Poldrack, 2006), particularly when the task generating the activity is taken into account (Hutzler, 2014).
With regard to the differences in the current study, WM probe period ERPs in the modified Sternberg task have been suggested to reflect retrieval, and are related to memory scanning and decision making (Ergen et al., 2012). WM probe period ERPs similar to those detected in our study have also been suggested to reflect a process that functions to inhibit widespread cortical regions, suppressing irrelevant neural activity from interrupting the WM relevant information (Klimesch et al., 2000). In memory tasks more generally, the FN400 has been suggested to reflect familiarity related processing (Curran & Cleary, 2003; Duarte et al., 2004), conceptual priming (Woodruff et al., 2006), and semantic processing (binding stimulus information to recalled information) (Federmeier & Laszlo, 2009; Yonelinas, 2002). FN400 activity is thought by some research to be generated in part by the left temporal region, and activation of this region during the FN400 is particularly related to attention mediated familiarity processing, with more negative activity in response to stimuli that are not familiar (Stróżak et al., 2016). Activity in the left temporal region is also associated with phonological processing in WM tasks (Vigneau et al., 2006), and left hemisphere effects are also more prominent for verbal WM tasks (Nagel et al., 2013; Osaka et al., 2007).
Based on this interpretation, the increased negativity in the temporal region in mindfulness meditators could suggest they were less familiar with the probe stimuli. However, a reduction in familiarity related processing in probe present trials would likely lead to reduced performance in those trials. Neither an interaction between group and trial type, nor reduced performance in probe present trials in meditators was found. As such, it seems more likely that the increased temporal negativity in mindfulness meditators reflects increased conceptual, semantic, or phonological processing. As well as the left temporal activity in the FN400, positive voltages in right frontal regions have been suggested to reflect general monitoring and decisional processes thought to be generated by the right DLPFC (Hayama et al., 2008). As such, the more right frontal positive voltage distribution in the meditation group might suggest they engage in more general monitoring and decision processes. More frontal distributions of the P3 have also been found in our previous research in mindfulness meditators (Wang et al., 2019), and we have previously suggested these alterations reflect enhanced attention.
As well as differences in the FN400, our results showed differences in the distribution and probable differences in the amplitude of the parietal old/new effect. The parietal old/new ERP following the FN400 has been associated with conscious recollection, with its amplitude reflecting attention orientation to recalled information (Duarte et al., 2004; Rugg & Curran, 2007; Rugg & Henson, 2002; Wagner et al., 2005; Woodruff et al., 2006; Yonelinas, 2002). In particular, the left parieto-occipital positivity (activity of which the meditation group showed lower amplitudes) is larger when items are consciously remembered and during successful memory performance (Friedman & Johnson Jr, 2000). Similar to the alterations in the FN400, the reduced left parieto-occipital positive voltage and reduced overall amplitude in the meditation group could then be expected to have resulted in lower performance, which was not the case. It may be that the meditation group performed the functions associated with the parietal old/new effect in other regions or more efficiently, leading to a reduction in the ERP without a reduction in performance.
While the distribution differences passed all multiple comparison controls (and as such likely represent true differences between the groups), the reduction in neural response strength was significant when using multiple comparison control for the duration of the effect, but not when controlling for all timepoints measured in the epoch. Future analyses focusing specifically on the significant window from our research are likely to show differences between groups, but our exploratory approach including the entire epoch cannot draw firm conclusions. The medium-to-large effect size of the GFP difference, and the fact that the length of the significant effect exceeded the global duration control by over twice the duration control length suggests the finding is likely to reflect a true difference present in the two populations sampled. This is particularly likely since global control multiple comparisons used in the current study is considered overly conservative (Koenig et al., 2011), and controls for the number of significant time points in the real data but do not consider the strength of significant differences. The Bayes factor analysis limited to the significant window also showed strong evidence for a difference between groups, providing further suggestion that the difference in GFP amplitudes reflects a true result.
Together, these differences suggest that rather than a simple increase in strength of the effective WM neural activity patterns that high performing meditation-naïve participants have shown in previous research (Maurer et al., 2015; Scheeringa et al., 2009), the meditation group engaged qualitatively different brain regions and thus showed an altered distribution of neural activity (and potentially a reduced overall amplitude). These differences might reflect altered neural processes in the meditation group, whereby frontally driven decision relevant processes might be activated earlier, allowing mindfulness meditators more processing time before responding, and thus enabling more accuracy with less overall neural response strength. In support of this explanation, the meditation group’s GFP amplitude peaked at about 300 ms after the probe while the control group’s GFP amplitude continued to increase until a larger and later peak at 400 ms (see microstate analyses in supplementary materials). While not specifically measured in the current study, it is worth noting that the P3 latency is thought to reflect processing speed (Pontifex et al., 2009). It should be emphasised, however, that none of these differences in neural activity were related to accuracy (a finding that is explored in a later section), and that while this explanation does fit the data, it is simply a possibility until further research explores the functional relevance of these differences in neural activity in more detail. As such, these interpretations should be considered as tentative, and further research is required to eliminate other potential explanations as well as replicate the current results before these explanations could be considered accurate.
