Abstract
Liquid crystal materials are a suitable environment for the synthesis of nanostructures of uniform size and shape due to their order and yet mobility at the molecular level. Dense liquid crystal phases allow nanoparticles to self-assemble, resulting in larger organized nanostructures. Although both types of lyotropic liquid crystal and thermotropic liquid crystal have been used for the synthesis and self-assembly of nanoparticles, the use of lyotropic liquid crystal types is more in the synthesis of nanoparticles, while the use of thermotropic liquid crystal phases is mainly related to the self-assembly of nanoparticles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
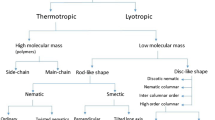
In the case of many materials (pure compounds or mixtures), liquid crystal phases also called mesophases occur in a multi-step process [1,2,3,4,5]. Liquid crystal state occurs when converting a regular crystalline state to a liquid state (or vice versa) through the formation of one or more intermediate phases [6,7,8,9]. Liquid crystals are anisotropic and somewhat regular fluids that are thermodynamically located between the crystalline solid state with a three-dimensional order and the isotropic liquid [10,11,12,13,14,15]. In fact, the anisotropy of the physical properties of liquid crystals is due to the directional order and anisotropy of the molecules that make up these materials [16,17,18,19]. These materials flow in the crystal phase like ordinary liquids, but show anisotropy properties similar to solid crystals [20,21,22,23,24]. In fact, the anisotropy of the physical properties of liquid crystals is due to the directional direction and anisotropy of the molecules that make up these materials. Liquid crystals are thermodynamically stable phases known for their anisotropic properties and lack a three-dimensional crystal lattice [25,26,27,28,29,30]. In this phase, each of the molecules is interested in being in the direction of a specific method called the unit vector n, which is called the guide vector [31,32,33,34,35]. With the passage of time and the rapid and increasing growth of photonics, many research fields have been created in various fields of photonics [36,37,38]. An important part of this research has included electro-optical studies of materials, which requires the knowledge of new optical materials and the study of their optical properties [39,40,41]. Among these, crystals are a group of materials with stable thermodynamic phases that simultaneously have the optical anisotropy properties of crystals and the fluidity of liquids [42,43,44,45]. These materials with relatively large organic molecules and anisotropic geometric shape due to having a long-range directional order show both linear optical properties and high refractive and nonlinear properties [46,47,48,49,50]. This has led to the penetration of applications of these materials in all areas of the photonics and optoelectronics industry. Research on liquid crystals has intensified over the past two decades [51,52,53]. Most studies of low power laser interaction (which is inconceivable in ordinary crystals due to their small nonlinearity) with optical environments such as liquid crystals and the physical and electro-optical properties of different types of liquid crystals have been considered [54,55,56,57,58]. The optical nonlinearity of nematic crystals is about 108 times larger than CS2, which encompasses almost all conceivable linear and nonlinear optical phenomena in all phases of liquid crystal, including dual stability, self-focal, self-focal, self-phase modulation, and the production of first coordinates, and second, the optical phase conjugate, the electro-optical effect of the chorus, the wave front wave fusion with gain, self-oscillation, and enables [59,60,61,62]. Since some of these properties are weak for industrial, medical, and applications in pure liquid crystals, the methods of mixing liquid crystals with nanoparticles and polymers, known as the guest-host method, are used to enhance their optical and electro-optical properties [63,64,65,66,67,68]. In this regard, the diffusion of suspended particles in crystals as a method to improve the physical and electro-optical properties of crystals due to their application potential in various fields such as photonics and bio photonics has been widely studied [69,70,71,72]. Figure 1 shows the phases and different shapes of the liquid crystal.
Phases and different shapes of the liquid crystal [1]
Individual pieces of cloth voluntarily associate themselves into a delineated and ordered structure or bigger pieces with minimum external direction is termed as self-assembly [73, 74]. Self-assembly is a well-known approach for achieving exceptional properties in both organic and inorganic nanostructures. The following is a list of the benefits of self-assembly in fabrication [21,22,23,24, 75,76,77,78,79].
-
Self-assembly might be a scalable, parallel process involving a large number of components in a short amount of time [80].
-
From the nanoscale to the macroscale, structural dimensions can vary with order of magnitude. When compared to top-down assembly, which usually uses enormous quantities of scarce resources, it is comparatively economical [81].
-
The natural mechanisms that promote self-assembly are usually quite repeatable. The repeatability of self-assembly is deeply engrossed in the existence of life [82].
Researchers all around the world are interested in using supramolecular self-assembly to create nanostructures and nanomaterial with unique physical and chemical features [83, 84]. The ability to create and build novel gadgets that can interact with living cells and create responses has been enabled with the organizing of molecules in these nano assemblies [85,86,87]. These are being studied not only as crucial components in the origin of cellular life, but also as materials that may be employed in a wide range of applications, including biomaterials, bioelectronics, energy production, catalysis, drug delivery, and nanocomposites [88,89,90,91,92]. For the manufacturing of nanostructures, two techniques are often used: top-down and bottom-up (Fig. 2). The first entails cutting out of a bigger block of matter the final nanostructure with a specific form and size. As a result, atomic-level control is not required for the technique [93,94,95,96,97]. The latter technique, on the other hand, entails constructing desired nanostructures from basic components using molecular recognition and self-assembly processes, which are essentially generated from the interactions of fundamental units to produce well-organized structures [98,99,100,101]. As a result, the latter technique allows for atomic or molecular level control over the production of nanostructures with changing the architectures of self-assembling molecular units.
Self-assembly techniques from the top-down and bottom-up [2]
2 Classification of Liquid Crystals
The transition between two phases for a system takes place with changing one or more thermodynamic parameters [102,103,104,105]. Liquid crystalline materials can undergo an intermediate phase due to changes in temperature or concentration or changes in both. Accordingly, these materials are classified into two categories, thermotropic liquid crystal, and lyotropic liquid crystal [106,107,108]. In thermotropic liquid crystal, the intermediate state is obtained due to temperature change and in lyotropic liquid crystal, the intermediate state is obtained due to change in temperature and concentration [109, 110]. The third group of liquid crystals are polymeric liquid crystals, which are placed separately in the other two categories and the order in the middle state of these materials is related to their polymer structure [31, 32, 111,112,113,114,115].
2.1 Thermotropic Liquid Crystal
Thermotropic liquid crystals form the liquid crystal phase only in a certain temperature range, the solid material melts at a certain temperature and turns into a turbid liquid which is a liquid crystal state, and then with further heating this material it becomes a liquid state [116,117,118]. Anisotropic is obtained, which is usually transparent. In some of these materials, the intermediate phase is observed at a temperature higher than the melting temperature of the material, and in both cooling and heating processes, the intermediate phase is observed, which is called the anthropic phase [33, 34]. We also have an exception for compounds in that the thermotropic phase of such compounds appears during the cooling process, not in the heating process, which is called the monotropic phase transition [119, 120]. The physical properties of thermotropic liquid crystals such as refractive index, dielectric constant, elastic constants, and viscosity are all temperature-dependent [121, 122]. Georges Friedel classified thermotropic liquid crystals into three basic categories in terms of fuzzy structure, which are nematic, cholestric, and sematic liquid crystals [35]. In Fig. 3, the different groups can be used to interact with ionic surfactants to form thermotropic liquid crystal molecules with various shapes. These molecules give rise to ordered structures in a certain range of temperature-driven with non-covalent interactions.
The different groups can be used to interact with ionic surfactants to form thermotropic liquid crystals molecules with various shapes. These molecules give rise to ordered structures in a certain range of temperature-driven with non-covalent interactions [3]
2.2 Nematic Liquid Crystal
Nematic liquid crystal has the lowest order and highest molecular symmetry compared to other types of liquid crystals. The difference between a nematic liquid crystal and ordinary liquids is the existence of a directional order with respect to a molecular direction, which is the long molecular axis characterized with the unit vector [123,124,125]. Due to the directional order, the liquid crystal molecules are symmetrically uniaxial, and the axis of symmetry of this uniaxial structure is the same as the preferred direction n. However, in the nematic phase, molecules are randomly distributed like normal liquids, and the molecules are allowed to rotate around their long axis, so the nematic liquid crystals are interested in optical and dielectric anisotropy [36, 37]. Figure 4 shows the schematic of different nematic liquid crystal.
Schematic of different nematic liquid crystal, a nematic liquid crystal phase, b semiotic liquid crystal A phase, c semiotic liquid crystal C phase, d cholesteric phase [4]
2.3 Semiotic Liquid Crystal
In addition to the directional order, the semiotic phase also has a short-range spatial order. The order and viscosity in this phase are higher than those in the nematic phase. From the point of view of the fuzzy structure, molecules in the semiotic phase have a layered structure, which is determined with the way the molecules are placed in the layers and the angle that the direction of the molecules of each layer is in the direction perpendicular to the plane [126,127,128,129]. This liquid crystal is divided into kind a, b, c semantics. The somatic of kind a is optically uniaxial and its molecular arrangement is such that the direction perpendicular to the planes of the layers is in the same direction as the direction of the optical axis, while in the somatic of kind c the long molecular axis is perpendicular to the axis perpendicular to the plates and also in somatic b, the molecules are placed on the layers in a hexagonal arrangement [38, 39, 130,131,132]. Figure 5 shows different kinds of Semiotic can be detected from their tissue under a polarizing microscope.
