Abstract
This paper reports a safe, simple, green, nontoxic, and environment-friendly approach for the synthesis of silver nanoparticles (AgNPs) using aqueous leaf extract of Trigonella foenum-graecum at room temperature. The aqueous leaf extract was capable to act as a reducing, capping, and stabilizing agent. UV-visible spectroscopic, Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), scanning electron microscope (SEM), and transmission electron microscopy (TEM) techniques were used to characterize the synthesized AgNPs. The synthesized AgNPs were found to be stable, face-centered cubic, crystalline, and spherical and in the range of 20–25 nm. The antimicrobial activities of the synthesized AgNPs were investigated against plant pathogenic fungi Alternaria alternata and plant pathogenic bacteria Pseudomonas syringae. The assay results showed that A. alternata and P. syringae were inhibited at the 100 ppm of AgNPs in the respective medium. Antifungal assay shows the disruption of fungal mycelium at various places. Similarly, antibacterial assay shows the inhibition zones. These results confirmed that this protocol is a green, environment-friendly, and nontoxic method for the synthesis of AgNPs. Assay results confirmed that the synthesized AgNPs can be used as an effective growth inhibitor for various pathogenic microorganisms and applicable to control microbial systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanotechnology is a rapidly developing field because of its wide range of applications in sciences, industries, biotechnology, and nanosciences. The word nanotechnology is generally used when referring to materials of 1–100 nm in size. When a material is reduced to nanosize, it acts differently and exhibits some new properties, which completely lacks in its macroscale or bulk form. Nanoparticles are of great scientific interest, because they bridge the gap between bulk materials and atomic and/or molecular structures [1, 2]. Nanoparticles are extremely important because of their exceptionally small size and large surface area to volume ratio, which determines their properties such as electrical, mechanical, chemical, physical, optical, solubility, and stability properties. Generally, nanoparticles are classified into organic and inorganic nanoparticles. Nanoparticles that contain carbon are considered organic nanoparticles whereas metallic nanoparticles, such as noble metal (gold, silver, and platinum) and semiconductor (titanium dioxide, zinc oxide, and zinc sulfide), are considered inorganic nanoparticles [2,3,4,5,6,7,8,9].
Silver nanoparticles (AgNPs) can absorb and scatter light as well as exhibit colors depending on the shape, size, and morphology. AgNPs have a range of diverse purpose in different applications [2,3,4,5,6,7]. AgNPs are gaining a lot of attention because of their unique properties like antimicrobial, physiochemical, catalytic, and optical properties [3,4,5,6].
The current nanoparticle synthesis methods use toxic chemicals and have hazardous effect on the environment. Therefore, it is necessary to develop ecofriendly and green methods for synthesizing nanoparticles using nontoxic chemicals and green and environment-friendly methods. The ecofriendly and “green” processes are becoming popular and are much needed as a result of worldwide problems associated with environmental concerns [1, 2, 10].
Agricultural production is broadly affected by various kinds of diseases due to pathogenic activities of microorganisms which leads to huge economic loss [11]. To manage these losses and to improve the agriculture production, “green perspective” plant-mediated synthesis of AgNPs can be used as an antimicrobial agent against agricultural pathogens. Several methods have been reported for the synthesis of AgNPs from different routes, and appreciation is given to the controlled size and shape of AgNPs. Hence, nanosilver with small particle size with no aggregation between the particles is considered favorable [2, 7, 12].
To the author’s knowledge, this is the first report of synthesis of AgNPs using the aqueous leaf extract of T. foenum-graecum. However, the synthesis of AgNPs using ethanolic leaf extract of T. foenum-graecum and its seed extract has been reported [13,14,15]. We have successfully synthesized AgNPs using the aqueous leaf extract of T. foenum-graecum which is a green and novel approach. T. foenum-graecum is an annual plant that belongs to the family Fabaceae. It is commonly known as Fenugreek in English and Methi in Hindi language. Fenugreek contains many compounds which include alkaloids, flavonoids, coumarins, vitamins, and saponins. Among others, the most prevalent alkaloids are trigonelline and coumarins [13,14,15,16]. The objectives of the present study are to show that AgNPs can be synthesized in a clear, simple, economical, and environment-friendly manner using the aqueous leaf extract of T. foenum-graecum and also to evaluate the in vitro antimicrobial activities and efficacy of AgNPs for the suppression of the plant pathogenic fungi Alternaria alternate and plant pathogenic bacteria Pseudomonas syringae. The synthesized AgNPs showed robust and enhanced antimicrobial activities.