Lack of Relationships Between Neural Activity Differences Between Groups and Performance Measures
Across the different ERP components that showed differences in neural activity, only the early component of the FN400 showed what could be reported as a trend towards a relationship between d-prime scores and the distribution of neural activity (p = 0.0672). This result was obtained assessing the relationship across all participants, and as such may be confounded by between-group differences in both neural activity and behavioural performance. Thus, it seems unlikely that any particular measure of altered neural activity in meditators that we detected is the explanation or reflects a direct mechanism of action for their improved WM performance. A number of potential interpretations for this are possible. The first (and in our view most likely) is that the improved WM performance is related to differences in neural processes that are causal for the differences in the measures we have detected, so that if we were to measure these deeper mechanisms (that lead to both the differences in the recall ERP and the improved performance) we would find a relationship to WM performance. As such, if future research detects these deeper mechanisms, they could be considered the mechanism of action underpinning improved WM accuracy in mindfulness meditators. The second potential explanation is that the differences in neural activity we detected do not relate to WM performance per se, but instead relate to differences in the experience of performing a WM task between the groups (perhaps differences in attention or non-judgemental acceptance that do not convert to differences in WM performance). If this explanation is true, we suggest that another (not yet detected) difference in neural activity between the groups is required to explain the differences in WM performance. Finally, it may be that the relationship between WM performance and these neural measures is non-linear or that the relationships are weak and require larger sample sizes to detect.
No Differences in WM Delay Period Theta, Alpha or 1/f Aperiodic Activity
Although research has suggested WM delay period parieto-occipital alpha and fronto-midline theta to be related to WM performance (Maurer et al., 2015; Scheeringa et al., 2009), differences in the BLC power, amplitude, centre frequency or bandwidth of these oscillations were not present in the meditation group (both when measured by traditional power computations across all electrodes, and measured independently of the 1/f aperiodic activity at specific electrodes of interest). As such, differences in these oscillations do not seem to be the explanation for the enhanced performance in the meditation group. In particular, frequentist analyses showed no significant differences in any of these measures. However, Bayes factor analyses showed only weak evidence against differences in the majority of alpha and theta measures, suggesting further research with larger sample sizes is required to confirm the null finding in these measures.
The lack of difference in alpha activity differs from the results of Jaiswal et al. (2019) which showed individuals high in trait mindfulness and low in anxiety (but without meditation experience) had a larger difference between alpha power in a high WM load condition compared to a low WM load condition than individuals low in trait mindfulness and high in anxiety (Jaiswal et al. 2019). However, Jaiswal et al. (2019) examined alpha activity during the WM probe period using a change detection task with coloured shapes as stimuli, rather than WM delay period alpha with letters as stimuli in a modified Sternberg task as per the current study. Jaiswal et al. (2019) also reported the comparison of a difference in alpha power between high and low WM load conditions, but not the results of a comparison between the high and low trait mindfulness groups in alpha power (only), while the current study only included a single WM load condition (and recruited groups matched in anxiety levels). As such, the results of the two studies are not directly comparable and may not be in conflict.
Although no differences were present in comparisons of oscillation measures such as amplitude, the meditation group in the current study did show a higher proportion of participants with fronto-midline theta peaks present above the 1/f aperiodic activity (with moderate evidence for this hypothesis shown by the Bayes factor analysis). Theta activity has been related to attentional function, particularly cognitive control (Cavanagh & Frank, 2014; Rac-Lubashevsky & Kessler, 2018; Sauseng et al., 2010; Sauseng et al., 2007), and previous research has indicated that meditators show increases in theta activity (Kerr et al., 2013; Lagopoulos et al., 2009; Tang et al., 2009). As such, it may be that mindfulness meditation practice enabled a higher proportion of the meditation group to generate theta oscillations to maintain attention on the task during the WM delay period. Having said that, it is also worth noting that when separated from the aperiodic signal, only a minority of participants in either group showed theta peaks during the WM delay period (18/58 in total). Previous research has suggested that theta activity is functionally relevant in the Sternberg task, but until now, this has not been tested with the 1/f aperiodic activity removed from oscillation measurements (Brookes et al., 2011; Jensen & Tesche, 2002; Kottlow et al., 2015; Maurer et al., 2015; Payne & Kounios, 2009; Scheeringa et al., 2009). This finding emphasises that it will be important for future research exploring the functional importance of different frequencies to measure oscillatory activity separately from 1/f aperiodic activity.