Structures of semiotic liquid crystal [5]
2.4 Cholestric Liquid Crystal
The first known liquid crystal was the cholestric liquid crystal. This type of liquid crystal is more orderly than the previous two types and is very similar to solids. In these materials, molecules form a molecular structure that has a semantic directional order in each layer [133,134,135,136]. Due to the spatial barrier of the molecules, the orientation of the molecules in the adjacent layers is not the same and the guide vector rotates in each layer at a certain angle to the adjacent layer, and a twisted structure according to the following figure that can be centered. It is created with the unit vector z shown [137,138,139,140]. The distance that rotates 180° with moving along z and n is called a screw (p) [39,40,41]. Figure 6 shows the structures of cholestric types.
The structures of cholestric types [6]
2.5 Lyotropic Liquid Crystal
Lyotropic liquid crystals are obtained with dissolving an appropriate amount of dual-friendly compounds in a solvent such as water, soaps, and detergents. These dual compounds contain molecules of a hydrophilic part that is highly water-absorbing and a hydrophobic part that are insoluble in water, such as soaps, detergents, and fats [140,141,142]. Most of the important changes in the formation of the intermediate phase will depend on the concentration of the solution. It is stretched together to form structures called micelles, which form with increasing concentration of lyotropic liquid crystal phase. The micelles first form a cubic state, then the rod states form a hexagon, and finally a layered state [42,43,44]. Figure 7 shows the phasing of a lyotropic liquid crystal as a function of temperature and concentration.
Liquid crystal under different temperatures and concentrations [7]
2.6 Polymeric Liquid Crystal
These types of liquid crystals are behaviorally similar to thermotropic liquid crystals and are structurally similar to single molecules of lyotropic liquid crystals. Polymers alone have flexible structures that consist of repeats of basic units or parts that are monomers. In these materials, parts of the hard polymer and rods must be able to take a directional or spatial order in a certain temperature range and express the property of liquid crystal [143,144,145]. In a polymer or main chain, the rigid structural parts resemble rod liquid crystal molecules separated with flexible hydrocarbon chains, whereas in lateral chain polymers, the hard structural parts of the liquid crystal are attached to the long polymer chain with very short flexible hydrocarbon chains [45, 46]. In Fig. 8, the structures of different types of polymeric liquid crystal can be seen.
The structures of different types of polymeric liquid crystal [45]
3 Synthesis Using Liquid Crystals and Liquid Crystal Molds
Because the properties of nanoscale materials depend on their size and shape, nanoparticles of uniform size and shape are required first of all to prepare functional nanomaterial. Liquid crystals combine order and mobility at the molecular level (nanoscale) and are therefore ideal options for controlled synthesis of nanoparticles [47].
3.1 Lyotropic Liquid Crystals as Surfactants and Phase Transfer Agents
A significant issue in nanoparticle synthesis is the preparation of air- and heat-stable particles with controlled size and diffusion, in such a way that it is possible to mix and re-separate them in organic solvents without irreversible aggregation and decomposition. Gold and silver nanorods were synthesized with the seed-mediated growth method [146,147,148,149,150]. In this method, spherical nanoparticles with a diameter of about 3.5 nm (grains) are prepared during the hydride reduction process of gold or silver salt in the presence of sodium citrate. These nanoparticles are then added to a solution containing acetyl trimethylammonium bromide (CTAB) and excess gold or silver salts. The resulting rod particles are collected using a centrifuge. CTAB is an ion-friendly dual compound that is able to form a type of lyotropic liquid crystal phase [48, 49]. This particular synthetic method prefers rod structure formation because CTAB acts as a guiding agent with creating a bilayer structure on gold nanorods [151,152,153,154]. The CTAB only allows them to grow in one direction with attaching more tightly to the side edges than the ends of the nanorods. Thus, CTAB plays an important role in the formation of one-dimensional nanostructures (Fig. 9). In addition to gold and silver nanorods, tellurium nanotubes and selenium nanowires were also prepared with growth method using lyotropic liquid crystal surfactant.
TEM images of gold nanorods synthesized from a 8-nm beads and b 16-nm beads in which CTAB is located on both samples [48]
3.2 Direct Molding of Liquid Crystal
Given the structural diversity of lyotropic liquid crystals, it is easy to see how these compounds are used as molds for the synthesis of porous nanostructures. This method leads to the production of materials with pore size, morphology, and uniform three-dimensional distribution, and in addition it is possible to control the properties and shape of the structure. Another advantage of lyotropic liquid crystal molding is that the size of the pores can be increased with adding a hydrophobic component and then expanding the inner part of the micelle [155,156,157,158]. Hence, this technique represents the concept of the term “direct liquid crystal molding” or “nanocasting” and is currently widely used in the synthesis of porous media with catalytic application or absorption [50, 51] (Fig. 10). Types of porous silicate and non-silicate nanomaterial have been synthesized with molding with lyotropic liquid crystal and using polymeric or oligomeric surfactant systems. Non-silica porous structures include metal oxides, CdS and CdSe composites, Pt/Ru alloy and Ni/Co alloy.
Nanocommanding, a crystalline phase of the main lyotropic liquid, b addition of continuous liquid phase, c precipitation of the crystalline phase of the main lyotropic liquid and formation of a porous material [50]
3.3 Reverse Molding
The lyotropic liquid crystal phase itself can be used to produce a regular arrangement of nanoparticles produced in the hydrophobic sections of reverse micelles or the hydrophilic sections of conventional micelles. In this case, the lyotropic liquid crystal acts as a nanospore or nanoreactor, and therefore with controlling the phase type of the liquid crystal, the size and shape of the nanoparticles that grow inside it can be controlled. Another advantage of this method is that the production of these nanoreactors is easily possible on a large scale. Lyotropic liquid crystals, which form layered or hexagonal columnar morphologies, are used to make metal nanoparticles or conductive nanostructured polymers [52, 53]. A solution of the metal salt is mixed with a sufficient amount of the liquid crystal host to form the desired lyotropic liquid crystal phase. The nanoparticles are then precipitated, which first aggregate into clusters and then form a single nanostructure. Because these nanostructures generally take the form of nanoreactors, the column phases of rod and cubic and layered phases usually form spherical and disk-like nanostructures (Fig. 11). After dispersing the crystalline phase of the lyotropic liquid, the resulting nanostructures are collected with centrifugation or filtration. Figure 12 shows BiOCl nanostructures prepared in different ways. While the crystalline phase of the layered lyotropic liquid produces nearly spherical particles with a diameter of 5 nm and the hexagonal phase produces arrow-shaped rods with a length of 250 nm and a width of 100 nm, in ordinary solution a set of discs are created with a diameter of 50 to 250 nm. A variety of nanostructures such as silver, copper, ZnS, CaSO4, and BaCO3 nanowires and spherical nanoparticles of bismuth, palladium, PbS, Fe3O4, and CoFe2O4 as well as spherical iron nanoparticles encapsulated in a thin film of gold are prepared with this method.
Synthesis of nanoparticles using lyotropic liquid crystals as nanoreactors [52]
TEM images of BiOCl compound in a ordinary solution, b hexagonal liquid crystal, c layered liquid crystal [53]
3.4 Crystalline Phases of Thermotropic Liquid in the Synthesis of Nanoparticles
Thermotropic liquid crystals, which, like lysotropic liquid crystal mesophases, self-assemble without the need to add solvent to regular structures, provide conditions that do not exist in the leotropic phase [159,160,161]. These conditions include inclined somatic smear (SmC) phases, somatic A bipolar or polar phases, and non-hexagonal column phases (such as Colr). As an example, a dense somatic phase has been used to synthesize fluorinated acrylate polymer nanostructures. This method involves mixing the monomer with the host thermotropic liquid crystal followed with polymerization at the appropriate temperature. The results show that the use of fluorinated nanoparticles leads to the formation of regular polymer structures, while this phenomenon is not observed in non-fluorinated counterparts of these compounds [54, 55]. This is due to the entrapment of nanoparticles within the liquid crystal phase during the polymerization process, which leads to the formation of regular polymer nanostructures (Fig. 13). Although thermotropic liquid crystal phases have significant efficiencies for organizing nanostructures with aiding in the self-assembly process, few studies have reported the synthesis of metal, semiconductor, or magnetic nanoparticles in the dense phase of these compounds. Therefore, more research is needed to develop methods for the application of dense thermotropic liquid crystal phase for the synthesis of nanoparticles.
a The monolayer structure and liquid crystal used to prepare polymer nanostructures, b show the resulting polymer texture (×200) [55]
4 Arrangement and Organization Using Liquid Crystals
Self-assembly of metal, magnetic, or semiconductor nanoparticles is a suitable technique for preparing larger organized structures because it is low cost and high efficiency and allows very special properties to be achieved [162, 163]. Nanoparticle self-assembly, which is an important goal in the field of nanotechnology advances, is essential for the application of nanomaterials in state-of-the-art devices. Most self-assembly methods result in enclosed arrangements of nanoparticles that do not allow the structure of the mass to be manipulated. The specific properties of nanoparticles depend on whether they are composed of regular alternating structures such as monolayer or multilayer films, or solutions of regular or randomly distributed materials [56,57,58]. To prepare alternating arrangements of nanoparticles, a variety of self-assembly methods include placement on a solid support using molecular imprinting techniques, filming in the air-water interfacial region (film). Langmuir-Blogget and the preparation of polymer matrices filled with nanoparticles has been used. The application of the concepts of supermolecular chemistry and molecular detection of low molecular weight liquid crystals in the organization of nanoparticles provides methods for controlling the self-arrangement of nanoscale systems into wider structures [164,165,166,167]. In recent years, self-assembly has been successfully performed with modifying nanoparticles with biological molecules such as DNA and proteins. Other methods include host-guest complexes, resorbable functional groups, metal complex formation, hydrogen bonding, and π-π interactions, the latter two of which are the most important causes. Self-assembly in many liquid crystal phases is lyotropic and thermotropic [59, 60]
4.1 Liquid Crystal Phase Formation of Nanomaterial
Anisotropic colloidal nanocrystals such as nanorods and nanodisks are not only synthesized using liquid crystal phases but can also be the basis for a new class of mineral-based liquid crystal materials that have unique and important, such as high thermal stability, show a rigid regular structure with weak interparticle interactions and low drift. The order in liquid crystals has been observed in a variety of rod nanoparticles. For example, the dense diffusion of CdSe nanorods shows the order of both the nematic and the semantic phases. The same is true for disk-like nanoparticles [61,62,63]. Colloidal CuS nanodisks with a diameter of 14–20 nm and a thickness of about 5 nm form self-assembled columnar structures (Fig. 14).
