Abstract
Breast cancer, the most common form of neoplasms in females, is the second death cause of cancer patients. Over the past few decades, the therapeutic potential of herbal medicine has been well studied. The ginger extract contains (6)-gingerol compound, which has several types of bioactivities including antioxidant, anticancer, antibacterial, and anti-inflammatory properties. In this study, we used chitosan-coated nanostructured lipid carriers (CS-NLCs) to deliver (6)-gingerol to MCF-7 breast cancer cells. The nanoparticles were characterized by conducting transmission electron microscopy (TEM), dynamic light scattering (DLS), Zeta potential, and Fourier transform infrared (FTIR) spectroscopy analysis. The CS-NLCs anticancer activity was evaluated by measuring the MCF-7 and HUVEC cells’ survival and determining the induced cell death type applying DAPI and Annexin V-FITC staining assays. Moreover, the cell’s apoptotic response was studied by providing apoptotic gene expression profile (caspase 3, 8, and 9). Finally, the CS-NLCs antioxidant activity was evaluated by DPPH method. The CS-NLCs exhibited selective apoptotic death on the MCF-7 breast cancer cells by overexpressing the apoptotic gene expression profile, which were verified by DAPI and Annexin V-FITC staining results. The meaningful increased DPPH inhibition rate following the increased CS-NLCs concentrations indicated its powerful antioxidant activity. In conclusion, the produced CS-NLCs significantly induce selective apoptotic death in the human MCF-7 breast cancer cells compared with the normal HUVEC cell line. The selective toxicity and antioxidant activity are the two main sufficient properties of the safe efficient anticancer compound. Therefore, the CS-NLCs has the potential to be used as the natural safe efficient anti-breast cancer drug delivery system. However, further in vivo studies are required to determine their pharmaceutical details.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer is developed by non-stop proliferation of epithelial cells [1,2,3,4]. However, the in-time screening and targeted treatment strategies can suppress its progression. Breast cancer remains one of the main leading death causes in women [5,6,7]. Cancer treatment strategies have recruited a wide field of methodologies including magnetotherapy, chemotherapy, and radiotherapy. However, herbal-extracted products are still the safest and efficient cancer therapy strategies [8,9,10]. They have assigned more than 50% of drugs in current clinical trials [11,12,13,14]. Herbal-based bioactive molecules found in medicinal plants have been used as the safe effective anticancer compounds [15,16,17]. Several types of medicinal plants have already been discovered suppressing the cancer progression [18,19,20]. There are several types of natural antioxidant molecules extracted from animal and herbal (ginger) originated sources. Gingerol, the herbal phenolic molecule, is an anti-inflammatory, antioxidant, anti-tumor, and antimicrobial compound found in edible ginger [21,22,23].
Chitosan coating layers as well as surfactant-formulated nano-emulsions due to their high absorption, controlled release, bio-adhesiveness, biocompatibility, and biodegradability have attracted the attention of researchers as the bio-compatible component of drug delivery systems [24, 25]. Chitosan-based nanocarriers are considered one of the most flexible and biocompatible systems among the other types of nanocarriers [26, 27]. Chitosan is a deacetylated form of chitin, an abundant polysaccharide found in many organisms (e.g., fungi, insects, plants, crustaceans) [28]. Nanocarriers such as polymeric, emulsions, and liposomes ranging up to 300 nm have been developed for drug delivery purposes [29,30,31,32]. Solid lipid nanoparticles (SLN), as nanostructured lipid carriers (CS-NLCs), are widely used as popular drug delivery platform. Nanostructured lipid carriers have the potential to gradually release their drug cargo depending their environmental pH, temperature, and other active molecular interactions [33, 34].
In the current study, (6)-gingerol was encapsulated by the chitosan-coated NLC as a nano drug delivery system to evaluate their selective anticancer activity on human breast MCF-7 cancer cells compared with human umbilical vein endothelial (HUVEC) normal cell line by conducting MTT, DAPI, V-FITC Annexin, and Q-PCR analysis. Finally, the antioxidant activity of CS-NLCs was studied by applying DPPH analysis.
2 Materials and Methods
2.1 Materials
Oleic acid, glycerol monostearate (GMS), lecithin, and polysorbagte-80 (Tween-80) were provided by Merck & Co., Inc. (US, New Jersey). Dimethyl sulfoxide (DMSO), 1,1-diphenyl-2-picrylhydrazyl (DPPH), and chitosan were obtained from Merck & Co., Inc. Propidium iodide (PI), 3,4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide (MTT), and FBS were procured from Sigma-Aldrich. The culture medium and penicillin streptomycin were purchased from Invitrogen company. SYBR green kit and cDNA synthesis kit were from Qiagen company. MCF-7 (breast cancer) and HUVEC cell lines were from the Bu Ali Research Institute (Iran).