Similarly, no differences were detected in 1/f aperiodic slope. 1/f aperiodic activity is generated by population spiking synchrony, which has been suggested to reflect excitation/inhibition balances (Peterson et al., 2018). These decreases or increases in proportions of inhibition and excitation could reflect alterations in numbers of the different types of neurons, or alterations in the strength of synaptic connections (Gao et al., 2017). Although the current results suggest that 1/f aperiodic activity measures do not differ in meditators, this is the first exploration of 1/f aperiodic activity in meditators, and the analyses were only post hoc exploratory analyses restricted to three electrodes in a single task. Additionally, Bayes factor analyses showed there was moderate evidence against a difference in 1/f aperiodic slope at Fz, but only weak evidence against the 1/f aperiodic slope and offset at PO7 and PO8, and the 1/f aperiodic offset at Fz. Further research with larger sample sizes is required to confirm the null finding in these measures. Further research is required before the current null result could be confirmed. It would also be interesting to compare meditators and controls in 1/f aperiodic activity in other tasks or during the meditation state. This research is worth pursuing, as the altered inhibition / excitation balances have been suggested as potential explanations underpinning the benefits of meditation (Edwards et al., 2012; Guglietti et al., 2013).
In contrast to the null results for alpha, theta and 1/f aperiodic slope, there was a trend towards an interaction between group and electrode for 1/f aperiodic offset at parieto-occipital electrodes, with meditators showing lower values at PO7 only. Offset is positively related to overall neuronal firing rates/spiking activity (Manning et al., 2009; Miller et al., 2012) and the fMRI bold signal (Winawer et al., 2013). This might reflect meditators showing less overall neural activity in the left parieto-occipital region during the retention period. However, Bayes factor analysis suggested only weak evidence for this interaction. Given the exploratory nature of the 1/f aperiodic analyses, the number of uncontrolled multiple comparisons, and the weak evidence supporting the presence of this interaction, we are hesitant to draw conclusions from this finding, and are uncertain if the result would replicate. Further research exploring 1/f aperiodic metrics in samples of experienced meditators is required.
Limitations and Future Research
Although the control group in our study was well-matched to the meditation group in terms of age, gender and years of education, the primary limitation of cross-sectional research is that it does not offer any information about causation. Longitudinal studies with active control comparison groups have suggested that mindfulness meditation is causal in WM performance improvements (Jha et al., 2010; Mrazek et al., 2013; Quach et al., 2016; Van Vugt & Jha, 2011; Zeidan et al., 2010). Similarly, reduced neural activity related to distractor flickering in stimuli in WM tasks has been shown to result from mindfulness training (Schöne et al., 2018). However, we cannot rule out the possibility that self-selection biases in those who choose to meditate underpin the differences in neural activity related to WM in the current study. As such, the most parsimonious and robust interpretation of the results of cross-sectional studies of experienced mindfulness meditators is that the differences relate to leading a life that involves mindfulness meditation (Wang et al., 2019).
In a related point, the meditators in our study were self-selected, and while steps were taken to ensure practices met the Kabat-Zinn definition of mindfulness, and were breath or body focused, the group practiced techniques from a variety of traditions. As such, the group may have shown more heterogeneity than samples selected from a specific tradition only, and conclusions cannot be drawn regarding specific mindfulness meditation techniques. However, the consistent neural activity in the meditation group shown in the topographical consistency test, combined with the significant differences in the meditation group suggest common changes in the group that were concurrent with enhanced WM performance. As such, including a variety of mindfulness meditation techniques in the sample could also be viewed as a strength of the study, suggesting that the results generalize across techniques.
Another important limitation of the current study is that in our primary analyses, we were unable to examine the probe present and probe absent related neural activity separately due to an insufficient number of accepted epochs in each group (higher epoch numbers are recommended over low epoch numbers for ERP comparisons to ensure sufficient signal to noise ratio (Kappenman & Luck, 2010)). Additionally, the two groups differed in the number of epochs available for analysis, which may have the potential to influence results. To address these limitations, we performed confirmation analyses, firstly splitting the probe present and probe absent epochs to analyse them separately, and secondly excluding random epochs from the meditation group so that individuals from each group had a matched number of epochs for analysis (reported in the supplementary materials). These confirmation analyses showed the same results as the primary analyses, suggesting that these potential confounds were not the explanation for the current results.
Lastly, there are some limitations on the implications that can be drawn from the study that we think are worth noting. Although the results of this study indicate that on average the meditation group showed differences in neural activity related to WM probe presentation and decisions as well as improved WM performance, it should be noted that the results do not demonstrate that mindfulness meditation reliably elicits these changes on an individual level. Medium effect sizes as per the current study suggest that the ‘average’ mindfulness meditator showed better WM performance and more altered neural activity than ~ 70% of the control group, with the average meditator correctly identifying whether the probe was present or not present in the WM memory set 3.35 more times than the average control (out of 52 trials, so correct 6.44% more often). However, averages reflect only the centre of a distribution. As such, some meditators showed less accurate performance than the average control. Similarly, the current results do not indicate mindfulness meditation ubiquitously improves cognitive function (nor can the results answer the question of whether mindfulness meditation only affects attention, or affects WM independently of attention, discussed further in the supplementary materials). Some of our research has shown null results in different measures of cognition (Bailey et al. 2019, Payne et al., 2020).