2 Materials and Methods
AgNO3 AR and all other reagents and solvents used in this work were purchased from Merck India Ltd., India, and were used without any purification. Single-strain bacteria Pseudomonas syringe (MTCC No. 673) and fungus Alternaria alternate (MTCC No. 1362) were purchased from Imtech, Chandigarh, India.
To confirm the formation of AgNPs, UV-Vis spectral analysis was performed using Shimadzu spectrophotometer (UV-Vis 1800, Japan). Fourier transform infrared (FT-IR) spectra of the AgNPs were obtained in the range of 4000–400 cm−1 with an FT-IR spectrophotometer (Perkin Elmer Spectrum 2000 FT-IR) using KBr pellets. X-ray diffraction (XRD, PANalytical, X’pert PRO-MPD, The Netherlands) was performed using CuKα radiation (λ = 0.15405 nm). The size and morphology of the AgNPs were analyzed by SEM (NOVA nano FE-SEM 450 FEI) and TEM (TECNAI-G-20).
2.1 Green Synthesis of Silver Nanoparticles
Aqueous leaf extract of the Trigonella foenum-graecum was used to synthesize the AgNPs on the basis of cost-effectiveness and ease of availability [13,14,15,16]. Fresh plant leaves of T. foenum-graecum were collected from Jagatpura, Jaipur, Rajasthan, India. Leaf surface was washed and cleaned with running tap water to remove debris and other contaminated organic contents, followed by double-distilled water and air-dried at room temperature. A 25 g of finely powdered leaves of T. foenum-graecum was kept in a round–bottom flask containing 250 mL double-distilled water and refluxed for 30 min. The extract was cooled down at room temperature and filtered with Whatman filter paper number 1 for synthesis process.
To a 90 mL, 1 mM AgNO3 aqueous solution, 15 mL of T. foenum-graecum aqueous leaf extract was added. The reaction mixture was stirred at room temperature till the color changes. During this period, the reduction of Ag+ to Ag0 was confirmed by the color change of solution from greenish brown to yellowish brown. AgNP formation was also confirmed using UV-visible spectroscopy that shows absorbance at ~ 410 nm.
2.2 Antimicrobial Activities
AgNPs synthesized using the aqueous leaf extract of T. foenum-graecum were used to screen for the in vitro antimicrobial activities against plant pathogenic fungus Alternaria alternate (MTCC No. 1362) and plant pathogenic bacteria Pseudomonas syringe (MTCC No. 673).
For antifungal activities, potato dextrose medium with 100 ppm AgNPs was used to screen the effect of AgNPs on mycelia growth of A. alternate compared with control (without NPs). A 100 ppm solution was prepared by dissolving 0.1 mL solutions of AgNPs in 1 L distilled water.
Similarly, for antibacterial activities, nutrient agar medium was used to culture P. syringae and a paper disc dipped in 100 ppm AgNPs placed in media containing P. syringae and compared with control (without NPs). These plates were incubated at 35 °C for ~ 24 h, and the obtained inhibition zones were measured and compared.
3 Results and Discussion
A green, biological, and environment-friendly method was used to synthesize AgNPs using AgNO3 as a precursor and the aqueous leaf extract of Trigonella foenum-graecum. The aqueous leaf extracts of Trigonella foenum-graecum act as both a stabilizing and reducing agent. The advantage of this synthesis is that it does not require the use of harsh and toxic chemical compounds.
3.1 Fourier Transform Infrared Spectroscopic Study
FT-IR measurement on AgNPs was performed to investigate the possible functional groups attached on the surface of the AgNPs. FT-IR spectroscopic analysis was executed to ascertain the involvement of possible plant biocompound responsible for the reduction of Ag+ ions and capping as well as the stabilization of bioreduced AgNPs. Figure 1 shows the FT-IR spectrum of the AgNPs synthesized using aqueous leaf extract of the T. foenum-graecum where the spectrum manifests prominent transmittance located at peaks 3247, 2919, 1587, 1376, and 1019 cm−1 which represents free OH, stretching -C=C- aromatic ring, and C-OH stretching vibrations, respectively, on AgNPs [13, 14]. These peaks suggested the presence of flavonoid and other phenolic groups on the surface of AgNPs synthesized using T. foenum-graecum aqueous leaf extract. The presence of flavonoid and other phenolic groups might be responsible for the bioreduction of Ag+ and formation of the AgNPs [13,14,15,16]. Consequently, the occurrence of these peaks in the FT-IR spectrum of the AgNPs synthesized using aqueous leaf extract of T. foenum-graecum evidently indicates the dual role of the T. foenum-graecum, both as a green reducing agent and also as a stabilizing agent.