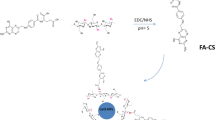
2.2 Synthesis of Chitosan-Coated NLC for (6)-Gingerol Delivery
To synthesize nanoparticles, both the ionic gelation and sonicate-mediated homogenization methods were conducted. The liquid and solid lipids including oleic acid and glyceryl monostearate, respectively were combined with lecithin. Then, (6)-gingerol was added to the lipid matrix by heating (up to 80 °C) under vigorous stirring conditions until melting. The selected temperature has been previously used for gingerol extraction in nonpolar solvents [35]. The aqueous phase was prepared using polysorbate 80 (Tween 80) and distilled water. Then it was incubated at 80 °C. The liquid and solid lipids were used to solve the (6)-gingerol and encapsulate the non-polar composition. The oil and aqueous phases were merged, and the solution was homogenized and then sonicated at 320 W for 10 min (8″ on 2″ off). The solution containing chitosan was dissolved in acetic acid and added to NLC. After incubation, the sample was placed on a stirrer for 2 h. Finally, the solution was centrifuged, and the precipitate was lyophilized. The Freeze dryer was set at − 80 °C and 0.05 Torr for 24 h [36].
2.3 Characterization of CS-NLCs
The CS-NLCs synthesized were characterized using dynamic light scattering (DLS), transmission electron microscopy (TEM), Zeta potential measurement, and Fourier transform infrared (FTIR) spectroscopy analysis. The DLS (Nano-ZS, Malvern, UK) method was used to determine the mean diameter and PDI, and shape was observed by TEM (JEOL, Tokyo, Japan). Zeta potential (Nano-ZS, Malvern, UK) was determined to estimate the stability of CS-NLCs, and Fourier transform infrared (FTIR) spectroscopy (PerkinElmer, Walthman, MA, USA) was performed to detect the functional groups of CS-NLCs in the range of 4000–400 cm−1.
2.4 DPPH Assay
The DPPH reduction process following the antioxidant exposure condition leads to a color change occurrence, which is detectable by measuring the DPPH absorbance at 517 nm wavelength. Briefly, different concentrations of CS-NLCs were added to ethanolic DPPH solution (0.05 mg/mL) and incubated for 30 min. A UV spectrophotometer was used to read the samples and standard butylated hydroxyanisole (BHA) absorbance at 517 nm [37]. The CS-NLCs radical scavenging activity was calculated following the equation:
2.5 Cell Culture
The complete RPMI culture medium supplemented by 10% fetal bovine serum (FBS) and penicillin/streptomycin mix was used culture cells under the standard conditions.
2.6 MTT Viability Assay
MCF-7 and HUVEC cells were seeded in 96-well plates. The 24-h cultured cells were exposed to different concentrations of CS-NLCs (3, 7.5, 15, 30, 60, 125, 250, 500, and 1000 µg/mL) and incubated for 48 h. Then, the culture medium was refreshed with MTT-supplemented fresh medium and incubated for further 4 h. Finally, the mediums were drained, and the cells were exposed to 100 µL of dimethyl sulfoxide (DMSO) for 10 min [38]. In the end, the viability of the cells was defined by MTT assay at 570 nm by using plate reader spectrophotometer (Epoch, Biotek, Winooski, VT, UK). The cell viability was calculated from the following equation:
2.7 DAPI Staining and Morphology Analysis
The 24-h cultured MCF-7 cancer cells were exposed to 125, 250, and 500 µg/mL concentrations of CS-NLCs in a 6-well plate. Then, the cells were fixed by methanol and washed to get ready for DAPI staining process. In this regard, the cells were exposed to DAPI dye and incubated for 15 min at room temperature. The stained cells were studied by a fluorescent microscope (Olympus DP27, Tokyo, Japan).
2.8 Apoptosis Detection by Annexin V-FITC Assay
The MCF-7 cells were cultured in 6-well plates, and after 24 h, the cells were exposed to CS-NLCs (125, 250, 500 µg/mL). To determine apoptosis response, an Annexin V-FITC apoptosis staining/detection kit (ab14085) was applied. The cells were stained as the suggested kit instrument. Briefly, the cells were precipitated by centrifuging at 2500 rpm for 5 min. Then, Binding Buffer 1X was added to the pelleted cells to re-disperse the cells. The cells were stained by annexin V (5 µL) and propidium iodide (PI) (5 µL). Finally, the stained cells were incubated in dark conditions for 5 min to get ready for flow cytometry analysis [39].