The current research was also conducted on mindfulness meditators with mostly over 2 years of experience, and a minimum of 2 h per week of current practice, with the average amount of experience of over 9 years and 5 h per week of practice. As such, it is unlikely the current results reflect changes that could be obtained from a typical eight-week mindfulness course. Further research is required to fully characterise the effects of mindfulness meditation across cognitive processes, at an individual level, across different durations of meditation practice, and to extend the research on the effect of meditation on WM related neural activity to clinical groups (which might show different changes to the healthy individuals sampled in the current study). Further research with larger sample sizes is also required to confirm our null results, as Bayes factor analyses suggested that while our sample size provided sufficient power to detect some meaningful effect sizes, the Bayes factors suggested only weak evidence in support of the null hypotheses for the majority of our null results.
Finally, we feel it is important to note that the current results require replication before we can be confident they reflect true differences between mindfulness meditators and non-meditators. Typically, this point is taken for granted in scientific research, but we think it is worth noting explicitly given the current replication crisis in psychology (Collaboration, 2015), recent non-replications in mindfulness research (Bailey et al., 2019, Payne et al., 2020), and the current media hype surrounding mindfulness research.
References
Aftanas, L. I., Varlamov, A. A., Pavlov, S. V., Makhnev, V. P., & Reva, N. V. (2002). Time-dependent cortical asymmetries induced by emotional arousal: EEG analysis of event-related synchronization and desynchronization in individually defined frequency bands. International Journal of Psychophysiology, 44(1), 67–82.
Baddeley, A. (2012). Working memory: Theories, models, and controversies. Annual Review of Psychology, 63, 1–29.
Baer, R. A., Smith, G. T., Hopkins, J., Krietemeyer, J., & Toney, L. (2006). Using self-report assessment methods to explore facets of mindfulness. Assessment, 13(1), 27–45.
Bailey, N., Freedman, G., Raj, K., Sullivan, C., Rogasch, N., Chung, S., Hoy, K., Chambers, R., Hassed, C., & Van Dam, N. (2019). Mindfulness meditators show altered distributions of early and late neural activity markers of attention in a response inhibition task. PLoS One, 14(8), e0203096.
Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Manual for the Beck Depression Inventory-II. San Antonio: Psychological Corporation.
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300.
Britton, W. B., Davis, J. H., Loucks, E. B., Peterson, B., Cullen, B. H., Reuter, L., Rando, A., Rahrig, H., Lipsky, J., & Lindahl, J. R. (2018). Dismantling mindfulness-based cognitive therapy: Creation and validation of 8-week focused attention and open monitoring interventions within a 3-armed randomized controlled trial. Behaviour Research and Therapy, 101, 92–107.
Brookes, M. J., Wood, J. R., Stevenson, C. M., Zumer, J. M., White, T. P., Liddle, P. F., & Morris, P. G. (2011). Changes in brain network activity during working memory tasks: A magnetoencephalography study. Neuroimage, 55(4), 1804–1815.
Busch, N. A., & Herrmann, C. S. (2003). Object-load and feature-load modulate EEG in a short-term memory task. Neuroreport, 14(13), 1721–1724.
Buttle, H. (2011). Attention and working memory in mindfulness–meditation practices. The Journal of Mind and Behavior, 32(2), 123–134.
Buttle, H. (2013). More than the sum of my parts: A cognitive psychologist reflects on mindfulness/meditation experience. Reflective Practice, 14(6), 766–773.
Cavanagh, J. F., & Frank, M. J. (2014). Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences, 18(8), 414–421.
Chang, Y. K., Huang, C. J., Chen, K. F., & Hung, T. M. (2013). Physical activity and working memory in healthy older adults: An ERP study. Psychophysiology, 50(11), 1174–1182.
Christophel, T. B., Klink, P. C., Spitzer, B., Roelfsema, P. R., & Haynes, J.-D. (2017). The distributed nature of working memory. Trends in Cognitive Sciences, 21(2), 111–124.
Collaboration, O. S. (2015). Estimating the reproducibility of psychological science. Science, 349(6251), aac4716.
Curran, T., & Cleary, A. M. (2003). Using ERPs to dissociate recollection from familiarity in picture recognition. Cognitive Brain Research, 15(2), 191–205.
Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21.
Duarte, A., Ranganath, C., Winward, L., Hayward, D., & Knight, R. T. (2004). Dissociable neural correlates for familiarity and recollection during the encoding and retrieval of pictures. Cognitive Brain Research, 18(3), 255–272.
Edwards, J., Peres, J., Monti, D. A., & Newberg, A. B. (2012). The neurobiological correlates of meditation and mindfulness. In A. Moreira-Almeida & F. S. Santos (Eds.), Exploring frontiers of the mind-brain relationship (pp. 97–112). Springer.
Ergen, M., Yildirim, E., Uslu, A., Gürvit, H., & Demiralp, T. (2012). P3 response during short-term memory retrieval revisited by a spatio-temporal analysis. International Journal of Psychophysiology, 84(2), 205–210.
Federmeier, K. D., & Laszlo, S. (2009). Time for meaning: Electrophysiology provides insights into the dynamics of representation and processing in semantic memory. Psychology of Learning and Motivation, 51, 1–44.
Finnigan, S., Humphreys, M. S., Dennis, S., & Geffen, G. (2002). ERP ‘old/new’ effects: Memory strength and decisional factor (s). Neuropsychologia, 40(13), 2288–2304.