TEM images of CuS and Cu2S nanodisks. (A) CuS nanodisks in monolayer mode, (b) linear chains of Cu2S nanodisks, (c) CuS nanodisks having a crystalline T-shaped structure, (d) self-assembly of nanodisks Cu2S oriented parallel to the substrate, (f) various self-arrangements of nanodisks (1) single layer, (2) hexagonal column self-arrangement with perpendicular to the substrate, (3) self-arrangement column with orientation parallel to the bed [61]
4.2 Nanoparticles Adorned with Liquid Crystal
Mineral, semiconductor, and mineral-derived nanomaterials are able to form liquid crystal phases without the need for organic mesogens. It is clear that this phenomenon is limited to nanomaterials that have a shape with unequal dimensions. To obtain liquid crystal properties in spherical nanoparticles that are symmetrical and have almost the same dimensions in all directions, one method is to decorate small spherical nanoclusters with thermotropic or mesogenic molecules [168,169,170,171]. Especially in the case of gold spherical nanoparticles, there are many examples that show the self-arrangement of these particles using agents that cover the crystal of thermotropic liquid and create a nematic or somatic phase [65, 66]. In addition to spherical metal nanoclusters, the formation of nematic phases has been reported with covering needle-shaped TiO2 particles and α-Fe2O3 and Fe3O4-coated nanorods or nanosheets coated with SiO2. Figure 15 shows nanoparticles scattered in liquid crystals are ordered [65].
Nanoparticles scattered in liquid crystals are ordered. We consider three scalar order parameters in this study: an orientational order parameter for a nematic phase, a one-dimensional translational order parameter for a smectic a phase, and a translational order parameter for a nanoparticles crystalline phase [65]
4.3 One-Dimensional Nanomaterial Suspension in Thermotropic Liquid Crystals
The organization and orientation of one-dimensional nanostructures with unequal dimensions using thermotropic liquid crystals, especially due to the possibility of order control in these systems, which leads to new optical and electron electronics applications, has been studied extensively [172,173,174,175]. Thermotropic liquid crystals, especially those with low molecular weight, have significant advantages over conventional liquid media for organizing one-dimensional nanomaterials. In addition to internal anisotropy properties (such as dielectric anisotropy), thermotropic liquid crystals have a directional order due to specific interactions with the surface, which can be in the presence of external electric, or magnetic fields with a relatively short response time should be used (Fig. 16a). Figure 16 b shows the first example of the use of thermotropic liquid crystals to produce organized arrangements of nanomaterials on the surface. In this method, called liquid crystal imprinting, the nanoparticles are dissolved in a non-chiral nematic liquid crystal under the influence of a magnetic field [176,177,178,179]. In this way, the crystalline phase of the nematic fluid aligner exerts its uniform orientation on the one-dimensional constituent nanocomposites and results in the production of organized thin films [66,67,68,69,70], to be deposited on the support.
a Specific interactions of liquid crystal molecules with modified surfaces (1) flat, (2) inclined, and (3) homeotropic. b Principles of forming single-axis molecular films with application of liquid crystal embedding in magnetic field [70]
5 The Overall Structure of a Liquid Crystal Molecule
Most liquid crystalline compounds are composed of one or more aromatic rings A or “A” interconnected with an X bonding group, as shown in Fig. 17. The aromatic rings shown can be a fully saturated cyclohexane; an unsaturated phenyl, biphenyl, or tri-phenyl; or a combination of the two. Compounds with long rings usually have a high melting point. The end groups R and “R” are usually small groups or often short chains. These groups play an important role in the dielectric anisotropy of liquid crystals [71]. The high value of dielectric anisotropy, in turn, lowers the threshold voltage of some switching devices based on liquid crystals. Selecting the right length of alkyl chains can stabilize the nematic phase and reduce the melting temperature of the material. The end groups often include alkyl, alkoxy, cyano-CN-isothiocyanate, sulfide, and halides such as OCF3, CL, F, and CF3. Among these, alkyl, alkoxy, and sulfide are weak polar groups and their effect on dielectric anisotropy is weak. NCS-, CN, F, and CL are strong polar groups, and materials containing one of these groups have relatively large dielectric anisotropy. X-bonding groups play an important role in the phase transition temperature as well as in the physical properties of liquid crystals [71,72,73]. The most popular X groups that bond phenyl rings can be saturated groups, esters, unsaturated groups, a double bond, or a triple bond. All the optical and physical properties of liquid crystals are determined with the properties of the constituent groups and how they are synthesized. Dielectric constants, elastic constants, viscosity, absorption spectra, transition temperatures, the presence of different mesophases, anisotropies, and nonlinear optical properties all depend on how the molecules are bonded and put together.
Molecular structure of a liquid crystal [71]
6 Applications of Liquid Crystals
One of the materials that will be extensively researched in the future is the filled nematics phase, which results from the suspension of fine particles (not necessarily nanoscale particles) such as titanium dioxide particles in the nematic liquid crystal matrix. Severe light scattering in these nematic phases accumulates in field-off conditions due to the large number of orientation defects caused with scattered particles [179,180,181,182,183]. With applying field-on, the accumulated nematic phase sandwich film becomes transparent due to the orientation of the nematic liquid crystal molecules with the external electric field [75,76,77,78]. A special feature of the accumulated nematic phases is the retention of light transmittance and transparency after the field is turned off, which is called the memory effect. The electron performance of these accumulated nematic phases strongly depends on the physical and chemical properties of the liquid crystal and filler particles, the concentration, and the properties of the applied electric field. Figure 18 shows a timeline of liquid crystal phase uses from their inception to the current day.
A timeline of liquid crystal phase uses from their inception to the current day [75]
6.1 Application of Nanocrystals in Environmental Processes
Cellulose and nanocrystals are one of the most popular items in recent years due to their biodegradability and low cost. In this research, cotton liner was used with acid hydrolysis method and ionic liquid application to prepare cellulose nanocrystals, and optimal conditions were provided for the formation of fine crystals [79, 80]. The effect of the method used on the particle size and crystals of cellulose nanocrystals was investigated with Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and scanning electron microscopy (SEM). The results indicate that the use of a combination of urea, sodium, and thiourea as ionic liquid caused the formation of fine crystals, which with examining the XRD spectra, the crystal size was about 1–10 nm and the crystallinity was 61–3%. Figures 19, 20, 21, and 22 show SEM, XRD, FTIR, and Raman spectra images which also show the fiber size in the nanometer range, which is approximately in the range of 30–20 nm.
SEM morphologies: a nanocrystals of (001)-oriented Ag2Se (orientation confirmed with X-ray diffraction analysis) after 1 h of solvothermal growth with methanol as the solvent; b oriented attachment toward the formation of a dendrite’s trunk after 3 h of solvothermal growth with methanol as the solvent; c close-up of a full dendrite formed after 12 h of solvothermal growth with d close-up of a full dendrite formed after 12 h of solvothermal growth with dodecagon as the solvent; e large-field view of dendrites formed under part c for the case of a relatively high nucleation density; and f large-field view of dendrites formed under part d for the case of a relatively low nucleation density [80]
Experimental X-ray diffraction analysis patterns of as-synthesized CoO nanocrystals for sample A and sample B. The bulk X-ray diffraction analysis pattern of cubic CoO is shown at bottom [81]
The Fourier transform infrared spectroscopy spectrum of silicon nanocrystals. Inset: the whole Fourier transform infrared spectroscopy spectrum without a break [82]
Raman spectra of silicon nanocrystals (solid line) and bulk silicon (dash line) [82]
6.2 Application of Nanocrystals in the Pharmaceutical Industry
Many drugs today are discarded in the early stages of research due to their low solubility in micronutrient and non-micronutrient solvents. A good way to solve this problem is to use nanocrystals that can be used in industry and laboratories [80]. There are methods for producing these structures such as using high-pressure homogenizer or using abrasion as well as sedimentation method. So far, these structures have been able to find a good place in the market and pharmaceutical research and introduce successful examples of formulations worldwide. Ease of construction and less side effects are the main features of these nanostructures, the main reason for which is the lack of complex formulations to insert the drug into the nanostructure and maintain the stability of the product. Drug nanocrystals are one of the most important formulations for soluble drugs that have been introduced so far. These formulations are simple and do not require the addition of other compounds (which themselves can cause side effects) for optimization. Given that the number of low-soluble drugs introduced is increasing, it is believed that nanocrystals may be able to have a good place among pharmaceutical formulations for the introduction of these drugs in the future [81]. Over the past decades, many designs have been made for pharmaceutical structures with the help of chemistry and computers, although about 60% of these structures are sparingly soluble in water. Formulation of these structures is one of the main problems of pharmaceutical researchers because these compounds, due to the speed of dissolution and slow dispersion, will have little bioavailability and absorption. For oral absorption, drugs must be able to create an appropriate concentration gradient between the gastrointestinal tract and the bloodstream. However, in the case of low-soluble drugs, this slope is low and the absorption is reduced. In non-oral ways, too, if the drug is sparingly soluble, it will not dissolve well at the injection site and, as a result, the proper level of the drug cannot be produced in the blood. To solve these problems, solutions such as using solvents, creating salt, or adding cyclodextrins (basket-like structures with a hole in the middle where compounds can enter this hole) or carriers such as liposomes and solids dispersion have been proposed. Unfortunately, it is not possible to use these solutions for all drugs; for example, some drugs are difficult to convert to ions to make salts or are not suitable for entering the structure of cyclodextrins. Also, additives used in formulations are not always helpful. For example, the injectable taxol solution currently available on the market contains high amounts of Cremophor EL, which has been used to improve the bioavailability of paclitaxol but causes side effects such as allergic shock. In the 1990s, pharmaceutical nanocrystals attracted the attention of pharmaceutical researchers. Drug nanocrystals are colloidal dispersions less than micrometers in size that contain approximately 100% of the active drug substance and are stabilized with the help of small amounts of stabilizers such as polymers or surfactants. The dispersing medium of these structures can be water, liquid, or non-liquid solutions (such as liquid polyethylene glycol or oils). Depending on the manufacturing technology, the solid particles created can be amorphous or crystalline, which is why these particles are also called nanocrystals in the amorphous structure [82].