3.2 UV-Vis Spectroscopy
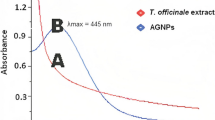
UV-Vis absorption spectroscopic analysis was performed to confirm the AgNP synthesis using aqueous leaf extract of the T. foenum-graecum. The green synthesis of AgNPs and its stability was confirmed by UV-Vis spectroscopy. It was observed that greenish brown color solution of leaves and AgNO3 changes to yellowish brown color within 30 min due to the formation of stable AgNPs. The UV-Vis spectrum of the synthesized AgNPs is shown in Fig. 2. It showed an optical absorption band at ~ 410 nm which is a characteristic band of AgNPs owing to the surface plasma resonance (SPR) of silver [7, 13, 14]. This SPR absorption band is because of the stimulation of free electrons in the outermost orbitals of AgNPs [13, 14].
3.3 XRD Analysis of the Synthesized AgNPs
The XRD analysis of the synthesized AgNPs was carried out to confirm the formation, structure, and crystalline nature of the synthesized AgNPs. Figure 3 shows the XRD patterns of the synthesized AgNPs. It shows the prominent diffraction peaks at 27.9°, 32.4°, 38.2°, and 46.3° which correspond to the (111), (200), (220), and (311) planes, respectively [7, 14]. These peaks are well attributed to the standard JCPDS data of the silver with face-centered cubic (fcc) crystal lattice structure (JCPDS No. 87-0720) [13]. This indicates and confirms the polycrystalline nature of the synthesized AgNPs. Similar patterns for the AgNPs have been also reported [7, 9, 13, 14]. No any impurity peaks were observed. The average crystallite size was calculated using Scherrer’s formula and found to be ~ 20 nm [7, 13, 14].
3.4 SEM, SAED, and TEM Studies of the Synthesized AgNPs
SEM analysis was carried out to investigate the morphology and size of the synthesized AgNPs. Figure 4a shows the SEM image of the synthesized AgNPs which were almost spherical in shape, well dispersed, less aggregated, uniform in size, and well crystalline in nature. The size of the AgNPs was ranging from 20 to 25 nm. The selected area electron diffraction (SAED) pattern (inset Fig. 4a) exhibits the well-resolved lattice fringes, diffraction cycles, and concentric rings correspondingly indexed to the (111), (200), (220), and (311) planes of fcc silver phase which is the characteristic crystal planes of elemental Ag0 of highly crystalline nature of the synthesized AgNPs. The SAED pattern is in good agreement with the ascribed result of XRD, which also suggests the similar reflections of the synthesized AgNPs.
TEM analysis was performed to know and confirm the size, shape, and morphology of the synthesized AgNPs. The TEM image (Fig. 4b) affirms that the AgNPs are in nanoscale and most of the particles are spherical in shape with an average diameter of 20–25 nm. The results clearly reveal that the synthesized colloidal AgNPs were well dispersed, homogeneous, predominantly spherical in shape, and well crystalline in nature as shown in Fig. 4b. Agglomeration of the AgNPs was not observed because of the encapsulation of the AgNPs with the leaf extract of the T. foenum-graecum. The encapsulation is owing to the presence of the alkaloids, flavonoids, polyphenols, etc., naturally found in the fenugreek plants.
3.5 Antifungal and Antibacterial Activities of the Synthesized AgNPs
Very few antifungal and antibacterial activities against the pathogens (fungi and bacteria) using AgNPs in one report have been reported. Hence, synthesized AgNPs were tested against the plant pathogenic fungi Alternaria alternata. In vitro study showed that AgNPs comprehensively inhibited the mycelial growth of the fungi Alternaria alternata. The growth of A. alternata was considerably reduced to 40–50% (Fig. 5) in potato dextrose medium with 100 ppm AgNPs compared with control (without NPs) experiment.