Friedman, D., & Johnson Jr., R. (2000). Event-related potential (ERP) studies of memory encoding and retrieval: A selective review. Microscopy Research and Technique, 51(1), 6–28.
Gao, R., Peterson, E. J., & Voytek, B. (2017). Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage, 158, 70–78.
Gramfort, A., Papadopoulo, T., Olivi, E., & Clerc, M. (2010). OpenMEEG: Opensource software for quasistatic bioelectromagnetics. Biomedical Engineering Online, 9(1), 45.
Gu, B.-M., van Rijn, H., & Meck, W. H. (2015). Oscillatory multiplexing of neural population codes for interval timing and working memory. Neuroscience & Biobehavioral Reviews, 48, 160–185.
Guglietti, C. L., Daskalakis, Z. J., Radhu, N., Fitzgerald, P. B., & Ritvo, P. (2013). Meditation-related increases in GABAB modulated cortical inhibition. Brain Stimulation, 6(3), 397–402.
Habermann, M., Weusmann, D., Stein, M., & Koenig, T. (2018). A student’s guide to randomization statistics for multichannel event-related potentials using Ragu. Frontiers in Neuroscience, 12.
Haller, M., Donoghue, T., Peterson, E., Varma, P., Sebastian, P., Gao, R., Noto, T., Knight, R. T., Shestyuk, A., & Voytek, B. (2018). Parameterizing neural power spectra. BioRxiv, 299859.
Hayama, H. R., Johnson, J. D., & Rugg, M. D. (2008). The relationship between the right frontal old/new ERP effect and post-retrieval monitoring: Specific or non-specific? Neuropsychologia, 46(5), 1211–1223.
Hofmann, S. G., Sawyer, A. T., Witt, A. A., & Oh, D. (2010). The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology, 78(2), 169–183.
Hutzler, F. (2014). Reverse inference is not a fallacy per se: Cognitive processes can be inferred from functional imaging data. Neuroimage, 84, 1061–1069.
Jaiswal, S., Tsai, S.-Y., Juan, C.-H., Muggleton, N. G., & Liang, W.-K. (2019). Low delta and high alpha power are associated with better conflict control and working memory in high mindfulness, low anxiety individuals. Social Cognitive and Affective Neuroscience, 14(6), 645–655.
Jensen, C. G., Vangkilde, S., Frokjaer, V., & Hasselbalch, S. G. (2012). Mindfulness training affects attention—Or is it attentional effort? Journal of Experimental Psychology: General, 141(1), 106–123.
Jensen, O., & Tesche, C. D. (2002). Frontal theta activity in humans increases with memory load in a working memory task. European Journal of Neuroscience, 15(8), 1395–1399.
Jha, A. P. (2002). Tracking the time-course of attentional involvement in spatial working memory: An event-related potential investigation. Cognitive Brain Research, 15(1), 61–69.
Jha, A. P., Stanley, E. A., Kiyonaga, A., Wong, L., & Gelfand, L. (2010). Examining the protective effects of mindfulness training on working memory capacity and affective experience. Emotion, 10(1), 54–64.
Kabat-Zinn, J. (2009). Wherever you go, there you are: Mindfulness meditation in everyday life. Hachette Books.
Kappenman, E. S., & Luck, S. J. (2010). The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology, 47(5), 888–904.
Kerr, C. E., Sacchet, M. D., Lazar, S. W., Moore, C. I., & Jones, S. R. (2013). Mindfulness starts with the body: Somatosensory attention and top-down modulation of cortical alpha rhythms in mindfulness meditation. Frontiers in Human Neuroscience, 7, 12.
Khoury, B., Sharma, M., Rush, S. E., & Fournier, C. (2015). Mindfulness-based stress reduction for healthy individuals: A meta-analysis. Journal of Psychosomatic Research, 78(6), 519–528.
Klimesch, W., Doppelmayr, M., Schwaiger, J., Winkler, T., & Gruber, W. (2000). Theta oscillations and the ERP old/new effect: Independent phenomena? Clinical Neurophysiology, 111(5), 781–793.
Klimesch, W., Sauseng, P., & Hanslmayr, S. (2007). EEG alpha oscillations: The inhibition–timing hypothesis. Brain Research Reviews, 53(1), 63–88.
Koenig, T., Kottlow, M., Stein, M., & Melie-García, L. (2011). Ragu: A free tool for the analysis of EEG and MEG event-related scalp field data using global randomization statistics. Computational Intelligence and Neuroscience, 2011, 4.
Kottlow, M., Schlaepfer, A., Baenninger, A., Michels, L., Brandeis, D., & Koenig, T. (2015). Pre-stimulus BOLD-network activation modulates EEG spectral activity during working memory retention. Frontiers in Behavioral Neuroscience, 9, 111.
Lagopoulos, J., Xu, J., Rasmussen, I., Vik, A., Malhi, G. S., Eliassen, C. F., Arntsen, I. E., Sæther, J. G., Hollup, S., & Holen, A. (2009). Increased theta and alpha EEG activity during nondirective meditation. The Journal of Alternative and Complementary Medicine, 15(11), 1187–1192.