2.9 Real-Time PCR
The MCF-7 cells were exposed to CS-NLCs (125, 250, and 500 µg/mL) for 48 h. The cells’ total RNA was isolated using the Rx BON kit. Briefly, the cells were lysed by 1 µL of lysis buffer solution and exposed to chloroform (250 µL) for 15 min. The samples were centrifuged (12,000 rpm, 4 °C), and cold propanol (600–800 µL) was added to the supernatant. The samples were kept at − 20 °C for 24 h and centrifuged for 45 min (12,000 rpm, 4 °C). The precipitate was mixed with cold ethanol (70%) and centrifuged at 4 °C conditions for 20 min. The resulting precipitate was dried at 25 °C and diluted with 20 µL of distilled water. The extracted RNA solution was stored at − 80 °C. Nano-Drop method was used to determine the quantity and quality of the extracted RNA before cDNA synthesis. To synthesize cDNA library, the Pars Tous cDNA synthesis kit was utilized. The target gene primer sets were designed at the exon junction site of the caspase 3, 8, and 9 applying Allel ID6 software (Table 1). The gene expression analysis was conducted by applying the SYBR Green PCR aster mix kit (Pars Tous, Iran). The Q-PCR thermocycling program was set at 39 cycles repeated as: 95 °C (15 s), 59 °C (20 s), and 72 °C (20 s). The gene expression results were normalized by applying the GAPDH control gene [40].
2.10 Statistical Analysis
The SPSS-21 software was utilized to conduct a one-way ANOVA test and determine the significant level at P < 0.05. The results were expressed as the mean ± SD.
3 Results
3.1 Characterization of CS-NLCs
According to Fig. 1, the average size of CS-NLCs was 197.77 nm. The reported polydispersity index (PDI) shows the size distribution of molecules or particles. A PDI of 0.0 reflects perfectly uniform particles, and a PDI between 0.1 and 0.4 indicates polydisperse molecules or particles with a moderate size distribution [41, 42]. The CS-NLCs polydispersity was obtained as 0.28, which reflected the CS-NLCs’ stability for a long time. The nano-carriers TEM images (Fig. 1A) showed the semi-spherical shape of CS-NLCs (Fig. 1B). The CS-NLCs surface charge was determined at + 8.41 mV, which prevents their aggregation in aqueous media and reflects their acceptable stability (Fig. 1C).
A Dynamical light scattering (DLS) plot of CS-NLCs. The average size of the particles was 197.77 nm, and the polydispersity index (PDI) was equal to 0.28. B Transmission electron microscopy (TEM) image of CS-NLCs revealed that the particles were morphologically quasi-spherical. C Zeta potential of CS-NLCs. The value of Zeta potential was + 8.41 mV
The FTIR spectrum of CS-NLCs has been provided in Fig. 2, displaying the nano-structure elements including chitosan, NLC, and gingerol by detecting the stretching vibrations of distinct structural bonds at 3306.06 cm−1, 2921.38 cm−1, 1468.16 cm−1, 1181.70 cm−1, and 721.22 cm−1 wavenumbers referring the chitosan, lipid ingredients, and gingerol structure, respectively (Table 2).
3.2 Antioxidant Activity
The ability of the CS-NLCs to neutralize DPPH free radicals was investigated at four concentrations. The results showed that CS-NLCs had significant antioxidant activity compared to BHA. As shown in Fig. 3, the increased CS-NLCs concentrations significantly enhanced the DPPH inhibition rate demonstrating a dose-dependent antioxidant activity for CS-NLCs. The minimum concentration of CS-NLCs inhibiting the 50% of DPPH radicals was estimated at 250 µg/mL.
3.3 Anti-Proliferative Activity
The CS-NLCs exhibited selective toxicity on the exposed cancerous MCF-7 cells compared with normal HUVEC cell line. As shown in Fig. 4, the normal cell survival was not meaningfully affected by CS-NLCs exposure. This is while the MCF-7 cell survival was significantly decreased following the increased CS-NLCs treatment doses (Fig. 5).
MTT cell toxicity assay. The CS-NLCs at different doses were incubated with MCF-7 cancer cells (24 and 48 h) and showed concentration- and time-dependent cytotoxicity against these cells. The IC50 value was equal to 250 µg/mL. *p < 0.05, **p < 0.01, and ***p < 0.001 indicated significant difference compared to the control
3.4 DAPI for Nuclear Staining
As displayed in Fig. 6, CS-NLCs induced changes in the nuclei of treated cells compared to untreated control cells. The CS-NLCs caused an increase in the cellular nuclear apoptotic bodies and chromatin density compared with the control cell nucleus. Also, chromatin fragmentation was observed in the cells treated with CS-NLCs, which is due the apoptosis occurrence in the MCF7 cells.
