Abstract
HLA-DR is a key marker of leukocyte activation, which suppression determines the effectiveness of immune response and prognosis in neonatal sepsis (NS). The aim of this study was to evaluate the HLA-DR expression on lymphocytes and monocytes in the peripheral blood of neonates with sepsis. The study involved 21 neonates with sepsis and 10 healthy neonates born during the study period. Bacteremia was detected in five cases (24%). The most frequent pathogens found were Klebsiella pneumoniae (n = 2) and Staphylococcus aureus (n = 2) followed by Streptococcus agalactae (n = 1). NS was associated with pneumonia (n = 17), meningitis (n = 3), and necrotizing enterocolitis (n = 1). The onset of NS was accompanied with high blood monocyte activity which ranged from 96 to 100%. Blood CD3+ lymphocyte population and CD4+ and CD8+ lymphocyte subset levels were decreased, with their median values being 1.7, 2.2, and 2.4 times lower than those in the control group, respectively (р = 0.005, р = 0.0002, р = 0.003). The HLA-DR expression on CD3+ lymphocytes, as well as on CD4+ and CD8+ lymphocyte subsets, was extremely low ranging from 0.1 to 7% both in the onset of the disease and in a week after admission to a hospital. Absolute counts of activated CD4+ and CD8+ lymphocyte subsets were significantly lower on the disease first days as compared to the control group (р = 0.02 and р = 0.003, respectively). These results demonstrate adaptive immunity to be non-effective in neonates with sepsis. Low expression of HLA-DR on CD3 lymphocytes as well as on CD4 and CD8 lymphocyte subsets is a reason for immunostimulating therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Neonatal sepsis (NS) is a most important infectious pathology in neonates that is due to its significant prevalence and mortality in this age group [1]. Immature immune system is a key risk factor of NS [2]. Therefore, preterm infants are especially predisposed to sepsis and are at a higher risk of the development of long-term complications and death than full-term neonates do [3]. The activity of immune system cells in NS, their ability to kill and eliminate different pathogens, and their toxins from the body determine a prognosis for the disease. Immune response in sepsis is known to be characterized by a succession of immune activation and immunosuppression [4]. The incipience of sepsis is characterized by activation of innate immunity cells predominantly and is accompanied with the formation of a systemic inflammatory response syndrome (SIRS) [5]. Current methods of evaluating the innate immunity cell functional activity involve the detection of markers and activation products (pro-inflammatory cytokines, C-reactive protein, and others) in the blood [6, 7]. Markers of immune system cell activation are different, for instance, CD11β and CD64 for neutrophils [7, 8] and HLA-DR for monocytes [9, 10]. HLA-DR, a MHC class II leukocyte surface marker, is a key marker of monocyte activation, which suppression determines the effectiveness of immune response and prognosis in severe forms of the disease [11,12,13]. A decreased monocyte HLA-DR expression was shown to be associated with a risk of death in sepsis [14]. Reduced HLA-DR expression on leukocyte’s surface might be due to either initial immaturity of immune system cells (especially in preterm neonates) or a resulting “depletion” of the immune system. At the same time, both innate and adaptive immunity cells are affected that manifests as reduced counts of dendritic cells and CD4 and CD8 lymphocyte subsets in the blood and different organs [5, 15]. Lymphocyte apoptosis which is the final stage of activation processes in T cells is the main cause of lymphopenia and immunosuppression [16]. Markers of Т cell activation are numerous, and CD69, CD25 (an interleukin-2 receptor), CD127 (an IL-7 receptor), HLA-DR, and other molecules are among them [16]. At the same time, CD69 and CD25 are early markers of lymphocyte activation, while HLA-DR being a late one [16]. The intensity of HLA-DR expression on lymphocytes in sepsis is understudied. The evaluation of HLA-DR expression on lymphocytes in NS would increase and advance our understanding of lymphocyte functional activity in this pathology in neonates and determine the possibilities of immunotherapy.