Lavie, N. (1995). Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception and Performance, 21(3), 451–468.
Lehmann, D., Ozaki, H., & Pal, I. (1987). EEG alpha map series: Brain micro-states by space-oriented adaptive segmentation. Electroencephalography and Clinical Neurophysiology, 67(3), 271–288.
Lin, F.-H., Witzel, T., Ahlfors, S. P., Stufflebeam, S. M., Belliveau, J. W., & Hämäläinen, M. S. (2006). Assessing and improving the spatial accuracy in MEG source localization by depth-weighted minimum-norm estimates. Neuroimage, 31(1), 160–171.
Lutz, A., Slagter, H. A., Dunne, J. D., & Davidson, R. J. (2008). Attention regulation and monitoring in meditation. Trends in Cognitive Sciences, 12(4), 163–169.
Lutz, A., Slagter, H. A., Rawlings, N. B., Francis, A. D., Greischar, L. L., & Davidson, R. J. (2009). Mental training enhances attentional stability: Neural and behavioral evidence. Journal of Neuroscience, 29(42), 13418–13427.
Lykins, E. L., Baer, R. A., & Gottlob, L. R. (2012). Performance-based tests of attention and memory in long-term mindfulness meditators and demographically matched nonmeditators. Cognitive Therapy and Research, 36(1), 103–114.
Ma, W. J., Husain, M., & Bays, P. M. (2014). Changing concepts of working memory. Nature Neuroscience, 17(3), 347–356.
MacLean, K. A., Ferrer, E., Aichele, S. R., Bridwell, D. A., Zanesco, A. P., Jacobs, T. L., King, B. G., Rosenberg, E. L., Sahdra, B. K., & Shaver, P. R. (2010). Intensive meditation training improves perceptual discrimination and sustained attention. Psychological Science, 21(6), 829–839.
Malinowski, P., Moore, A. W., Mead, B. R., & Gruber, T. (2017). Mindful aging: The effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness, 8(1), 78–94.
Manning, J. R., Jacobs, J., Fried, I., & Kahana, M. J. (2009). Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. Journal of Neuroscience, 29(43), 13613–13620.
Maurer, U., Brem, S., Liechti, M., Maurizio, S., Michels, L., & Brandeis, D. (2015). Frontal midline theta reflects individual task performance in a working memory task. Brain Topography, 28(1), 127–134.
Michel, C. M., Murray, M. M., Lantz, G., Gonzalez, S., Spinelli, L., & de Peralta, R. G. (2004). EEG source imaging. Clinical Neurophysiology, 115(10), 2195–2222.
Miller, K. J., Hermes, D., Honey, C. J., Hebb, A. O., Ramsey, N. F., Knight, R. T., Ojemann, J. G., & Fetz, E. E. (2012). Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Computational Biology, 8(9), e1002655.
Mrazek, M. D., Franklin, M. S., Phillips, D. T., Baird, B., & Schooler, J. W. (2013). Mindfulness training improves working memory capacity and GRE performance while reducing mind wandering. Psychological Science, 24(5), 776–781.
Murray, M. M., Brunet, D., & Michel, C. M. (2008). Topographic ERP analyses: A step-by-step tutorial review. Brain Topography, 20(4), 249–264.
Nagel, B. J., Herting, M. M., Maxwell, E. C., Bruno, R., & Fair, D. (2013). Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain and Cognition, 82(1), 58–68.
Osaka, M., Komori, M., Morishita, M., & Osaka, N. (2007). Neural bases of focusing attention in working memory: An fMRI study based on group differences. Cognitive, Affective, & Behavioral Neuroscience, 7(2), 130–139.
Palmer, J. A., Makeig, S., Kreutz-Delgado, K., & Rao, B. D. (2008). Newton method for the ICA mixture model (pp. 1805-1808). 2008 IEEE international conference on acoustics, Speech and Signal Processing. Las Vegas, NV.
Pascual-Marqui, R. D. (2002). Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods and Findings in Experimental and Clincal Pharmacology, 24(Suppl D), 5–12.
Payne, J., Baell, O., Geddes, H., Fitzgibbon, B., Emonson, M., Hill, A., Van Dam, N., Humble, G., Fitzgerald, P., & Bailey, N. (2020). Experienced meditators exhibit no differences to demographically-matched controls in theta phase synchronisation, P200, or P300 during an auditory oddball task. Mindfulness, 11(3), 643–659.
Payne, L., & Kounios, J. (2009). Coherent oscillatory networks supporting short-term memory retention. Brain Research, 1247, 126–132.
Perrin, F., Pernier, J., Bertrand, O., & Echallier, J. (1989). Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology, 72(2), 184–187.
Peterson, E. J., Rosen, B. Q., Campbell, A. M., Belger, A., & Voytek, B. (2018). 1/f neural noise is a better predictor of schizophrenia than neural oscillations. BioRxiv, 113449.