DAPI stained for monitoring apoptotic nucleus changes in the MCF-7 cells exposed to CS-NLCs (125, 250, and 500 µg/mL). The green arrow indicates normal cells, and the red arrow shows apoptotic cells. The cell nucleus in the control group was morphologically normal, but chromatin fragmentation was evident in the cells treated with CS-NLCs × 40 magnification
3.5 Annexin Staining
Annexin V binds to membrane phospholipids in a Ca2+-dependent manner. It has a high-binding affinity for phosphatidylserine. In apoptotic cells, Annexin V is expelled out of the cells by being translocated from the inner to the outer leaflet of the plasma membrane, allowing this assay to discern alive from necrotic and early form of the late apoptotic cells. According to results, MCF-7 cells exposed to CS-NLCs underwent apoptosis. This is while the control cells viability remained 92.5%. After 48-h exposure to CS-NLCs, the cells ratio in Annexin V-FITC-positive area has gradually increased from 6.4 to 52.8%. The Fig. 7 presents the percentage of alive cells (double negative), early apoptotic (Annexin V positive/PI negative), necrotic (Annexin V negative/ PI positive), and late apoptotic (double positive) cells in the control group at different concentrations.
Annexin V-FITC staining. Treatment with CS-NLCs induced apoptosis in MCF-7 cells (24 h), as evidenced by an increase in the ratio of Annexin V-FITC-positive cells with time. At the concentrations of 125, 250, and 500 µg/mL, the percentage of late apoptotic cells was recorded as 6.4, 47.3, and 52.8, respectively
3.6 Apoptotic Genes’ Expression
The transcripts of the caspase-3, caspase-8, and caspase-9 genes were quantified using real-time PCR in cells treated with different concentrations of CS-NLCs (125, 250, and 500 µg/mL). By increasing the concentration of CS-NLCs, the expression of apoptotic genes (Cas3/Cas8/Cas9) significantly increased compared with control untreated cells. The effect of CS-NLCs was more pronounced on the caspase-3 gene than on caspases 9 and 8 genes (Fig. 8).
4 Discussion
The present study focused on the synthesis of lipid nanostructures coated with chitosan for the efficient delivery of (6)-gingerol to breast cancer cells, as well as the investigation of the antioxidant and pro-apoptotic effects of the delivered compound. The findings of this study contribute to the growing field of nanotechnology-based drug delivery systems for targeted cancer therapy.
The physicochemical characterization of the synthesized CS-NLCs revealed their suitability as drug carriers. The particle size and Zeta potential measurements indicated that the CS-NLCs were within the nanoscale range, which is desirable for efficient cellular uptake and improved biodistribution. The small particle size also suggests a higher surface area-to-volume ratio, facilitating drug encapsulation and release. The positive Zeta potential of the CS-NLCs may contribute to their enhanced cellular uptake through electrostatic interactions with the negatively charged breast cancer cell membranes.
In the present study, we aimed to assess whether (6)-gingerol could be used as an effective substance to suppress cancer or not. The results showed that (6)-gingerol prevented MCF-7 cancer cells’ growth via apoptosis induction. Ginger has been widely studied for its medicinal properties, which belong to its main bioactive compound, (6)-gingerol [43]. It has been reported as an antioxidative, anticancer, and anti-inflammatory compound [44,45,46,47]. The anticancer effects of (6)-gingerol seem to be mediated through apoptosis induction [48]. Considering the medicinal properties of (6)-gingerol, we aimed to assess its toxicity on the MCF-7 breast cancer cells. Nevertheless, the poor water solubility and low bioavailability of (6)-gingerol decrease its therapeutic efficacy [49]. Therefore, to improve the efficiency of (6)-gingerol, a suitable carrier is needed to deliver this agent, and nanostructured carriers are promising vehicles for this purpose. During recent decades, lipid-based drug delivery systems have evolved into nanoscale drug carriers to increase the solubility and bioavailability of water-insoluble drugs [50, 51]. In this regard, CS-NLCs are promising drug carriers for (6)-gingerol and can act as an alternative drug delivery system to bypass the first-pass metabolism, prolonging the drug’s circulation time [52].