The aim of this study was to evaluate the HLA-DR expression on lymphocytes and monocytes in peripheral blood of neonates with sepsis.
1 Materials and Methods
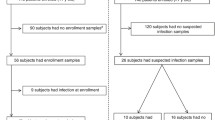
This study is “case-control” involving 31 neonates, which were divided into 2 groups. The first group consisted of 21 neonates with sepsis who were admitted to a neonatal intensive care unit of the children’s hospital no. 1 of Kazan. Eleven neonates (52%) including five babies (23%) with a very low birth weight (VLBW) were preterm. There were 52 and 48% of boys and girls, respectively, in this group. In accordance with the Report of the Expert Meeting on Neonatal and Pediatric Sepsis [17], sepsis was defined as the presence of at least two clinical symptoms and at least two laboratory signs in the presence of or as a result of a suspected or proven infection (positive blood culture). The clinical symptoms are as follows: (1) body temperature instability; (2) cardiovascular instability; (3) skin and subcutaneous lesions such as petechial rash or sclerema; (4) apnea or increased oxygen requirement, or requirement for ventilation support; (5) feeding intolerance or abdominal distension; and (6) irritability, lethargy, or hypotonia. Its laboratory signs are (1) a white blood cell (WBC) count of < 4 or > 20 × 109 cells/L; (2) an immature to total neutrophil ratio (I/T) of > 0.2; (3) a platelet count of < 100 × 109/L; (4) C-reactive protein (CRP) levels of > 15 mg/dL; (5) blood glucose values of > 180 mg/dL or hypoglycemia (< 40 mg/dL) confirmed at least twice; and (6) metabolic acidosis as characterized by a base excess (BE) of ≤ 10 mmol/L [17].
Bacteremia was detected in five cases (24%). The most frequent pathogens found were Klebsiella pneumoniae (n = 2), producing extended-spectrum beta-lactamases (ESBL), and Staphylococcus aureus (n = 2) followed by Streptococcus agalactae (n = 1). NS was associated with pneumonia (17 cases, 81%), meningitis (3 children, 14%), and necrotizing enterocolitis (1 case, 5%). All patients were on mechanical ventilation of various duration. The disease resulted in two deaths: a baby with purulent meningitis and the formation of leukomalacia, and a baby with necrotizing enterocolitis complicated by peritonitis. The second group consisted of 10 neonates who had no signs of infection. Three neonates (30%) were born preterm. The characteristics of patients are given in Table 1.
Serum C-reactive protein levels were measured with immunoturbidimetry on an analyzer “Cobas Integra 800” using a reagent kit manufactured by Roche (Switzerland) according to the manufacturer’s instruction.
Innate and adaptive immunity factors were evaluated. Blood monocyte, lymphocyte, and CD3+ lymphocyte subset (CD4+ и CD8+) counts were evaluated. Immunophenotyping of lymphocyte subsets was performed with flow cytofluorometry using appropriate monoclonal antibodies (Becton Dickinson, USA). The activity of monocytes and lymphocyte subsets was assessed by the HLA-DR expression on their surfaces. Blood was collected twice: within the first 2 days since sepsis clinical and laboratory manifestations and during the disease progression, in 7 days.
Statistical analysis was made with the Mann-Whitney nonparametric method using Statistica 6.1 for Windows (Statsoft, Tulsa, OK, USA). Significance was established at a value of p < 0.05.
The Ethics Committee of the City Children’s Hospital approved this study and a written informed consent was obtained from the parents in accordance with the principles authorized under this protocol (the Federal Law N323-FL dated on 21.11. 2011 “On Protection of Health of the Citizens in the Russian Federation”).