7 Advantages of Nanocrystals
-
Increase dissolution rate
As the particle size decreases, the contact surface between the particle and the environment increases, resulting in a faster dissolution rate (which is related to the contact surface area) [81].
-
The possibility of creating amorphous structures and its benefits
Amorphous structures have a higher rate of dissolution, so if these structures are created during production, the time required for dissolution will be shortened and the effect of the drug will increase.
-
Improve biological characteristics
Nanoparticles perform better than larger particles in terms of adsorption and efficiency.
-
Smaller nanocrystals
In the first generation, most of the particles created are less than 200 nm in size, but in the second generation this range is smaller and reaches below 100 nm.
-
Increase physical stability
With methods such as abrasion and homogenization in the second generation of nanocrystals, the particle size is actually reduced in two ways, which helps to reduce the particle size range. The more similar the size of the particles, the less likely they are to accumulate and clump, which is one of the advantages of the second generation over the first generation [82].
8 Calculation
The reproducible synthesis of nanoscale materials has attracted much attention due to their important role in the production of high-tech tools. The production of nanostructured porous materials is important because of their use in fuel cells, energy emission control, and catalytic applications such as hydrogenation reactions. Lyotropic liquid crystals have been used as a mold for the synthesis of porous nanostructures with pores of uniform size and shape. These compounds have also been used for the synthesis of spherical nanoparticles and nanorods, as well as nanoreactors in the synthesis of nanomaterial of uniform size and shape. The dense phase of thermotropic liquid crystals also provides a suitable environment for the preparation of regular polymer nanostructures and the self-arrangement of spherical nanoparticles. Design of functionalized liquid crystal nanocomposites is one of the main fields of future research in the field of nanotechnology.
References
Martin, A. N., & Sinko, P. J. (2011). Martin’s physical pharmacy and pharmaceutical sciences. In Physical Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences (6th ed., pp. 112–143). Lippincott Williams & Wilkins.
Lancelot, A., Sierra, T., & Serrano, J. L. (2014). Nanostructured liquid crystalline particles for drug delivery. Expert Opinion on Drug Delivery, 11(4), 547–564.
Muller-Goymann, C. C. (2004). Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. European Journal of Pharmaceutics and Biopharmaceutics, 58, 343–356.
Rochow, T. G. (1994). Compound Microscopes Using Reflected Light. In T. G. Rochow & P. A. Tucker (Eds.), Introduction to microscopy by means of light, electrons, X rays, or acoustics (pp. 56–98). Springer US.
Clercq, K. D. E. (2012). Formulation and characterization of liquid crystal platforms for the treatment of periodontal diseases [dissertation]. Ghent University.
Li, Y., Dong, C., Cun, D., Liu, J., Xiang, R., & Fang, L. (2016). Lamellar liquid crystal improves the skin retention of 3-o. AAPS PharmSciTech, 17(3), 767–777.
Calixto, G. M., Bernegossi, J., de Freitas, L. M., Fontana, C. R., & Chorilli, M. (2016). Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: A review. Molecules, 21, 342–350.
Guo, C., Wang, J., Cao, F., Lee, R. J., & Zhai, G. (2010). Lyotropic liquid crystal systems in drug delivery. Drug Discovery Today, 15(23-24), 1032–1040.
Hussain, A., Pina, A. S., & Roque, A. C. A. (2009). Bio-recognition and detection using liquid crystals. Biosensors & Bioelectronics, 25, 1–8.
Kawamoto, H. (2002). The history of liquid crystal display. Proceedings of the IEEE, 90(4), 460–499.
Oswald, P., & Pieranski, P. (2005). Nematic and cholesteric liquid crystals: Concepts and physical properties (pp. 17–20). CRC Press.
Kelker, H., & Hatz, R. (1980). Handbook of liquid crystals (pp. 121–134). Verlag Chemie.
Stevenson, C. L., Bennett, D. B., & Lechuga-Ballesteros, D. (2005). Pharmaceutical liquid crystals: The relevance of partially ordered systems. Journal of Pharmaceutical Sciences, 94, 1861–1880.
Jakli, A., & Saupe, A. (2006). One- and two-dimensional fluids: Physical properties of smectic, lamellar and columnar liquid crystals (pp. 256–270). CRC Press.
Attwood, D. (1983). Surfactant systems: Their chemistry, pharmacy and biology (pp. 121–145). Springer.
Siddig, M. A., & Radiman, S. (2004). Structure of cubic phases in ternary systems glucopone/water/hydrocarbon. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 236(1-3), 57–67.
Carvalho, F. C., Barbi, M. S., Sarmento, V. H., Chiavacci, L. A., Netto, F. M., & Gremião, M. P. (2010). Surfactant systems for nasal zidovudine delivery: Structural, rheological and mucoadhesive properties. The Journal of Pharmacy and Pharmacology, 62(4), 430–439.
Rosevear, F. B. (1968). Liquid crystals: the mesomorphic phases of surfactant compositions. Journal of the Society of Cosmetic Chemists, 19, 581–594.
Shah, M. H., Biradar, S. V., & Paradkar, A. R. (2006). Spray dried glyceryl monooleate-magnesium trisilicate dry powder as cubic phase precursor. International Journal of Pharmaceutics, 323, 18–26.
Mohammady, S. Z., Pouzot, M., & Mezzenga, R. (2009). Oleoylethanolamide-based lyotropic liquid crystals as vehicles for drug delivery of amino acids in aqueous environment. Biophysical Journal, 96, 1537–1546.
Boyd, B. J., Whittaker, D. V., Khoo, S. M., & Davey, G. (2006). Lyotropic liquid crystalline phases formed from glycerate surfactants as sustained release drug delivery systems. International Journal of Pharmaceutics, 309, 218–226.
Fraser, S., Separovic, F., & Polyzos, A. (2009). Cubic phases of ternary amphiphile-water systems. European Biophysics Journal, 39(1), 83–90.
Clogston, J., & Caffrey, M. (2005). Controlling release from the lipidic cubic phase. Amino acids, peptides, proteins and nucleic acids. Journal of Controlled Release, 107(1), 97–111.
Fontell, K. (1974). Liquid crystals and plastic crystals. In G. W. Gray & P. A. Winsor (Eds.), Liquid crystals and plastic crystals: Physico-chemical properties and methods of investigation (pp. 80–109). Ellis Horwood Publishers.
Khoo, I. C. (1987). Liquid crystals: Physical properties and nonlinear optical phenomena (pp. 214–285). Wiley-Interscience.
de Gennes PG, Prost J. The physics of liquid crystals. : Oxford University Press; 1993. 25-6, 175-179.
Gao, M., Kim, Y. K., Zhang, C., Borshch, V., Zhou, S., Park, H. S., et al. (2014). Direct observation of liquid crystals using cryo-TEM: Specimen preparation and low-dose imaging. Microscopy Research and Technique, 77(10), 754–772.
Burylov, S. V., & Raikher, Y. L. (1994). Orientation of a solid particle embedded in a monodomain nematic liquid crystal. Physical Review. E, Statistical Physics, Plasmas, Fluids, and Related Interdisciplinary Topics, 50, 358–367.
Murgia, S., Bonacchi, S., Falchi, A. M., Lampis, S., Lippolis, V., Meli, V., et al. (2013). Drug-loaded fluorescent cubosomes: Versatile nanoparticles for potential theranostic applications. Langmuir, 29(22), 6673–6679.
Monteiro, N., Martins, A., Reis, R. L., & Neves, N. M. (2014). Liposomes in tissue engineering and regenerative medicine. J R Soc Interface, 11(101), 1–24.
Roux, D., Nallet, F., & Diat, O. (1993). Rheology of lyotropic lamellar phases. Europhysics Letters, 15, 53–58.
Bertelsen, B. M., Korsholm, K. S., Rose, F., Nordly, P., Franzyk, H., Andersen, P., et al. (2013). The supramolecular structure is decisive for the immunostimulatory properties of synthetic analogues of a mycobacterial lipid in vitro. RSC Advances, 3, 20673–20683.
Malmsten, M. (2006). Surfactants and polymers in drug delivery (pp. 116–212). Marcel Dekker Inc.