Among many platforms that can be used in nanostructured lipid carriers, we used chitosan to augment the biological effects of (6)-gingerol. Chitosan-coated nanoparticles were synthesized using an ionic gel with sodium tripolyphosphate as a cross-linking agent. The TEM, FTIR, and DLS techniques were used to further characterize the particles. Chitosan and its derivatives have many applications in pharmaceutical and biomedical sciences [53,54,55]. Hence, a chitosan-coated system can significantly increase the accumulation of CS-NLCs at the target site, increasing the bioavailability of encapsulated drugs [56, 57]. As a result, modifying the surface of CS-NLCs with chitosan offers various advantages, such as increasing drug retention at the target site [58]. Clinical studies, as well as in vivo and in vitro experiments have suggested that several signaling pathways, including ROS/NF-κB/COX-2, ERK1/2, SAPK/JNK, Nrf2, p65/NF-κB, TNF-α, Bax/Bcl2, and p38/MAPK, as well as caspases-3, -9, and p53, can contribute to the anticancer, anti-proliferative, and anti-inflammatory activities of (6)-gingerol and ginger derivatives [59, 60]. In the present study, we provided evidence that (6)-gingerol could trigger a caspase-dependent apoptotic pathway with an increase in the expression of caspase 3, 8, and 9 genes in MCF-7 cells.
The evaluation of cellular uptake demonstrated the ability of the CS-NLCs to target breast cancer cells. The positive charge of chitosan promotes electrostatic interactions with the negatively charged cell membranes facilitates the CS-NLCs internalization into cancer cells. This selective cellular uptake can enhance the therapeutic efficacy of (6)-gingerol by concentrating the drug within the tumor microenvironment, minimizing systemic exposure, and reducing off-target effects on healthy tissues.
Assessment of the cytotoxicity and selectivity of the (6)-gingerol-loaded CS-NLCs revealed their potential as an effective anticancer therapy. The enhanced cytotoxicity observed in breast cancer cells compared to normal cells suggests the specific targeting of cancer cells by the delivered (6)-gingerol. This selectivity is crucial in minimizing the adverse effects associated with traditional chemotherapy, which often affects healthy cells and tissues.
The improved selectivity of the CS-NLCs may be attributed to the enhanced cellular uptake and intracellular release of (6)-gingerol within cancer cells. Furthermore, the investigation of the antioxidant and pro-apoptotic effects of (6)-gingerol delivered through CS-NLCs provided valuable insights into the potential mechanisms of action. The pro-apoptotic effects of (6)-gingerol can induce programmed cell death in breast cancer cells, further inhibiting tumor growth and metastasis. The findings suggest that the delivery of (6)-gingerol through CS-NLCs enhances its therapeutic effects, potentially leading to improved outcomes in breast cancer treatment.
Overall, this study demonstrates the potential of lipid nanostructures coated with chitosan for the targeted delivery of (6)-gingerol to breast cancer cells. The successful encapsulation, cellular uptake, cytotoxicity, and selectivity of the formulation support its application as an effective therapeutic approach [61,62,63,64]. The investigation of the antioxidant and pro-apoptotic effects further highlights the potential mechanisms of action underlying the observed therapeutic effects. Future studies may focus on in vivo experiments to validate the efficacy and safety of the developed NLC formulation in animal models, ultimately leading to the translation of this promising approach into clinical trials for breast cancer treatment.
The antioxidant property of (6)-gingerol was evaluated by the DPPH test. The nanoparticles showed a high antioxidant activity and inhibited DPPH free radicals by 76%, confirming that (6)-gingerol possessed a high antioxidant activity. In another study, the antioxidant activity of (6)-gingerol was evaluated by the DPPH, hydroxyl radical, and superoxide radical tests, and the results of these tests demonstrated a significant antioxidant activity for (6)-gingerol with the IC50 values of 26.3, 4.62, and 4.05 mM, respectively [65]. Overall, gingerol and chitosan seem to show additive antioxidant activity as confirmed by the DPPH test in the present study.
5 Conclusion
The anticancer and antioxidant activities of CS-NLCs were investigated in this study. As observed in the DPPH assay, CS-NLCs showed dose-dependent antioxidant activity. The nanoparticles also have considerable cytotoxic activity against MCF-7 cancer cells but not against HUVEC normal cells. Our study suggests that CS-NLCs can induce apoptosis in a breast cancer cell line (as confirmed by DAPI and Annexin V staining) and, therefore, can be regarded as a therapeutic agent for this cancer.
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper.
References
Javad Farhangi, M., et al. (2021). MOF-Mediated Synthesis of CuO/CeO2 Composite nanoparticles: Characterization and estimation of the cellular toxicity against breast cancer cell line (MCF-7). Journal of Functional Biomaterials, 12(4), 53.
Es-haghi, A., et al. (2019). The expression of antioxidant genes and cytotoxicity of biosynthesized cerium oxide nanoparticles against hepatic carcinoma cell line. Avicenna Journal of Medical Biochemistry, 7(1), 16–20.
Mohammadzadeh, V., et al. (2023). Poly-γ-glutamic acid nanoparticles as adjuvant and antigen carrier system for cancer vaccination. Journal of Controlled Release, 362, 278–296.