2 Results
It was found that the incipience of NS was accompanied with a high blood monocyte activity which ranged from 96 to 100% (Table 2). At the same time, the median of monocyte absolute counts and the HLA-DR expression on them did not significantly differ from the control group values both in the sepsis initial stage and in a week after the treatment was started (Table 3). Neither monocyte count nor their activity in children with NS depended on the gestational age (p = 0.1). None of the children including those with an unfavorable disease outcome had the HLA-DR expression levels lower than 96%. However, the monocyte absolute count in the patients who died was quite low being 0.395 and 0.798 × 109/L, respectively.
The evaluation of adaptive immunity parameters demonstrated that the incipience of NS proceeded in the setting of a decreased peripheral blood lymphocyte count, which median being 1.7-fold lower as compared to the control group (p = 0.0003). There was absolute lymphopenia (less than 3 × 109/L) in seven children (33%) with NS. Blood CD3+ lymphocyte population and CD4+ and CD8+ lymphocyte subset levels were noted to decrease, with their median values being 1.7, 2.2, and 2.4 times lower than those in the control group, respectively (р = 0.005, р = 0.0002, р = 0.003). It should be noted that children with an unfavorable disease outcome had the lowest CD4+ and CD8+ lymphocyte subset counts. When the disease progressed, in 7 days, total blood lymphocyte and lymphocyte subset counts increased, with their levels being similar to those in the control group (p > 0.05). The expression of HLA-DR on CD3+ lymphocytes, as well as on CD4+ и CD8+ lymphocyte subsets, was extremely low ranging from 0.1 to 7% both in the onset of the disease and in a week after admission to hospital. However, absolute counts of activated CD4+ и CD8+ lymphocyte subset was significantly lower on the disease first days as compared to the control group (р = 0.02 and р = 0.003, respectively). Moreover, an activated CD8+ lymphocyte count was lower than that in the control group even in a week after the onset of the disease (р = 0.04). Given that the immune system in preterm children is characterized by originally immature adaptive immunity, the activity of lymphocyte subsets was compared in children with NS taking into account the gestational age. The degree of activation of CD4+ and CD8+ lymphocyte subset was found to be almost similar in full-term and preterm children within the first days of the disease (р = 0.4 and р = 0.6, respectively). In the second phase of NS, an activated CD4 lymphocyte count in preterm babies was 3.1-fold lower as compared to full-term children (p = 0.02). The median of activated CD8 lymphocytes in the NS second phase in preterm children was similar to that in full-term ones (p = 1.0).
3 Discussion
Innate immunity factors play a key role in providing protection against infection in neonates, while adaptive immunity being immature [18] and manifests as a decreased ability to synthesize immunoglobulins, low T cell functional activity, and naive Т cell dominance in this age [18]. The activation of innate immunity cells (monocytes, dendritic cells) during the incipience of sepsis is followed by synthesis of pro-inflammatory cytokines and the development of SIRS [19], which is targeted to localize an inflammatory process. High WBC and neutrophil counts with the predominance of immature cell forms are expected but not informative laboratory indicators of sepsis. In our study, high WBC and neutrophil counts were observed in the initial sepsis stage only in 19 and 33% of cases, respectively. Two babies had leukopenia (less than 5 × 109/L). That is, there was no pronounced inflammatory process in the peripheral blood in the majority of neonates with sepsis. A degree of the increase of WBC and neutrophil counts in peripheral blood seems to be primarily determined by a degree of the activity of monocytes and dendritic cells as well as their ability to synthesize pro-inflammatory cytokines. A low monocyte activity and a decreased cytokine production might cause generalization of an infection [20]. On the other hand, excessive cell activation and resulting pronounced synthesis of pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) (“cytokine storm”) might cause the development of septic shock and death in the early stages of the disease [19]. The activation of innate immunity cells occurred within the first hours of a septic process is quickly followed by immunosupression or “immunoparalysis” which are at present recognized as the main cause of an unfavorable outcome in patients with sepsis in the later period of the disease (after third day of the onset of sepsis). Persistent HLA-DR expression on monocytes less than 60% is interpreted as immunosupression and the expression of less than 30% as immunoparalysis [21]. Monneret G. et al. found out that reduced HLA-DR expression on monocytes of less than 40%, persisting for 5 days of the onset of septic shock, was associated with an unfavorable outcome [22]. An increased production of interleukin-10 (IL-10) and re-stimulation by gram-negative bacteria endotoxin are the main factors which promote suppression of monocyte activation. Wolk K. et al. showed that initial stimulation of monocytes with endotoxin resulted in the activation of these cells with evident pro-inflammatory response; however, there was tolerance to endotoxin with its reintroduction and a pronounced reduction of monocyte HLA-DR expression levels [23]. Therefore, a low monocyte HLA-DR expression is considered as one of predictors of an unfavorable outcome in sepsis [24, 25]. Consequently, the detection of reduced HLA-DR expression on monocytes in patients with sepsis should serve as a ground for immunostimulating therapy with colony-stimulating agents. In our study, the expression of HLA-DR on monocytes was quite high in all neonates with sepsis both in the onset of the disease and in a week even in the cases with a lethal outcome. However, it should be emphasized that no septic shock occurred in the infants studied, and both babies died in the later stages of the disease. Two out of five NS cases confirmed with the isolation of a causative organism from the blood were caused by K. pneumoniae. Although no detection of blood endotoxin levels was performed in our study, a high monocyte HLA-DR expression level in children with NS might be attributed to short-term and transient endotoxinemia. The monocyte HLA-DR expression appeared unexpectedly as high in the controls as in the group of children with NS (р = 0.6). Such a significant activated monocyte count in healthy neonates can be explained by antigen stimulation of normal bacterial flora that actively colonize the previously sterile gastrointestinal tract.
Despite the central role innate immunity cells play in rapid immune response in early sepsis, adaptive immunity factors such as B and T cells as well as immunoglobulins are crucial in the formation of specific immunity and pathogen elimination from the body [2, 15]. Septic shock was shown in adult patient studies to occur in the setting of decreased В cell counts, CD19+CD23+ in particular [26]. Blood low IgG-antibody levels in preterm neonates born before 32 weeks of the gestation are associated with an increased risk of NS [2, 27]. At the same time, study results of adaptive immunity parameters in sepsis have been conducted in adults and neonates within the last decade demonstrated a significant decrease of not only a В cell population but also that of Т cells, especially CD4+ lymphocytes, in the blood [28,29,30]. Moreover, a marked decrease of CD4+ lymphocyte subset count was shown to occur in different organs (the spleen, the intestine, lymph nodes) in patients who died of sepsis [31,32,33]. CD4 T cells are known to play a key role in providing interactions between different components of immunity. When being a “conductor” in an immunity “orchestra” which is complex in composition, CD4 T cells determine the direction of an immune response [34]. Among various CD4 T cell subpopulations, there are two major ones such as Th1, whose formation is stimulated by interferon-ɣ (IFN-ɣ), and Th2, activated in the presence of interleukin-4 (IL-4) [34]. CD4 T cells are skewed toward Th2 responses in healthy neonates due to low production of IFN-ɣ [17]. In sepsis, the activation of CD4 T cells also has a prominent Th2-tendency with the synthesis of anti-inflammatory cytokines IL-4 and IL-10 [34, 35]. The development of lymphopenia in sepsis may occur during the first 24 h of the onset of the disease due to both peripheral blood lymphocyte recruitment into a site of infection and activation of apoptosis [36, 37]. What is more, not only circulating lymphocytes