Wahlgren, S., Lindstrom, A. L., & Friberg, S. E. (1984). Liquid crystals as a potential ointment vehicle. Journal of Pharmaceutical Sciences, 73, 1484–1486.
Geraghty, P. B., Attwood, D., Collett, J. H., & Dandiker, Y. (1996). In vitro release of some antimuscarinic drugs from monoolein / water lyotropic liquid crystalline gels. Pharmaceutical Research, 13, 1265–1271.
Makai, M., Csanyi, E., Nemeth, Z., Palinkas, J., & Eros, I. (2003). Structure and drug release of lamellar liquid crystals containing glycerol. International Journal of Pharmaceutics, 256, 95–107.
Shah, J. C., Sadhale, Y., & Chilukuri, D. M. (2001). Cubic phase gels as drug delivery systems. Advanced Drug Delivery Reviews, 47, 229–250.
Wallin, R., & Arnebrant, T. (1994). The activity of lipase at the cubic liquid crystalline phase/water interface. Journal of Colloid and Interface Science, 164, 16–20.
Engstrom, E., Lindahl, L., Wallin, R., & Engblom, J. (1992). A study of polar lipid drug carrier system undergoing a thermo reversible lamellar to cubic phase transformation. International Journal of Pharmaceutics, 86, 137–145.
Wyatt, D., & Dorschel, D. (1992). A cubic-phase delivery system composed of glyceryl monooleate and water for sustained release of water-soluble drugs. PharmTech, 16, 116–130.
Lawrence, M. J. (1994). Surfactant systems: Their use in drug delivery. Chemical Society Reviews, 23, 417–423.
Chung, H., Kim, J., Um, Y. J., Kwon, I. C., & Jeong, S. Y. (2002). Self-assembled nanocubicle as a carrier for peroral insulin delivery. Diabetologia, 45, 448–451.
Kuntsche, J., Westesen, K., Drechsler, M., Koch, M. H., & Bunjes, H. (2004). Supercooled smectic nanoparticles: A potential novel carrier system for poorly water soluble drugs. Pharmaceutical Research, 21, 1834–1843.
Kuntsche, J., Bunjes, H., Fahr, A., Pappinen, S., Ronkko, S., Suhonen, M., et al. (2008). Interaction of lipid nanoparticles with human epidermis and an organotypic cell culture model. International Journal of Pharmaceutics, 354, 180–195.
Nag, O. K., Naciri, J., Oh, E., Spillmann, C. M., & Delehanty, J. B. (2016). Lipid raft-mediated membrane tethering and delivery of hydrophobic cargos from liquid crystal-based nano-carriers. Bioconjugate Chemistry, 27(4), 982–993.
Sallam, A. L., Hamudi, F. F., & Khalil, E. A. (2015). Effect of ethyl cellulose and propylene glycol on the controlled-release performance of glyceryl monooleate-metronidazole periodontal gel. Pharmaceutical Development and Technology, 20(2), 159–168.
Swarnakar, N. K., Thanki, K., & Jain, S. (2014). Bicontinuous cubic liquid crystalline nanoparticles for oral delivery of doxorubicin: Implications on bioavailability, therapeutic efficacy, and cardio toxicity. Pharmaceutical Research, 31(5), 1219–1238.
Hongbing, W., Jianxu, L., Qizhi, Z., Xiluan, Y., Liangran, G., Xiaoling, G., et al. (2012). A novel small odorranalctin-bearing cubosomes: Preparation brain delivery and pharmacodynamics study on amyloid-β25-35treated rats following intranasal administration. European Journal of Pharmaceutics and Biopharmaceutics, 80, 368–378.
Shah, M. H., & Paradkar, A. (2005). Cubic liquid crystalline glyceryl monooleate matrices for oral delivery of enzyme. International Journal of Pharmaceutics, 294, 161–171.
Sung, K. H., Jin, Y. M., & Jin-Chul, K. (2012). Preparation of iron oxide nanoparticles within monoolein cubic phase. Industrial and Engineering Chemistry Research, 18, 1977–1982.
Bei, D., Marszalek, J., & Youan, B.-B. C. (2009). Formulation of dicarbazineloaded cubosomes-Part II: Influence of process parameters. AAPS PharmSciTech, 10, 1040–1047.
Jin, X., Zhang, Z., Sun, E., Tan, X., Li, S., Cheng, X., et al. (2013). Enhanced oral absorption of 20(S)-protopanaxadiol by self-assembled liquid crystalline nanoparticles containing piperine: in vitro and in vivo studies. International Journal of Nanomedicine, 8, 641–652.
Zhiwen, Y., Yinhe, T., Meiwan, C., Linghui, D., Ziyun, S., Xinsheng, P., et al. (2012). Development of amphotericin B-loaded cubosomes through the SolEmuls technology for enhancing the oral bioavailability. AAPS Pharm SciTech, 13(4), 1483–1491.
Jain, S., Rathi, V. V., Jain, A. K., Das, M., & Godugu, C. (2012). Folate-decorated PLGA nanoparticles as a rationally designed vehicle for the oral delivery of insulin. Nanomedicine (London, England), 7(9), 1311–1337.
Nguyen, T. H., Hanley, T., Porter, C. J., & Boyd, B. J. (2011). Nanostructured liquid crystalline particles provide long duration sustained-release effect for a poorly water soluble drug after oral administration. Journal of Controlled Release, 153(2), 180–186.
Thapa, R. K., Baskaran, R., Madheswaran, T., Rhyu, J. Y., Kim, J. O., Yong, C. S., et al. (2013). Effect of saturated fatty acids on tacrolimus-loaded liquid crystalline nanoparticles. Journal of Drug Delivery Science and Technology, 23(2), 137–141.
Lee, K. W. Y., Nguyen, T. H., Hanley, T., & Boyd, B. J. (2009). Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. International Journal of Pharmaceutics, 365(1-2), 190–199.
Esposito, E., Cortesi, R., Drechsler, M., Paccamiccio, L., Mariani, P., Contado, C., et al. (2005). Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharmaceutical Research, 22(12), 2163–2173.
Chen, Y., Lu, Y., Zhong, Y., Wang, Q., Wu, W., & Gao, S. (2012). Ocular delivery of cyclosporine A based on glyceryl monooleate/poloxamer 407 liquid crystalline nanoparticles: Preparation, characterization, in vitro corneal penetration and ocular irritation. Journal of Drug Targeting, 20(10), 856–863.
Sadhale, Y., & Shah, J. C. (1999). Stabilization of insulin against agitation-induced aggregation by the GMO cubic phase gel. International Journal of Pharmaceutics, 1919(1), 51–64.
Sadhale, Y., & Shah, J. C. (1999). Biological activity of insulin in GMO gels and the effect of agitation. International Journal of Pharmaceutics, 191(1), 65–74.
Sadhale, Y., & Shah, J. C. (1998). Glyceryl monooleate cubic phase gel as chemical stability enhancer of cefazolin and cefuroxime. Pharmaceutical Development and Technology, 3(4), 549–556.
Spillmann, C. M., Naciri, J., Algar, W. R., Medintz, I. L., & Delehanty, J. B. (2014). Multifunctional liquid crystal nanoparticles for intracellular fluorescent imaging and drug delivery. ACS Nano, 8(7), 6986–6997.
Nilsson, C., Barrios-Lopez, B., Kallinen, A., Laurinmäki, P., Butcher, S. J., Raki, M., et al. (2013). SPECT/CT imaging of radiolabeled cubosomes and hexosomes for potential the ranostic applications. Biomaterials, 34(33), 8491–8503.
Boyd, B. J., Khoo, S. M., Whittake, D. V., Davey, G., & Porter, C. J. H. (2007). A lipid-based liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poorly water soluble drug in rats. International Journal of Pharmaceutics, 340(1-2), 52–60.
Boyd, B. J., Whittaker, D. V., Khoo, S. M., & Davey, G. (2006). Hexosomes formed from glycerate surfactants-formulation as a colloidal carrier for irinotecan. International Journal of Pharmaceutics, 318(1-2), 154–162.
Lopes, L. B., Speretta, F. F. F., & Bentley, M. V. (2007). Enhancement of skin penetration of vitamin K using monoolein-based liquid crystalline systems. European Journal of Pharmaceutical Sciences, 32(3), 209–215.
Swarnakar, N. K., Jain, V., Dubey, V., Mishra, D., & Jain, N. K. (2007). Enhanced oromucosal delivery of progesterone via hexosomes. Pharmaceutical Research, 24(12), 2223–2230.
Fong, W. K., Hanley, T., & Boyd, B. J. (2009). Stimuli responsive liquid crystals provide ‘on-demand’ drug delivery in vitro and in vivo. Journal of Controlled Release, 135(3), 218–226.
Lee, D. R., Park, J. S., Bae, I. H., Lee, Y., & Kim, B. M. (2016). Liquid crystal nanoparticle formulation as an oral drug delivery system for liver-specific distribution. International Journal of Nanomedicine, 11, 853–871.
Swarnakar, N. K., Thanki, K., & Jain, S. (2014). Lyotropic liquid crystalline nanoparticles of coq10: implication of lipase digestibility on oral bioavailability, in vivo antioxidant activity, and in vitro – in vivo relationships. Molecular Pharmaceutics, 11(5), 1435–1449.
Okawara, M., Hashimoto, F., Todo, H., Sugibayashi, K., & Tokudome, Y. (2014). Effect of liquid crystals with cyclodextrin on the bioavailability of a poorly water-soluble compound, diosgenin, after its oral administration to rats. International Journal of Pharmaceutics, 472(1), 257–261.