The SEM analysis was used to observe the morphological changes on the surface of the mycelium treated with AgNPs. Disruption of fungal mycelium was observed at various places. Similarly, Kim et al. [17] reported antifungal effects of AgNPs against various plant pathogenic fungi and observed similar disruption of the fungal mycelium. Ibrahim et al. [18] also reported that AgNPs showed efficient antimicrobial property compared with other reports due to their extremely large surface area providing better attachment with the cell wall of the microorganisms. AgNPs may directly attach and penetrate the cell membrane, although penetration of AgNPs into microbial cell membranes is not completely understood [19]. Vahabi et al. [20] observed that antifungal activity of AgNPs may be due to suppression of enzymes and toxins used by the fungal pathogens for pathogenesis. This confirms that the synthesized AgNPs can be used as antifungal agents.
AgNPs due to their antimicrobial properties have been used widely in the health industry, medicine, food storage, wound dressing, dye reduction, textile coatings, antiseptic creams, and a number of environmental applications [21]. An antibacterial assay was performed against P. syringae using the synthesized AgNPs via disc diffusion method, i.e., comparing the inhibition zone on the agar plates in the presence or absence of the synthesized AgNPs. The results of antibacterial activities of the synthesized AgNPs were evaluated by measuring the inhibition zone. A strong inhibition zone of 3–5 mm was observed around a paper disc dipped in 100-ppm AgNPs placed in nutrient agar medium inoculated with P. syringae (Fig. 6a). Based on the zone of inhibition produced, synthesized AgNPs prove to exhibit a good antibacterial activity against P. syringae. Figure 6b shows the bacterial cell disruption after treating with the synthesized AgNPs. Velmurugan et al. [22] reported the green synthesis of silver and gold nanoparticles using Zingiber officinale root extract, and similar antibacterial activities of the AgNPs against food pathogens were observed. From this study, it was found that the AgNPs synthesized using aqueous leaf extract of T. foenum-graecum exhibited antimicrobial activities by damaging the cell membranes.
4 Conclusions
In this study, a simple, green, and one-pot green synthesis of stable AgNPs using aqueous leaf extract of T. foenum-graecum at room temperature was reported. The synthesis of AgNPs using T. foenum-graecum is a clean, green, inexpensive, ecofriendly, reliable, and safe method. The UV-visible spectroscopic, FT-IR, XRD, SEM, and TEM techniques confirmed the synthesis and well-dispersed nature of the AgNPs. The synthesized AgNPs showed antimicrobial activities against plant pathogenic fungi Alternaria alternata and plant pathogenic bacteria Pseudomonas syringae. Hence, the synthesized AgNPs can be used as an antimicrobial agent for food safety, improvement of agricultural production, and a range of applications in real life for the benefit of human beings.
References
Khan, A. U., Khan, M., Malik, N., Cho, M. H., & Khan, M. M. (2019). Recent progress of algae and blue–green algae-assisted synthesis of gold nanoparticles for various applications. Bioprocess and Biosystems Engineering, 42, 1–15.
Khan, A. U., Malik, N., Khan, M., Cho, M. H., & Khan, M. M. (2018). Fungi-assisted silver nanoparticles synthesis and their application. Bioprocess and Biosystems Engineering, 41, 1–20.
Savithramma, N., Rao, M. L., Rukmini, K., & Devi, P. S. (2011). Antimicrobial activity of silver nanoparticles synthesized by using medicinal plants. International Journal of ChemTech Research, 3(3), 1394–1402.
Xu, R., Wang, D., Zhang, J., & Li, Y. (2006). Shape-dependent catalytic activity of silver nanoparticles for the oxidation of styrene. Chemistry – An Asian Journal, 1(6), 888–893.
Shahverdi, A. R., Fakhimi, A., Shahverdi, H. R., & andMinaian, S. (2007). Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine: Nanotechnology, Biology and Medicine, 3(2), 168–171.
Evanoff, D. D., & Chumanov, G. (2005). Synthesis and optical properties of silver nanoparticles and arrays. Chemphyschem, 6(7), 1221–1231.
Khan, M. M., Kalathil, S., Lee, J., & Cho, M. H. (2012). Synthesis of cysteine capped silver nanoparticles by electrochemically active biofilm and their antibacterial activities. Bulletin of the Korean Chemical Society, 33(8), 2592–2596.