Haddad-Mashadrizeh, A. A., Bahrami, A. R., Moghaddam, M. M., Saebnia, N. (2023). Introns and their therapeutic applications in biomedical researches. Iranian Journal of Biotechnology, 21(4), 31–46.
Akbarzadeh, I., et al. (2022). Gingerol/letrozole-loaded mesoporous silica nanoparticles for breast cancer therapy: In-silico and in-vitro studies. Microporous and Mesoporous Materials, 337, 111919.
Ashna, M., et al. (2022). Greener synthesis of cerium oxide nanoemulsion using pollen grains of Brassica napus and evaluation of its antitumour and cytotoxicity properties. Materials Technology, 37(8), 525–532.
Youssry, S. A., El-Sheredy, H. G., & Shalaby, T. I. (2022). In vitro evaluation of antitumor and immunomodulatory potential of curcumin nano-emulsion on breast cancer. BioNanoScience, 12(3), 841–850.
Taghavizadeh Yazdi, M. E., et al. (2022). Antimycobacterial, anticancer, antioxidant and photocatalytic activity of biosynthesized silver nanoparticles using Berberis Integerrima. Iranian Journal of Science and Technology, Transactions A: Science, 46(1), 1–11.
Mousavi-Kouhi, S. M., et al. (2021). Silver-zinc oxide nanocomposite: From synthesis to antimicrobial and anticancer properties. Ceramics International, 47(15), 21490–21497.
Modarres, M., & TaghavizadehYazdi, M. E. (2021). Elicitation improves phenolic acid content and antioxidant enzymes activity in salvia leriifolia cell cultures. Iranian Journal of Science and Technology, Transactions A: Science, 45(3), 849–855.
Sekiwa, Y., Kubota, K., & Kobayashi, A. (2000). Isolation of novel glucosides related to gingerdiol from ginger and their antioxidative activities. Journal of Agricultural and Food Chemistry, 48(2), 373–377.
Amiri, M. S., Yazdi, M. E. T., & Rahnama, M. (2021). Medicinal plants and phytotherapy in Iran: Glorious history, current status and future prospects. Plant Science Today, 8(1), 95–111.
Nadaf, M., et al. (2023). Ethnobotanical diversity of trees and shrubs of Iran: A comprehensive review. International Journal of Plant Biology, 14(1), 120–146.
Yazdi, M. E. T., et al. (2020). Green synthesis of silver nanoparticles using helichrysum graveolens for biomedical applications and wastewater treatment. BioNanoScience, 10(4), 1121–1127.
Joung, Y. H., et al. (2014). Combination of AG490, a Jak2 inhibitor, and methylsulfonylmethane synergistically suppresses bladder tumor growth via the Jak2/STAT3 pathway. International journal of oncology, 44(3), 883–895.
Ghorani-Azam, A., et al. (2022). Resveratrol-mediated gold-nanoceria synthesis as green nanomedicine for phytotherapy of hepatocellular carcinoma. Frontiers in Bioscience-Landmark, 27(8), 227.
Yazdi, T., et al. (2020). Assessment of phytochemical components and antioxidant activity of Rheum turkestanicum Janisch. Studies in Medical Sciences, 31(2), 75–81.
Kamatou, G. P., et al. (2008). Antimalarial and anticancer activities of selected South African Salvia species and isolated compounds from S. radula. South African Journal of Botany, 74(2), 238–243.
Darroudi, M., M.E.T. Yazdi, and M.S. Amiri, Plant-mediated biosynthesis of nanoparticles, in 21st Century Nanoscience–A Handbook. 2020, CRC Press. 1–1–1–18.
Khalil Abad, M. H., Nadaf, M., & TaghavizadehYazdi, M. E. (2023). Taghavizadeh Yazdi, Biosynthesis of ZnO. Ag2O3 using aqueous extract of Haplophyllum obtusifolium: Characterization and cell toxicity activity against liver carcinoma cells. Micro & Nano Letters, 18(6), 12170.
Oyagbemi, A. A., Saba, A. B., & Azeez, O. I. (2010). Molecular targets of [6]-gingerol: Its potential roles in cancer chemoprevention. BioFactors, 36(3), 169–178.
Prasad, S., & Tyagi, A. K. (2015). Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterology Research and Practice, 25. https://doi.org/10.1155/2015/142979
Srinivasan, K. (2014). Antioxidant potential of spices and their active constituents. Critical Reviews in Food Science and Nutrition, 54(3), 352–372.
Gebelein, C. G., & Dunn, R. L. (2013). Progress in biomedical polymers. Springer Science & Business Media.
Hashemzadeh, M. R., et al. (2021). Stem cell therapy in the heart: Biomaterials as a key route. Tissue and Cell, 71, 101504.