Yaghmur, A., Kriechbaum, M., Amenitsch, H., Steinhart, M., Laggner, P., & Rappolt, M. (2010). Effects of pressure and temperature on the self-assembled fully hydrated nanostructures of monoolein-oil systems. Langmuir, 26(2), 1177–1185.
Sallam, A. S., Khalil, E., Ibrahim, H., & Freij, I. (2002). Formulation of an oral dosage form utilizing the properties of cubic liquid crystalline phases of glyceryl monooleate. European Journal of Pharmaceutics and Biopharmaceutics, 53(3), 343–352.
Negrini, R., & Mezzenga, R. (2011). pH-responsive lyotropic liquid crystals for controlled drug delivery. Langmuir, 27(9), 5296–5303.
Negrini, R., Fong, W. K., Boyd, B. J., & Mezzenga, R. (2015). pH-Responsive lyotropic liquid crystals and their potential therapeutic role in cancer treatment. Chemical Communications, 51(30), 6671–6674.
Yaghmur, A., Larsen, S. W., Schmitt, M., Østergaard, J., Larsen, C., Jensen, H., et al. (2011). In situ characterization of lipidic bupivacaine-loaded formulations. Soft Matter, 7(18), 8291–8295.
Yaghmur, A., Rappolt, M., Oostergaard, J., Larsen, C., & Larsen, S. W. (2012). Characterization of bupivacaine-loaded formulations based on liquid crystalline phases and micro emulsions: The effect of lipid composition. Langmuir, 28(5), 2881–2889.
Yuli, A., & Garti, N. (2005). Transitions induced by solubilized fat into reverse hexagonal mesophases. Colloids and Surfaces. B, Biointerfaces, 43(2), 72–82.
Li, Z. Z. (2008). Shui, Long, Jie Yu, Ka Wai Wong, Lin Yang, Zhang, and Woon Ming Lau. Using elemental Se and Ag to grow pure Ag2Se dendrites/dendritic-films of highly oriented (001) nanocrystals. The Journal of Physical Chemistry C, 112(8), 2845–2850. https://doi.org/10.1021/jp710190j
Deori, K., & Deka, S. (2013). Morphology oriented surfactant dependent CoO and reaction time dependent Co3O4 nanocrystals from single synthesis method and their optical and magnetic properties. CrystEngComm, 15, 8465.
Tan, D., Ma, Z., Xu, B., Dai, Y., Ma, G., He, M., Jin, Z., & Qiu, J. (2011). Surface passivated silicon nanocrystals with stable luminescence synthesized by femtosecond laser ablation in solution. Physical Chemistry Chemical Physics, 13(45), 20255–20261. https://doi.org/10.1039/c1cp21366k
Salimi, M., Pirouzfar, V., & Kianfar, E. (2017). Enhanced gas transport properties in silica nanoparticle filler-polystyrene nanocomposite membranes. Colloid & Polymer Science, 295, 215–226. https://doi.org/10.1007/s00396-016-3998-0
Kianfar, E. (2018). Synthesis and characterization of AlPO4/ZSM-5 catalyst for methanol conversion to dimethyl ether. Russian Journal of Applied Chemistry, 91, 1711–1720. https://doi.org/10.1134/S1070427218100208
Kianfar, E. (2019). Ethylene to propylene conversion over Ni-W/ZSM-5 catalyst. Russian Journal of Applied Chemistry, 92, 1094–1101. https://doi.org/10.1134/S1070427219080068
Kianfar, E., Salimi, M., Kianfar, F., et al. (2019). CO2/N2 separation using polyvinyl chloride iso-phthalic acid/aluminium nitrate nanocomposite membrane. Macromolecular Research, 27, 83–89. https://doi.org/10.1007/s13233-019-7009-4
Kianfar, E. (2019). Ethylene to Propylene over Zeolite ZSM-5: Improved catalyst performance by treatment with CuO. Russian Journal of Applied Chemistry, 92, 933–939. https://doi.org/10.1134/S1070427219070085
Kianfar, E., Shirshahi, M., Kianfar, F., et al. (2018). Simultaneous prediction of the density, viscosity and electrical conductivity of pyridinium-based hydrophobic ionic liquids using artificial neural network. Silicon, 10, 2617–2625. https://doi.org/10.1007/s12633-018-9798-z
Salimi, M., Pirouzfar, V., & Kianfar, E. (2017). Novel nanocomposite membranes prepared with PVC/ABS and silica nanoparticles for C2H6/CH4 separation. Polymer Science, Series A, 59, 566–574. https://doi.org/10.1134/S0965545X17040071
Kianfar, F., & Kianfar, E. (2019). Synthesis of isophthalic acid/aluminum nitrate thin film nanocomposite membrane for hard water softening. Journal of Inorganic and Organometallic Polymers, 29, 2176–2185. https://doi.org/10.1007/s10904-019-01177-1
Kianfar, E., Azimikia, R., & Faghih, S. M. (2020). Simple and strong dative attachment of α-diimine nickel (II) catalysts on supports for ethylene polymerization with controlled morphology. Catalysis Letters, 150, 2322–2330. https://doi.org/10.1007/s10562-020-03116-z
Kianfar, E. (2019). Nanozeolites: Synthesized, properties, applications. Journal of Sol-Gel Science and Technology, 91, 415–429. https://doi.org/10.1007/s10971-019-05012-4
Liu, H., & Kianfar, E. (2020). Investigation the synthesis of nano-SAPO-34 catalyst prepared by different templates for MTO process. Catalysis Letters, 151, 787–802.
Kianfar, E., Salimi, M., Hajimirzaee, S., & Koohestani, B. (2018). Methanol to gasoline conversion over CuO/ZSM-5 catalyst synthesized using sonochemistry method. International Journal of Chemical Reactor Engineering, 17(2), 20180127.
Kianfar, E., Salimi, M., Pirouzfar, V., & Koohestani, B. (2018). Int J Appl CeramTechnol., 15, 734–741.
Kianfar, E., Salimi, M., Pirouzfar, V., & Koohestani, B. (2018). International Journal of Chemical Reactor Engineering, 16, 1–7.
Kianfar, E. (2019). Comparison and assessment of zeolite catalysts performance dimethyl ether and light olefins production through methanol: A review. Reviews in Inorganic Chemistry, 39, 157–177.
Kianfar, E., & Salimi, M. (2020). A review on the production of light olefins from hydrocarbons cracking and methanol conversion. In J. C. Taylor (Ed.), Chapter: 1 book: Advances in Chemistry Research (Vol. 59). Nova Science Publishers, Inc.
Kianfar, E., & Razavi, A. (2020). Zeolite catalyst based selective for the process MTG: A review. In A. Mahler (Ed.), Chapter: 8 book: Zeolites: Advances in Research and Applications. Nova Science Publishers, Inc.
Kianfar, E. (2020). Zeolites: Properties, applications, modification and selectivity. In A. Mahler (Ed.), Chapter: 1 book: Zeolites: Advances in Research and Applications. Nova Science Publishers, Inc.
Kianfar, E., Hajimirzaee, S., Musavian, S. S., & Mehr, A. S. (2020). Zeolite-based catalysts for methanol to gasoline process: A review. Microchemical Journal, 156, 104822.
Kianfar, E., Baghernejad, M., & Rahimdashti, Y. (2015). Study synthesis of vanadium oxide nanotubes with two template hexadecylamin and hexylamine. Biological Forum, 7, 1671–1685.
Kianfar, E. (2020). Synthesizing of vanadium oxide nanotubes using hydrothermal and ultrasonic method (pp. 1–80). Lambert Academic Publishing.
Kianfar, E., Pirouzfar, V., & Sakhaeinia, H. (2017). An experimental study on absorption/stripping CO2 using mono-ethanol amine hollow fiber membrane contactor. Journal of the Taiwan Institute of Chemical Engineers, 80, 954–962.
Kianfar, E., & Viet, C. (2021). Polymeric membranes on base of PolyMethyl methacrylate for air separation: a review. Journal of Materials Research and Technology, 10, 1437–1461.
S.S. Nmousavian,P Faravar, ,M.G. Monjezi,E. Kianfar Z Zarei, R Zimikia.Modeling and simulation absorption of CO2 using hollow fiber membranes (HFM) with mono-ethanol amine with computational fluid dynamics. Journal of Environmental Chemical Engineering 8, 4, 103946 (2020).
Yang, Z., Zhang, L., Zhou, Y., Wang, H., Wen, L., & Kianfar, E. (2020). Investigation of effective parameters on SAPO-34 Nano catalyst the methanol-to-olefin conversion process: A review. Reviews in Inorganic Chemistry, 40(3), 91–105. https://doi.org/10.1515/revic-2020-0003
Gao, C., Liao, J., Lu, J., Ma, J., & Kianfar, E. (2020). The effect of nanoparticles on gas permeability with polyimide membranes and network hybrid membranes: a review, Reviews in Inorganic Chemistry. https://doi.org/10.1515/revic-2020-0007
Kianfar, E., Salimi, M., & Koohestani, B. (2020). Zeolite CATALYST: A review on the production of light olefins (pp. 1–116). Lambert Academic Publishing ISBN:978-620-3-04259-7.
Kianfar, E. (2020). Investigation on catalysts of methanol to light olefins (pp. 1–168). Lambert Academic Publishing ISBN: 978-620-3-19402-9.
Kianfar, E. (2020). Application of nanotechnology in enhanced recovery oil and gas Importance & Applications of Nanotechnology (Vol. 5, Chapter 3, pp. 16–21). MedDocs Publishers.