but also those in lymphoid organs undergo apoptosis [31,32,33]. At the same time, apoptosis is a final stage of activation processes in lymphocytes which is preceded by the expression of activation and proliferation molecules on their surface [16]. Studies of the Т cell activity in sepsis demonstrated the enhanced expression of some activation markers (CD69, CD25) and the decreased expression of others (CD127) [31, 38]. Single studies where HLA-DR was used to evaluate the activity of lymphocytes in sepsis demonstrated a decrease in the CD3+ lymphocyte activity [39]. Our study detected the extremely low activity of CD3+ lymphocytes and CD4+ and CD8+ lymphocyte subsets in the onset of sepsis and its progression, in 7 days. Percentages of HLA-DR-positive CD3+ lymphocytes and HLA-DR-expressing CD4+ and CD8+ lymphocyte subsets in the blood of children with NS did not almost differ from those in the control group (р > 0.05). Furthermore, a HLA-DR-expressing CD4+ and CD8+ lymphocyte subset absolute count in the NS incipience was even lower than that in the control group (р = 0.0002 and р = 0.03, respectively), while the activated CD8+ lymphocyte level remained lower than that of the controls even in 7 days after the disease onset (р = 0.04). A low expression of HLA-DR on surface of CD4+ and CD8+ lymphocytes in children with NS indicates failure of these cells of an immunity adaptive component to respond effectively to infectious hazards. A low activity of lymphocytes, a decrease of their proliferative capacity, and apoptosis are key causes of immunosuppression in late stage of sepsis that contributes to an impairment of elimination mechanisms of the immune system and is associated with a risk of death [5, 40]. Lymphocyte immaturity is a contributory cause of low adaptive immunity capacity in neonates. Despite a higher blood T cell level in neonates as compared to adults, the activity of these cells is low with the predominance of naive T cells against low memory T cell counts [41]. The activity of blood Т cells in healthy neonates in our study was extremely low: the median of HLA-DR-positive CD3+ cells was only 2%, while a percentage of HLA-DR-expressing lymphocytes in healthy adults reaches 10–15% according to the published data [39]. Blood lymphocyte levels in neonates might be influenced by a gestational age: CD4 and CD8 lymphocyte counts were demonstrated to be markedly lower in preterm neonates than in full-term ones [42]. Moreover, the expression of CD127, which is a marker of naive and mature Т cell proliferation and differentiation, was less marked in preterm neonates as compared to full-term ones [42]. In our study, the degree of CD4+ and CD8+ lymphocyte subset activation did not depend upon a gestational age in the onset of sepsis; however, a retest in 7 days detected that HLA-DR-positive CD4 lymphocyte count was 3.1-fold lower in preterm neonates than in full-term ones (р = 0.02). Reduced blood concentrations of activated CD4+ and CD8 lymphocyte subsets might be caused by a decrease of blood circulating CD4+ and CD8 lymphocyte subpopulation counts due to above-mentioned sepsis-induced apoptosis.
4 Conclusion
NS is accompanied by a high monocyte HLA-DR expression level that in general represents effective functioning of innate immunity cells in the children studied. However, the HLA-DR expression on CD3 lymphocytes as well as on CD4 and CD8 lymphocyte subsets was extremely low in the same children that indicates non-effectiveness of adaptive immunity and is a reason for immunostimulating therapy.
References
Liu, L., Oza, S., Hogan, D., et al. (2016). Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet, 388(10063), 3027–3035.
Camacho-Gonzales, A., Spearman, P. W., & Stoll, B. J. (2013). Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatric Clinics of North America, 60, 367–389.
Wynn, J. L. (2016). Defining neonatal sepsis. Current Opinion in Pediatrics, 28, 135–140.
Hotchkiss, R. S., Coopersmith, G. M., McDunn, J. E., & Ferguson, T. A. (2009). Nature Medicine, 15(5), 496–497.
Boomer, J. S., Green, J. M., & Hotchkiss, R. S. (2014). The changing immune system in sepsis: is individualized immune-modulatory therapy the answer? Virulence, 5(1), 45–56.
Reinhart, K., Bauer, M., Riedemann, N. C., et al. (2012). New approaches to sepsis: molecular diagnostics and biomarkers. Clinical Microbiology Reviews, 25(4), 609–634.