Dubey, S. K., et al. (2022). Application of chitosan modified nanocarriers in breast cancer. International Journal of Biological Macromolecules, 194, 521–538.
Yazdi, M. E. T., Amiri, M. S., & Darroudi, M. (2020). Biopolymers in the synthesis of different nanostructures, 29–43. https://doi.org/10.1016/B978-0-12-803581-8.10560-0
Zarharan, H., et al. (2023). The anti-angiogenesis and antioxidant activity of chitosan-mediated synthesized selenium-gold nanostructure. Arabian Journal of Chemistry, 16(7), 104806.
Müller, R. H., et al. (2016). Nanostructured lipid carriers (NLC): The second generation of solid lipid nanoparticles. In Percutaneous penetration enhancers chemical methods in penetration enhancement: Nanocarriers (pp. 161–185). https://doi.org/10.1007/978-3-662-47862-2_11
Mousavi-Kouhi, S. M., et al. (2023). Plant gel-mediated synthesis of gold-coated nanoceria using Ferula gummosa: Characterization and estimation of its cellular toxicity toward breast cancer cell lines. Journal of Functional Biomaterials, 14(7), 332.
Alabyadh, T., et al. (2022). ZnO/CeO2 Nanocomposites: Metal-organic framework-mediated synthesis, characterization, and estimation of cellular toxicity toward liver cancer cells. Journal of Functional Biomaterials, 13(3), 139.
Seyedi, Z., et al. (2023). Icariin: A promising natural product in biomedicine and tissue engineering. Journal of Functional Biomaterials, 14(1), 44.
Üner, M. (2006). Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Die Pharmazie-an International Journal of Pharmaceutical Sciences, 61(5), 375–386.
Sadat Khadem, F., et al. (2022). The loaded Ferula assa-foetida seed essential oil in solid lipid nanoparticles (FSEO-SLN) as the strong apoptosis inducer agents in human NTERA-2 embryocarcinoma cells. Materials Technology, 37(9), 1120–1128.
Ok, S., & Jeong, W.-S. (2012). Optimization of extraction conditions for the 6-shogaol-rich extract from ginger (Zingiber officinale Roscoe). Preventive Nutrition and Food Science, 17(2), 166.
Rabelo, R. S., et al. (2018). Chitosan coated nanostructured lipid carriers (NLCs) for loading vitamin D: A physical stability study. International Journal of Biological Macromolecules, 119, 902–912.
Ravichandran, V., et al. (2016). Green synthesis of silver nanoparticles using Atrocarpus altilis leaf extract and the study of their antimicrobial and antioxidant activity. Materials Letters, 180, 264–267.
Tolosa, L., Donato, M. T., & Gómez-Lechón, M. J. (2015). General cytotoxicity assessment by means of the MTT assay. Protocols in In Vitro Hepatocyte Research, 333–348. https://doi.org/10.1007/978-1-4939-2074-7_26
Kumar, R., Saneja, A., & Panda, A. K. (2021). An annexin V-FITC—propidium iodide-based method for detecting apoptosis in a non-small cell lung cancer cell line. Lung Cancer: Methods and Protocols, 213–223. https://doi.org/10.1007/978-1-0716-1278-1_17
Raymaekers, M., et al. (2009). Checklist for optimization and validation of real-time PCR assays. Journal of clinical laboratory analysis, 23(3), 145–151.
Stetefeld, J., McKenna, S. A., & Patel, T. R. (2016). Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophysical Reviews, 8(4), 409–427.
Baboota, S., et al. (2007). Design, development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta pharmaceutica, 57(3), 315–332.
Bhattarai, S., Tran, V. H., & Duke, C. C. (2007). Stability of [6]-gingerol and [6]-shogaol in simulated gastric and intestinal fluids. Journal of pharmaceutical and biomedical analysis, 45(4), 648–653.
Choi, J. W., et al. (2017). Neuroprotective effect of 6-paradol enriched ginger extract by fermentation using Schizosaccharomyces pombe. Journal of Functional Foods, 31, 304–310.
Semwal, R. B., et al. (2015). Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry, 117, 554–568.
Justo, O. R., et al. (2008). Evaluation of the antioxidant potential of plant extracts obtained by supercritical fluid extraction. Química Nova, 31, 1699–1705.
Duan, H., Wang, D., & Li, Y. (2015). Green chemistry for nanoparticle synthesis. Chemical Society Reviews, 44(16), 5778–5792.
Nigam, N., et al. (2010). Induction of apoptosis by [6]-gingerol associated with the modulation of p53 and involvement of mitochondrial signaling pathway in B [a] P-induced mouse skin tumorigenesis. Cancer chemotherapy and pharmacology, 65, 687–696.