Kianfar, E. (2020). Catalytic properties of nanomaterials and factors affecting it Importance & Applications of Nanotechnology (Vol. 5, Chapter 4, pp. 22–25). MedDocs Publishers.
Kianfar, E. (2020). Introducing the application of nanotechnology in lithium-ion battery Importance & Applications of Nanotechnology (Vol. 4, Chapter 4, pp. 1–7). MedDocs Publishers.
Kianfar, E., & Mazaheri, H. (2020). Synthesis of nanocomposite (CAU-10-H) thin-film nanocomposite (TFN) membrane for removal of color from the water. Fine Chemical Engineering, 1, 83–91.
Kianfar, E. (2020). Simultaneous prediction of the density and viscosity of the ternary system water-ethanol-ethylene glycol using support vector machine. Fine Chemical Engineering, 1, 69–74.
Kianfar, E., Salimi, M., & Koohestani, B. (2020). Methanol to gasoline conversion over CuO / ZSM-5 catalyst synthesized and influence of water on conversion. Fine Chemical Engineering, 1, 75–82.
Kianfar, E. (2020). An experimental study PVDF and PSF hollow fiber membranes for chemical absorption carbon dioxide. Fine Chemical Engineering, 1, 92–103.
Kianfar, E., & Mafi, S. (2020). Ionic liquids: Properties, application, and synthesis. Fine Chemical Engineering, 2, 22–31.
Faghih, S. M., & Kianfar, E. (2018). Modeling of fluid bed reactor of ethylene dichloride production in Abadan Petrochemical based on three-phase hydrodynamic model. International Journal of Chemical Reactor Engineering, 16, 1–14.
Kianfar, E., & Mazaheri, H. (2020). Methanol to gasoline: A sustainable transport fuel. In J. C. Taylor (Ed.), Chapter: 4 book: Advances in Chemistry Research (Vol. 66). Nova Science Publishers, Inc.
Kianfar, E. (2020). A comparison and assessment on performance of zeolite catalyst based selective for theprocess methanol to gasoline: A review. In Advances in Chemistry Research (Vol. 63, Chapter 2). Nova Science Publishers, Inc.
Kianfar, E., Hajimirzaee, S., Faghih, S. M., et al. (2020). Polyvinyl chloride + nanoparticles titanium oxide Membrane for Separation of O2 / N2. Advances in Nanotechnology. Nova Science Publishers, Inc.
Kianfar, E. (2020). Synthesis of characterization nanoparticles isophthalic acid / aluminum nitrate (CAU-10-H) using method hydrothermal. In Advances in Chemistry Research. Nova Science Publishers, Inc.
Kianfar, E. (2020). CO2 capture with ionic liquids: A review. In Advances in Chemistry Research (Vol. 67). Nova Science Publishers, Inc.
Kianfar, E. (2020). Enhanced light olefins production via methanol dehydration over promoted SAPO-34. In Advances in Chemistry Research (Vol. 63, Chapter: 4). Nova Science Publishers, Inc.
Kianfar, E. (2020). Gas hydrate: applications, structure, formation, separation processes. In J. C. Taylor (Ed.), Chapter: 8 Thermodynamics. Advances in Chemistry Research (Vol. 62). Nova Science Publishers, Inc.
Kianfar, M., Kianfar, F., & Kianfar, E. (2016). The effect of nano-composites on the mechanic and morphological characteristics of NBR/PA6 blends. American Journal of Oil and Chemical Technologies, 4(1), 29–44.
Kianfar, E. (2016). The effect of nano-composites on the mechanic and morphological characteristics of NBR/PA6 Blends. American Journal of Oil and Chemical Technologies, 4(1), 27–42.
Kianfar, F., Reza, S., Moghadam, M., & Kianfar, E. (2015). Energy optimization of Ilam gas refinery unit 100 by using HYSYS Refinery Software. Indian Journal of Science and Technology, 8(S9), 431–436.
Kianfar, E. (2015). Production and identification of vanadium oxide nanotubes. Indian Journal of Science and Technology, 8(S9), 455–464.
Kianfar, F., Reza, S., Moghadam, M., & Kianfar, E. (2015). Synthesis of spiro pyran by using silica-bonded N-propyldiethylenetriamine as recyclable basic catalyst. Indian Journal of Science and Technology, 8(11), 68669.
Kianfar, E. (2019). Recent advances in synthesis, properties, and applications of vanadium oxide nanotube. Microchemical Journal, 145, 966–978.
Hajimirzaee, S., Mehr, A. S., & Kianfar, E. (2020). Modified ZSM-5 zeolite for conversion of LPG to aromatics. Polycyclic Aromatic Compounds, 42(5), 2334–2347. https://doi.org/10.1080/10406638.2020.1833048
Kianfar, E. (2021). Investigation of the effect of crystallization temperature and time in synthesis of SAPO-34 catalyst for the production of light olefins. Petroleum Chemistry, 61, 527–537. https://doi.org/10.1134/S0965544121050030
Huang, X., Zhu, Y., & Kianfar, E. (2021). Nano biosensors: Properties, applications and Electrochemical Techniques. Journal of Materials Research and Technology, 12, 1649–1672. https://doi.org/10.1016/j.jmrt.2021.03.048
Kianfar, E. (2021). Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. Journal of Nanbiotechnology, 19, 159. https://doi.org/10.1186/s12951-021-00896-3
Kianfar, E. (2022). A review of recent advances in carbon dioxide absorption–stripping by employing a gas–liquid hollow fiber polymeric membrane contactor. Polymer Bulletin. https://doi.org/10.1007/s00289-022-04626-z
Salahdin, O. D., Sayadi, H., Solanki, R., et al. (2022). Graphene and carbon structures and nanomaterials for energy storage. Applied Physics A: Materials Science & Processing, 128, 703. https://doi.org/10.1007/s00339-022-05789-2
Kianfar, E. (2022). The effects of SiO2/Al2O3 and H2O/Al2O3 molar ratios on SAPO-34 catalyst in the methanol to olefin process. Silicon, 15, 381–396. https://doi.org/10.1007/s12633-022-02008-8
Smaisim, G. F., Mohammed, D. B., Abdulhadi, A. M., et al. (2022). Nanofluids: Properties and applications. Journal of Sol-Gel Science and Technology, 104, 1–35. https://doi.org/10.1007/s10971-022-05859-0
Smaisim, G. F., Abed, A. M., Al-Madhhach, H., et al. (2022). Graphene-based important carbon structures and nanomaterials for energy storage applications as chemical capacitors and supercapacitor electrodes: A review. BioNanoScience, 13, 219–298. https://doi.org/10.1007/s12668-022-01048-z
Hachem, K., Ansari, M. J., Saleh, R. O., et al. (2022). Methods of chemical synthesis in the synthesis of nanomaterial and nanoparticles by the chemical deposition method: A review. BioNanoScience, 12, 1032–1057. https://doi.org/10.1007/s12668-022-00996-w
Ansari, M. J., Kadhim, M. M., Hussein, B. A., et al. (2022). Synthesis and stability of magnetic nanoparticles. BioNanoScience, 12, 627–638. https://doi.org/10.1007/s12668-022-00947-5
Abderrahmane, A., Mourad, A., Mohammed, S., Smaisim, G. F., Toghraie, D., Koulali, A., et al. (2023). Second law analysis of a 3D magnetic buoyancy-driven flow of hybrid nanofluid inside a wavy cubical cavity partially filled with porous layer and non-Newtonian layer. Annals of Nuclear Energy, 181, 109511.
Wang, Y., Zheng, J., Smaisim, G. F., & Toghraie, D. (2022). Molecular dynamics simulation of phase transition procedure of water-based nanofluid flow containing CuO nanoparticles. Alexandria Engineering Journal, 61(12), 12453–12461.
Xiao, M., & Smaisim, G. F. (2022). Joint chance-constrained multi-objective optimal function of multi-energy microgrid containing energy storages and carbon recycling system. Journal of Energy Storage, 55, 105842.
Mourad, A., Aissa, A., Abed, A. M., Smaisim, G. F., Toghraie, D., Fazilati, M. A., et al. (2022). The numerical analysis of the melting process in a modified shell-and-tube phase change material heat storage system. Journal of Energy Storage, 55, 105827.
Cheng, H., Abed, A. M., Alizadeh, A. A., Ghabra, A. A., Altalbawy, F. M., Sabetvand, R., et al. (2022). The effect of temperature and external force on the thermal behavior of oil-based refrigerant inside an atomic nanochannel using molecular dynamics simulation. Journal of Molecular Liquids, 369, 120893.
Smaisim, G. F., Abed, A. M., & Alavi, H. (2022). Analysis of pollutant emission reduction in a coal power plant using renewable energy. International Journal of Low-Carbon Technologies, 18, 38–48.
Abderrahmane, A., Jamshed, W., Abed, A. M., Smaisim, G. F., Guedri, K., Akbari, O. A., et al. (2022). Heat and mass transfer analysis of non-Newtonian power-law nanofluid confined within annulus enclosure using Darcy-Brinkman-Forchheimer model. Case Studies in Thermal Engineering, 40, 102569.
Tan, X., Obaid, R. F., Smaisim, G. F., Esfahani, M. M., Alsaikhan, F., Baghaei, S., et al. (2022). Investigation of addition of calcium phosphate ceramic to multilayer scaffold for bone applications with improved mechanical properties: Fuzzy logic analysis. Ceramics International, 49(5), 8339–8349.
Mir, S., Abed, A. M., Akbari, O. A., Toghraie, D., Marzban, A., Smaisim, G. F., et al. (2023). Effects of curvature existence, adding of nanoparticles and changing the circular minichannel shape on behavior of two phase laminar mixed convection of Ag/water nanofluid. Alexandria Engineering Journal, 66, 707–730.