Bhandari, V. (2014). Effective biomarkers for diagnosis of neonatal Sepsis. Journal of the Pediatric Infectious Diseases Society, 3(3), 234–245.
Shah, B. A., & Padbury, J. F. (2014). Neonatal sepsis: an old problem with new insights. Virulence, 54, 449–457.
Venet, F., Lepape, A., & Monneret, G. (2011). Clinical review: flow cytometry perspectives in the ICU—from diagnosis of infection to monitoring of injury-induced immune dysfunctions. Critical Care, 15, 231.
Gille, C., & Orlikowsky, T. W. (2007). Flow cytometric methods in the detection of neonatal infection. Transfusion Medicine and Hemotherapy, 34, 157–163.
Monneret, G., & Venet, F. (2014). Monocyte HLA-DR in sepsis: shall we stop following the flow? Critical Care, 18, 102. https://doi.org/10.1186/cc13179.
Zhuang, Y., Peng, H., Chen, Y., et al. (2017). Dynamic monitoring of monocyte HLA-DR expression for the diagnosis, prognosis, and prediction of sepsis. Frontiers in Bioscience Landmark, 22, 1344–1354.
Monneret, G. (2005). How to identify systemic sepsis-induced immunoparalysis. Advances in Sepsis, 4(2), 42–49.
Wu, J.-F., Ma, J., Chen, J., et al. (2011). Changes of monocyte human leukocyte antigen-DR expression as a reliable predictor of mortality in severe sepsis. Critical Care, 15, R220.
Rimmele, T., Payen, D., Cantaluppi, V., et al. (2016). Immune cell phenotype and function in sepsis. Shock, 45(3), 282–291.
Litvinova, L. S., Gutsol, A. A., Sokhonevich, N. A., et al. (2014). Basic surface markers of functional activity T-lymphocytes. Medical Immunology (Russia), 16(1), 7–26.
Rossi, P., Botgros, R., Tibby S. et al. (2010). Report on the Expert Meeting on Neonatal and Paediatric Sepsis. London: European Medicines Agency, http://www.ema.europa.eu/docs/enGB/documentlibrary/Report/2010/12/WC500100199.pdf.
Rundolph, A. G., & Mc Culloh, R. J. (2014). Pediatric sepsis. Virulence, 5(1), 179–189.
Hotchkiss, R. S., & Karl, I. E. (2003). The pathophysiology and treatment of sepsis. The New England Journal of Medicine, 348(2), 138–150.
Perez, A., Bellon, J. M., Gurbindo, M. D., et al. (2010). Impairment of stimulation ability of very-preterm neonatal monocytes in response to lipopolysaccharide. Human Immunology, 71, 151–157.
Frazier, W. J., & Hall, M. W. (2008). Immunoparalysis and adverse outcomes from critical illness. Pediatric Clinics of North America, 55, 647–668.
Monneret, G., Elmenkouri, N., Bohe, J., et al. (2002). Analytical requirements for measuring monocytic HLA-DR by flow cytometry. Clinical Chemistry, 48, 1589–1592.
Wolk, K., Kunz, S., Crompton, N. E., et al. (2003). Multiple mechanisms of reduced major histocompatibility complex class II expression in endotoxin tolerance. The Journal of Biological Chemistry, 278(20), 18030–18036.
Monneret, G., Lepape, A., Voirin, N., et al. (2006). Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Medicine, 32(8), 1175–1183.
Drewry, A. M., Ablordeppey, E. A., Murray, E. T., et al. (2016). Comparison of monocyte human leukocyte antigen-DR expression and stimulated tumor necrosis factor alpha production as outcome predictors in severe sepsis: a prospective observational study. Critical Care, 20, 334.
Monserrat, J., de Pablo, R., Diaz-Martín, D., et al. (2013). Early alterations of B cells in patients with septic shock. Critical Care, 17(3), R105.