Peterson, E. J., & Voytek, B. (2017). Alpha oscillations control cortical gain by modulating excitatory-inhibitory background activity. BioRxiv, 185074.
Poldrack, R. A. (2006). Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences, 10(2), 59–63.
Pontifex, M. B., Hillman, C. H., & Polich, J. (2009). Age, physical fitness, and attention: P3a and P3b. Psychophysiology, 46(2), 379–387.
Quach, D., Mano, K. E. J., & Alexander, K. (2016). A randomized controlled trial examining the effect of mindfulness meditation on working memory capacity in adolescents. Journal of Adolescent Health, 58(5), 489–496.
Rac-Lubashevsky, R., & Kessler, Y. (2018). Oscillatory correlates of control over working memory gating and updating: An eeg study using the reference-back paradigm. Journal of Cognitive Neuroscience, 30(12), 1870–1882.
Rauss, K. S., Pourtois, G., Vuilleumier, P., & Schwartz, S. (2009). Attentional load modifies early activity in human primary visual cortex. Human Brain Mapping, 30(5), 1723–1733.
Riley, M. R., & Constantinidis, C. (2016). Role of prefrontal persistent activity in working memory. Frontiers in Systems Neuroscience, 9, 181.
Rouder, J. N., Morey, R. D., Verhagen, J., Swagman, A. R., & Wagenmakers, E.-J. (2017). Bayesian analysis of factorial designs. Psychological Methods, 22(2), 304–321.
Rugg, M. D., & Curran, T. (2007). Event-related potentials and recognition memory. Trends in Cognitive Sciences, 11(6), 251–257.
Rugg, M. D., & Henson, R. N. (2002). Episodic memory retrieval: An (event-related) functional neuroimaging perspective. In A. M. Park, E. L. Wilding, & T. J. Bussey (Eds.), The cognitive neuroscience of memory. East Sussex, UK: Psychology Press.
Rugg, M. D., Mark, R. E., Walla, P., Schloerscheidt, A. M., Birch, C. S., & Allan, K. (1998). Dissociation of the neural correlates of implicit and explicit memory. Nature, 392(6676), 595–598.
Sauseng, P., Griesmayr, B., Freunberger, R., & Klimesch, W. (2010). Control mechanisms in working memory: A possible function of EEG theta oscillations. Neuroscience & Biobehavioral Reviews, 34(7), 1015–1022.
Sauseng, P., Hoppe, J., Klimesch, W., Gerloff, C., & Hummel, F. C. (2007). Dissociation of sustained attention from central executive functions: Local activity and interregional connectivity in the theta range. European Journal of Neuroscience, 25(2), 587–593.
Scheeringa, R., Petersson, K. M., Oostenveld, R., Norris, D. G., Hagoort, P., & Bastiaansen, M. C. (2009). Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage, 44(3), 1224–1238.
Schmeichel, B. J., Volokhov, R. N., & Demaree, H. A. (2008). Working memory capacity and the self-regulation of emotional expression and experience. Journal of Personality and Social Psychology, 95(6), 1526–1540.
Schöne, B., Gruber, T., Graetz, S., Bernhof, M., & Malinowski, P. (2018). Mindful breath awareness meditation facilitates efficiency gains in brain networks: A steady-state visually evoked potentials study. Scientific Reports, 8(1), 13687.
Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., Hergueta, T., Baker, R., & Dunbar, G. C. (1998). The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59 Suppl 20:22–33.
Slagter, H. A., Lutz, A., Greischar, L. L., Nieuwenhuis, S., & Davidson, R. J. (2009). Theta phase synchrony and conscious target perception: Impact of intensive mental training. Journal of Cognitive Neuroscience, 21(8), 1536–1549.
Steer, R., & Beck, A. (1997). Beck anxiety inventory manual. San Antonio, TX: Psychological Corporation.
Sternberg, S. (1966). High-speed scanning in human memory. Science, 153(3736), 652–654.
Stróżak, P., Abedzadeh, D., & Curran, T. (2016). Separating the FN400 and N400 potentials across recognition memory experiments. Brain Research, 1635, 41–60.
Tadel, F., Baillet, S., Mosher, J. C., Pantazis, D., & Leahy, R. M. (2011). Brainstorm: A user-friendly application for MEG/EEG analysis. Computational Intelligence and Neuroscience, 2011, 8.
Tallon-Baudry, C., Bertrand, O., Delpuech, C., & Pernier, J. (1997). Oscillatory γ-band (30–70 Hz) activity induced by a visual search task in humans. Journal of Neuroscience, 17(2), 722–734.
Tang, Y.-Y., Ma, Y., Fan, Y., Feng, H., Wang, J., Feng, S., Lu, Q., Hu, B., Lin, Y., & Li, J. (2009). Central and autonomic nervous system interaction is altered by short-term meditation. Proceedings of the National Academy of Sciences, 106(22), 8865–8870.
Tang, Y.-Y., Ma, Y., Wang, J., Fan, Y., Feng, S., Lu, Q., Yu, Q., Sui, D., Rothbart, M. K., & Fan, M. (2007). Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences, 104(43), 17152–17156.