Ruhani, B., Andani, M. T., Abed, A. M., Sina, N., Smaisim, G. F., Hadrawi, S. K., & Toghraie, D. (2022). Statistical modeling and investigation of thermal characteristics of a new nanofluid containing cerium oxide powder. Heliyon, 8(11), e11373.
Cai, W., Sabetvand, R., Abed, A. M., Toghraie, D., Hekmatifar, M., Rahbari, A., et al. (2022). Thermal analysis of hydration process in the vicinity of the copper matrix using molecular dynamics simulation for application in thermal engineering. Energy Reports, 8, 7468–7475.
Moarrefzadeh, A., Morovvati, M. R., Angili, S. N., Smaisim, G. F., Khandan, A., & Toghraie, D. (2022). Fabrication and finite element simulation of 3D printed poly L-lactic acid scaffolds coated with alginate/carbon nanotubes for bone engineering applications. International Journal of Biological Macromolecules, 224, 1496–1508.
Hai, T., Abidi, A., Wang, L., Abed, A. M., Mahmoud, M. Z., El Din, E. M. T., & Smaisim, G. F. (2022). Simulation of solar thermal panel systems with nanofluid flow and PCM for energy consumption management of buildings. Journal of Building Engineering, 58, 104981.
Fadhil Smaisim, G., Abed, A. M., Hadrawi, S. K., & Shamel, A. (2022). Parametric investigation of thermal behaviour of salt-gradient solar pool for climatic conditions. Clean Energy, 6(5), 693–704.
Smaisim, G. F., Gholami, M., Toghraie, D., Hashemian, M., & Abed, A. M. (2022). Numerical investigation of the flow and heat transfer of Al2O3/water nanofluid in a tube equipped with stationary and self-rotating twisted tapes. Progress in Nuclear Energy, 151, 104335.
Jiang, Y., Smaisim, G. F., Mahmoud, M. Z., Li, Z., Aybar, H. Ş., & Abed, A. M. (2022). Simultaneous numerical investigation of the passive use of phase-change materials and the active use of a nanofluid inside a rectangular duct in the thermal management of lithium-ion batteries. Journal of Power Sources, 541, 231610.
Tian, M. W., Abed, A. M., Yan, S. R., Sajadi, S. M., Mahmoud, M. Z., Aybar, H. Ş., & Smaisim, G. F. (2022). Economic cost and numerical evaluation of cooling of a cylindrical lithium-ion battery pack using air and phase change materials. Journal of Energy Storage, 52, 104925.
Alharbi, K. A. M., Smaisim, G. F., Sajadi, S. M., Fagiy, M. A., Aybar, H. Ş., & Elkhatib, S. E. (2022). Numerical study of lozenge, triangular and rectangular arrangements of lithium-ion batteries in their thermal management in a cooled-air cooling system. Journal of Energy Storage, 52, 104786.
Wu, W., Smaisim, G. F., Sajadi, S. M., Fagiry, M. A., Li, Z., Shamseldin, M. A., & Aybar, H. Ş. (2022). Impact of phase change material-based heatsinks on lithium-ion battery thermal management: A comprehensive review. Journal of Energy Storage, 52, 104874.
Tian, M. W., Smaisim, G. F., Yan, S. R., Sajadi, S. M., Mahmoud, M. Z., Aybar, H. Ş., & Abed, A. M. (2022). Economic cost and efficiency analysis of a lithium-ion battery pack with the circular and elliptical cavities filled with phase change materials. Journal of Energy Storage, 52, 104794.
Brontowiyono, W., AbdulHussein, W. A., Smaisim, G. F., Mahmoud, M. Z., Singh, S., Lafta, H. A., et al. (2022). Annealing temperature effect on structural, magnetic properties and methyl green degradation of Fe2O3 nanostructures. Arabian Journal for Science and Engineering, 48(1), 375–382.
Tian, Y., Patra, I., Majdi, H. S., Ahmad, N., Sivaraman, R., Smaisim, G. F., et al. (2022). Investigation of atomic behavior and pool boiling heat transfer of water/Fe nanofluid under different external heat fluxes and forces: A molecular dynamics approach. Case Studies in Thermal Engineering, 38, 102308.
Smaisim, G. F., Al-Madhhachi, H., & Abed, A. M. (2022). Study the thermal management of Li-ion batteries using looped heat pipes with different nanofluids. Case Studies in Thermal Engineering, 37, 102227.
Smaisim, G. F., Abed, A. M., & Shamel, A. (2022). Modeling the thermal performance for different types of solar chimney power plants. Complexity, 2022
Smaisim, G. F., Bidgoli, M. O., Goh, K. L., & Bakhtiari, H. (2022). Review of thermoelastic, thermal properties and creep analysis of functionally graded cylindrical shell. Australian Journal of Mechanical Engineering, 1–12.
Smaisim, G. F., Abed, A. M., Hadrawi, S. K., & Shamel, A. (2022). Modeling and thermodynamic analysis of solar collector cogeneration for residential building energy supply. Journal of Engineering, 2022.
Mozafarifard, M., Azimi, A., Sobhani, H., Smaisim, G. F., Toghraie, D., & Rahmani, M. (2022). Numerical study of anomalous heat conduction in absorber plate of a solar collector using time-fractional single-phase-lag model. Case Studies in Thermal Engineering, 34, 102071.
Sharba, Z. M., Smaisim, G. F., & Arani, A. A. A. (2022). Thermal Performance of Inline and Staggered Bank of Tubes with Laminar Cross Flow. In 2022 5th International Conference on Engineering Technology and its Applications (IICETA) (pp. 78–84). IEEE.
Smaisim, G. F., Prabu, N. M., Senthilkumar, A. P., & Abed, A. M. (2022). Synthesis of biodiesel from fish processing waste by nano magnetic catalyst and its thermodynamic analysis. Case Studies in Thermal Engineering, 35, 102155.
AbdulHussein, W. A., Abed, A. M., Mohammed, D. B., Smaisim, G. F., & Baghaei, S. (2022). Investigation of boiling process of different fluids in microchannels and nanochannels in the presence of external electric field and external magnetic field using molecular dynamics simulation. Case Studies in Thermal Engineering, 35, 102105.
Ahamad, S., Mohseni, M., Shekher, V., Smaisim, G. F., & Tripathi, A. (2022). A Detailed Analysis of the Critical Role of artificial intelligence in Enabling High-Performance Cloud Computing Systems. In 2022 2nd International Conference on Advance Computing and Innovative Technologies in Engineering (ICACITE) (pp. 156–159). IEEE.
Doss, A. N., Shah, D., Smaisim, G. F., Olha, M., & V, S. (2022). A Comprehensive Analysis of Internet of Things (IOT) in Enhancing Data Security for Better System Integrity-A Critical Analysis on the Security Attacks and Relevant Countermeasures. In 2022 2nd International Conference on Advance Computing and Innovative Technologies in Engineering (ICACITE) (pp. 165–167). IEEE.
Lefteh, A., Houshmand, M., Khorrampanah, M., & Smaisim, G. F. (2022). Optimization of modified adaptive neuro-fuzzy inference system (MANFIS) with artificial bee colony (ABC) algorithm for classification of bone cancer. In 2022 Second International Conference on Distributed Computing and High Performance Computing (DCHPC) (pp. 78–81). IEEE.
Sallal, A. S., Smaisim, G. F., & Thahab, S. M. (2021). The heat transfer from fined perforated pipe improved due to nano-fluid. In Journal of Physics: Conference Series 1973 (p. 012075). IOP Publishing.
Al-Madhhachi, H., & Smaisim, G. F. (2021). Experimental and numerical investigations with environmental impacts of affordable square pyramid solar still. Solar Energy, 216, 303–314.
Smaisim, G. F. (2018). Investigation on heat transfer augmentation using continuous and broken ribs on a plate of heat exchanger. International Journal of Energy and Environment, 9(3).
Smaisim, G. F. (2017). Augmentation of heat transfer in corrugated tube using four-start spiral wall. Al-Qadisiya Journal for Engineering Sciences, 10(4), 451–467.
Smaisim, G. F. (2017). Enhancement heat transfer of Cu-water nanofluids with thermophysical properties modeling by artificial neural network. Journal of University of Babylon, 25(5), 1721–1735.
Smaisim, G. F., Fatta, O., Valera-Medina, A., Rageb, A., & Syred, N. (2016). Investigation of heat transfer and fluid mechanics across a heated rotating circular cylinder in crossflow. In 54th AIAA Aerospace Sciences Meeting (p. 0494)
Smaisim, G., Fatla, O., Valera Medina, A., Rageb, A. M., & Syred, N. (2016). Experimental and theoretical investigation of the effect of rotating circular cylinder speed on the lift and drag forces. International Journal of Energy and Environment, 7(1), 23–36.
Acknowledgements
The authors thank the Department of Chemical Engineering, Arak Branch, Islamic Azad University, Arak, Iran, and the Young Researchers and Elite Club, Gachsaran Branch, Islamic Azad University, Gachsaran, Iran.
Availability of Data and Material
Not applicable
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
GFS: investigation, writing—original draft; KJM: reviewing and editing; SKH: reviewing and editing; HK: reviewing and editing; EK: investigation; writing—original draft; reviewing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for Publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Smaisim, G.F., Mohammed, K.J., Hadrawi, S.K. et al. Properties and Application of Nanostructure in Liquid Crystals: Review. BioNanoSci. 13, 819–839 (2023). https://doi.org/10.1007/s12668-023-01082-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01082-5