Capasso, L., Borrelli, A. C., Cerullo, J., et al. (2015). Role of immunoglobulins in neonatal sepsis. Translational Medicine, 11(5), 28–33.
Hotchkiss, R. S., Osmon, S. B., Chang, K. C., et al. (2005). Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. The Journal of Immunology, 174, 5110–5118.
Khaertynov Kh., S., Boichuk, S. V., Anohin, V. A., et al. (2014). Activity rates of apoptosis of lymphocytes in children with neonatal sepsis. Geny i kletki, 9(3), 267–271.
Khaertynov Kh., S., Anohin, V. A., Mustafin, I. G., et al. (2015). Specific features of immunity in neonatal infants with localized and generalized bacterial infections. Russian Bulletin of Perinatology and Pediatrics, 5, 168–173.
Boomer, J. S., To, K., Chang, K. C., et al. (2011). Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA, 306, 2594–2605.
Toti, P., De Felice, C., Occhini, R., et al. (2004). Spleen depletion in neonatal sepsis and chorioamnionitis. American Journal of Clinical Pathology, 122, 765–771.
Felmet, K. A., Hall, M. W., Clark, R. S., et al. (2005). Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. Journal of Immunology, 174, 3765–3772.
Cabrera-Perez, J., Condotta, S. A., Badovinac, V. P., et al. (2014). Impact of sepsis on CD4 T cell immunity. Journal of Leukocyte Biology, 96(5), 767–777.
Wilson, C. B., Rowell, E., & Sekimata, M. (2009). Epigenetic control of T-helper cell differentiation. Nature Reviews Immunology, 9, 91–105.
Boomer, J. C., Shuherk-Shaffer, J., Hotchkiss, R. S., et al. (2012). A prospective analysis of lymphocytes phenotype and function over the course of acute sepsis. Critical Care, 16, R112.
Wesche, D. E., Lomas-Neira, J. L., Perl, M., et al. (2005). Leukocyte apoptosis and its significance in sepsis and shock. Journal of Leukocyte Biology, 78, 325–337.
Venet, F., Foray, A.-P., Villars-Mechin, A., et al. (2012). IL-7 restores lymphocytes function in septic patients. Journal of Immunology, 189(10), 5073–5081.
Holub, M., Kluckova, Z., Beneda, B., et al. (2000). Changes in lymphocyte subpopulations and CD3+/DR+ expression in sepsis. Clinical Microbiology and Infection, 6, 657–660.
Delano, M. J., & Ward, P. A. (2016). Sepsis-induced immune dysfunction: can immune therapies reduce mortality? The Journal of Clinical Investigation, 126(1), 23–31.
Walker, J. C., Smolders, M. A., Gemen, E. F., et al. (2011). Development of lymphocyte subpopulations in preterm infants. Scandinavian Journal of Immunology, 73, 53–58.
Correa-Rocha, R., Perez, A., Lorente, R., et al. (2012). Preterm neonates show marked leukopenia and lymphopenia that are associated with increased regulatory T-cell values and diminished IL-7. Pediatric Research, 71(5), 590–597.
Funding
This study was supported by the Russian Government Program of Competitive Growth of the Kazan Federal University. Albert A. Rizvanov was supported by the state assignment 20.5175.2017/6.7 of the Ministry of Education and Science of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Ethics Committee of the City Children’s Hospital approved this study, and a written informed consent was obtained from the parents in accordance with the principles authorized under this protocol (the Federal Law N323-FL dated on 21.11. 2011 “On Protection of Health of the Citizens in the Russian Federation”).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Khaertynov, K.S., Anokhin, V.A., Mustafin, I.G. et al. Changes in HLA-DR Expression on Monocytes and Lymphocytes in Neonatal Sepsis. BioNanoSci. 8, 647–653 (2018). https://doi.org/10.1007/s12668-018-0519-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-018-0519-2