Khan, M. M., Lee, J., & Cho, M. H. (2013). Electrochemically active biofilm mediated bio-hydrogen production catalyzed by positively charged gold nanoparticles. International Journal of Hydrogen Energy, 38, 5243–5250.
Khan, M. M., Adil, S. F., & Mayouf, A. A. (2015). Metal oxides as photocatalysts. Journal of Saudi Chemical Society, 19, 462–464.
Thuesombat, P., Hannongbua, S., Akasit, S., & Chadchawan, S. (2014). Ecotoxicology and environmental safety effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicology and Environmental Safety, 104, 302–309.
Mishra, S., & Singh, H. B. (2015). Biosynthesized silver nanoparticles as a nanoweapon against phytopathogens: exploring their scope and potential in agriculture. Applied Microbiology and Biotechnology, 99(3), 1097–1107.
Kamyar, S., Ahmad, M. B., Zamanian, A., Sangpou, P., Shabanzadeh, A. Y., & Zargar, M. (2012). Green biosynthesis of silver nanoparticles using Curcuma longa tuber powder. International Journal of Nanomedicine, 7, 5603–5610.
Senthil, B., Devasena, T., Prakash, B., & Rajasekar, A. (2017). Non-cytotoxic effect of green synthesized silver nanoparticles and its antibacterial activity. Journal of Photochemistry and Photobiology, B: Biology, 177, 1–7.
Varghese, R., Almalki, M. A., Ilavenil, S., Rebecca, J., & Choi, K. C. (2019). Silver nanopaticles synthesized using the seed extract of Trigonella foenum-graecum L. and their antimicrobial mechanism and anticancer properties. Saudi Journal of Biological Sciences, 26(1), 148–154.
Goyal, S., Gupta, N., Kumar, A., Chatterjee, S., & Nimesh, S. (2018). Antibacterial, anticancer and antioxidant potential of silver nanoparticles engineered using Trigonella foenum-graecum seed extract. IET Nanobiotechnology, 12(4), 526–533.
Ouzir, M., El Bairi, K., & Amzazi, S. (2016). Toxicological properties of fenugreek (Trigonella foenum graecum). Food and Chemical Toxicology, 96, 145–154.
Kim, S. W., Jung, J. H., Lamsal, K., Kim, Y. S., Min, J. S., & Lee, Y. S. (2012). Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology, 40(1), 53–58.
Ibrahim, H. M. M. (2015). Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. Journal of Radiation Research and Applied Science, 8(3), 265–275.
Morones, J. R., Elechiguerra, J. L., Camacho, A., Holt, K., Kouri, J. B., Ramírez, J. T., & Yacaman, M. J. (2005). The bactericidal effect of silver nanoparticles. Nanotechnology, 16, 2346–2353.
Vahabi, K., Mansoori, G. A., & Karimi, S. (2011). Biosynthesis of silver nanoparticles by fungus Trichoderma Reessei. Insciences Journal, (1), 65–79.
Gao, X., Yourick, J. J., Topping, V. D., Black, T., Olejnik, N., & Keltner, Z. (2015). Toxicogenomic study in rat thymus of F1 generation offspring following maternal exposure to silver ion. Toxicology Reports, 2, 341–350.
Velmurugan, P., Anbalagan, K., Manosathyadevan, M., Lee, K. J., Cho, M., Lee, S. M., & Oh, B. T. (2014). Green synthesis of silver and gold nanoparticles using Zingiber officinale root extract and antibacterial activity of silver nanoparticles against food pathogens. Bioprocess and Biosystems Engineering, 37(10), 1935–1943.
Acknowledgments
M. M. Khan would like to thanks the Chemical Sciences, Faculty of Science, University Brunei Darussalam, Brunei Darussalam for the support to complete this review article. A. U. Khan would like to thanks to Dr. Sandeep Bakshi, Chancellor of Jaipur National University, Jaipur 302 017, India for providing the research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Research Involving Humans and Animals Statement
None.
Informed Consent
None.
Funding Statement
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, A.U., Khan, M. & Khan, M.M. Antifungal and Antibacterial Assay by Silver Nanoparticles Synthesized from Aqueous Leaf Extract of Trigonella foenum-graecum. BioNanoSci. 9, 597–602 (2019). https://doi.org/10.1007/s12668-019-00643-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-019-00643-x