Yagihashi, S., Miura, Y., & Yagasaki, K. (2008). Inhibitory effect of gingerol on the proliferation and invasion of hepatoma cells in culture. Cytotechnology, 57, 129–136.
Beloqui, A., et al. (2016). Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine: Nanotechnology, Biology and Medicine, 12(1), 143–161.
Balamurugan, M., Kaushik, S., & Saravanan, S. (2016). Green synthesis of gold nanoparticles by using Peltophorum Pterocarpum flower extracts. Nano Biomed. Eng, 8(4), 213–218.
Ferreira, M., et al. (2015). Optimization of nanostructured lipid carriers loaded with methotrexate: A tool for inflammatory and cancer therapy. International Journal of Pharmaceutics, 492(1–2), 65–72.
Soltani, M., et al. (2022). Incorporation of Boswellia sacra essential oil into chitosan/TPP nanoparticles towards improved therapeutic efficiency. Materials Technology, 37(11), 1703–1715.
Eslamieh-Ei, F. M., et al. (2023). Synthesis and its characterisation of selenium/silver/chitosan and cellular toxicity against liver carcinoma cells studies. Natural Product Research, 1–9. https://doi.org/10.1080/14786419.2023.2256023
Rahimi, E., et al. (2023). Chitosan coated copper/silver oxide nanoparticles as carriers of breast anticancer drug: Cyclin D1/P53 expressions and cytotoxicity studies. Inorganic Chemistry Communications, 158, 111581. https://doi.org/10.1016/j.inoche.2023.111581
Liu, M., Zhong, X., & Yang, Z. (2017). Chitosan functionalized nanocochleates for enhanced oral absorption of cyclosporine A. Scientific reports, 7(1), 1–10.
Seyedi, S. M. R., Asoodeh, A., & Darroudi, M. (2022). The human immune cell simulated anti-breast cancer nanorobot: The efficient, traceable, and dirigible anticancer bio-bot. Cancer Nanotechnology, 13(1), 1–24.
Ling, J. T. S., Roberts, C. J., & Billa, N. (2019). Antifungal and mucoadhesive properties of an orally administered chitosan-coated amphotericin B nanostructured lipid carrier (NLC). An Official Journal of the American Association of Pharmaceutical Scientists, 20(3), 136.
de Lima, R. M. T., et al. (2018). Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phytotherapy Research, 32(10), 1885–1907.
Hashemy, S. I., & Seyedi, S. M. R. (2021). ROS impacts on cell cycle checkpoint signaling in carcinogenesis. Handbook of oxidative stress in cancer: Mechanistic aspects (pp. 1–19). Springer.
Taghavizadeh Yazdi, M. E., et al. (2023). Recent advances in nanoparticles applications in respiratory disorders, a review. Frontiers in Pharmacology, 14, 1059343.
Mobaraki, F., et al. (2022). Apoptotic, antioxidant and cytotoxic properties of synthesized AgNPs using green tea against human testicular embryonic cancer stem cells. Process Biochemistry, 119, 106–118. https://doi.org/10.1016/j.procbio.2022.05.021
Mousavi-Kouhi, S. M., et al. (2022). Biological synthesis and characterization of gold nanoparticles using Verbascum speciosum Schrad. and cytotoxicity properties toward HepG2 cancer cell line. Research on Chemical Intermediates, 48(1), 167–178.
Mobaraki, F., et al. (2021). Plant-derived synthesis and characterization of gold nanoparticles: Investigation of its antioxidant and anticancer activity against human testicular embryonic carcinoma stem cells. Process Biochemistry, 111, 167–177.
Dugasani, S., et al. (2010). Comparative antioxidant and anti-inflammatory effects of [6]-gingerol,[8]-gingerol,[10]-gingerol and [6]-shogaol. Journal of ethnopharmacology, 127(2), 515–520.
Acknowledgements
The authors appreciatively acknowledge the support and assistance provided by Islamic Azad University.
Funding
None.
Author information
Authors and Affiliations
Contributions
RKMA: doing experiments; NA, MRR, and AE: conceptualization and experimental design; both RKMA and NA contributed to the final version of the manuscript; M.R: edit the manuscript; A.E supervised the project.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Research Involving Humans and Animals Statement
None.
Informed Consent
None.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Ziyadi, R.K.M., Hayati, N., Rezaei, M.R. et al. Preparation and Characterization of Chitosan-Coated Nanostructured Lipid Carriers (CS-NLC) Containing (6)-Gingerol and Investigating their Toxicity Against MCF-7 Breast Cancer Cell Line. BioNanoSci. 14, 153–163 (2024). https://doi.org/10.1007/s12668-023-01261-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01261-4