Tran, T. T., Hoffner, N. C., LaHue, S. C., Tseng, L., & Voytek, B. (2016). Alpha phase dynamics predict age-related visual working memory decline. NeuroImage, 143, 196–203.
Van Vugt, M. K., & Jha, A. P. (2011). Investigating the impact of mindfulness meditation training on working memory: A mathematical modeling approach. Cognitive, Affective, & Behavioral Neuroscience, 11(3), 344–353.
Vigneau, M., Beaucousin, V., Herve, P.-Y., Duffau, H., Crivello, F., Houde, O., Mazoyer, B., & Tzourio-Mazoyer, N. (2006). Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage, 30(4), 1414–1432.
Vogel, E. K., Woodman, G. F., & Luck, S. J. (2005). Pushing around the locus of selection: Evidence for the flexible-selection hypothesis. Journal of Cognitive Neuroscience, 17(12), 1907–1922.
Voytek, B., Kramer, M. A., Case, J., Lepage, K. Q., Tempesta, Z. R., Knight, R. T., & Gazzaley, A. (2015). Age-related changes in 1/f neural electrophysiological noise. Journal of Neuroscience, 35(38), 13257–13265.
Wagner, A. D., Shannon, B. J., Kahn, I., & Buckner, R. L. (2005). Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences, 9(9), 445–453.
Walach, H., Buchheld, N., Buttenmüller, V., Kleinknecht, N., & Schmidt, S. (2006). Measuring mindfulness—The Freiburg mindfulness inventory (FMI). Personality and Individual Differences, 40(8), 1543–1555.
Wang, M. Y., Freedman, G., Raj, K., Fitzgibbon, B. M., Sullivan, C., Tan, W.-L., Van Dam, N., Fitzgerald, P. B., & Bailey, N. W. (2019). Mindfulness meditation alters neural activity underpinning working memory during tactile distraction. BioRxiv, 790584.
Winawer, J., Kay, K. N., Foster, B. L., Rauschecker, A. M., Parvizi, J., & Wandell, B. A. (2013). Asynchronous broadband signals are the principal source of the BOLD response in human visual cortex. Current Biology, 23(13), 1145–1153.
Wolpaw, J. R. (2002). Memory in neuroscience: Rhetoric versus reality. Behavioral and Cognitive Neuroscience Reviews, 1(2), 130–163.
Woodruff, C. C., Hayama, H. R., & Rugg, M. D. (2006). Electrophysiological dissociation of the neural correlates of recollection and familiarity. Brain Research, 1100(1), 125–135.
Yonelinas, A. P. (2002). The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language, 46(3), 441–517.
Zeidan, F., Johnson, S. K., Diamond, B. J., David, Z., & Goolkasian, P. (2010). Mindfulness meditation improves cognition: Evidence of brief mental training. Consciousness and Cognition, 19(2), 597–605.
Data Availability Statement
Participants involved in the study did not provide consent to data sharing, and data sharing was not approved by the Alfred Hospital ethics committee, so the results reported in the paper comprise the complete data available for sharing. The Alfred Hospital ethics committee can be contacted via research@alfred.org.au.
Funding
PBF has received equipment for research from MagVenture A/S, Medtronic Ltd., Cervel Neurotech and Brainsway Ltd. and funding for research from Neuronetics and Cervel Neurotech. PBF is supported by a National Health and Medical Research Council of Australia Practitioner Fellowship (6069070). NCR is supported by a National Health and Medical Research Council of Australia Fellowship (1072057). These funders supported the researchers for other projects but no funding contribution was made to this specific project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
NWB designed and executed the study, assisted with data collection, performed data analysis and wrote the paper. GF collected data and contributed to editing the final manuscript. KR collected data and contributed to editing the final manuscript. KNS contributed to data analysis and editing of the final manuscript. LRP contributed to data analysis and editing of the final manuscript. CMS contributed to data collection, analysis and editing of the final manuscript. SWC contributed to data analysis and editing of the final manuscript. ATH contributed to data analysis and editing of the final manuscript. NCR contributed to study design, advised on analysis and editing of the final manuscript. PBF contributed to study design and editing of the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of both The Alfred Hospital and Monash University ethical research committee and with the 1964 Helsinki declaration and its later amendments.
Competing Interests
PBF has received equipment for research from MagVenture A/S, Medtronic Ltd., Cervel Neurotech and Brainsway Ltd. and funding for research from Neuronetics and Cervel Neurotech. PBF is on the scientific advisory board for Bionomics Ltd. All other authors have no conflicts to report. PBF is supported by a National Health and Medical Research Council of Australia Practitioner Fellowship (6069070). NCR is supported by a National Health and Medical Research Council of Australia Fellowship (1072057).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 840 kb)
Rights and permissions
About this article
Cite this article
Bailey, N.W., Freedman, G., Raj, K. et al. Mindfulness Meditators Show Enhanced Accuracy and Different Neural Activity During Working Memory. Mindfulness 11, 1762–1781 (2020). https://doi.org/10.1007/s12671-020-01393-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12671-020-01393-8