Abstract
As a new proposal, natural tears collected from the Schirmer strips were studied using surface pressure-area, π-A, isotherms obtained with the Langmuir technique. Parameters such as limit surface pressure, πlimit, elastic modulus at the maximum, CS−1max, and surface pressure at the maximum, πmax, were correlated with clinical tests, such as the Schirmer (SCH) and the tear film breakup-time (TFBUT) tests. The analysis from the chi-square test indicates dependence between the SCH test and the type of isotherms with a value of P < 0.05. A moderate Pearson correlation coefficient with statistical significance (P < 0.05) between the values of the SCH test and the πlimit was obtained. On the other hand, a low Pearson correlation coefficient with no statistical significance (P > 0.05) between the values of the TFBUT test and the πlimit was obtained. Additionally, considering only the isotherms classified as good, moderate Pearson correlation coefficients with statistical significance (P < 0.05) between the values of the TFBUT test and πlimit or πmax can be obtained. This study shows that the isotherms obtained with the Langmuir technique correlate well with the tear secretion, and when the later is moderate or good, the isotherm parameters afford information about the tear film stability and the behaviour of the tear lipids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The tear film presents a lipid layer at the outermost part that contains a fraction of polar lipids as well as non-polar lipids. The Meibomian glands, located in the eyelid margins, secrete mainly the lipid layer. The Langmuir technique is used to study polar lipids at the air/water interface. The Langmuir technique has been applied to the study of Meibomian lipids [1,2,3,4,5], their interaction with proteins [6,7,8,9], to the study of the instability and breakup of model tear films [10] and to the study of lipid-containing artificial tears [11]. The study of natural tear films is of great importance in the fields of ophthalmology and optometry [12, 13]. Dysfunctions in the secretion, stability and composition of the tear film are one of the causes of the syndrome known as dry eye [14].
The study of the Langmuir behaviour of natural tear films has been done, in previous works, directly using Meibomian lipids, collected from the eyelids or from contact lenses and dissolved in chloroform [3,4,5]. Several clinical methods are of common use in the ocular surface field. The Schirmer (SCH) test evaluates aqueous tear secretion and then is related with the tear volume, and the tear film breakup time (TFBUT) test assesses the tear film stability [14,15,16,17].
In this work, we propose a new method to study the characteristics of the tear film collecting it with a SCH strip and transferring the strip content to a Langmuir trough. As this work is meanly used to establish the procedure technique, for that a limited sample is used. After transfer, the surface pressure-area isotherms, π-A, are registered and some characteristics of them will be correlated with clinical tests results. Among these characteristics, the inverse of the compressibility modulus, or elastic modulus (Eq. 1), will be used.
In our study, we use the whole tear film extract collected with a SCH strip, a specific paper strip, and for that, the tear film components in the strip are lipids, proteins, electrolytes and others. Between them, the most surface-active components are lipids. In addition, our study permits a more direct correlation between the clinical tests of the tear film of individuals and the corresponding Langmuir behaviour.
2 Materials and Methods
2.1 Materials
SCH strips were of standard Whatman 41 paper (size 5 mm × 35 mm). These strips are folded at 5 mm from the end in order to place it on the lid margin, and on it, there is a millimetric scale. Powder-free nitrile gloves were used for strip manipulation. Water was ultrapure Milli-Q (18.2 MΩ cm). Sodium fluorescein strips wetted in saline solution were used for the TFBUT test.
2.2 Techniques and Equipment
2.2.1 Clinical Tests
For the Schirmer test, SCH strips were placed on the temporal side of the lower lid margin, leaving 5 mm of the SCH strip inside the conjunctival sac, during 5 min, without anaesthesia and with the eyes closed. After removal of the strip, the impregnated length is measured in millimetres, and the strip is kept in a sterile tube for further experiments and placed in a fridge at 4 °C.
For tear film breakup time (TFBUT) measurements, sodium fluorescein strips wetted in saline solution were placed in contact with the tear, in order to transfer sodium fluorescein to the later, and a bio-microscope (TOPCON SL-D701) incorporating a blue cobalt filter, for superior fluorescent viewing, was used for observation. After 1 min, the patient had to stop blinking. The time passing between the last blink and the moment of breaking of the tear was taken as the TFBUT value. Three measurements were made in each eye. The ethical committee of the Institute of Ocular Microsurgery (Barcelona), where clinical tests were done, approved those.
2.2.2 Langmuir Experiments
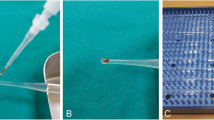
The isotherm experiments have been done in a NIMA Teflon trough, model 1232D1D2 (from NIMA Technology, Coventry, UK), with two movable barriers and using a Wilhelmy plate to measure the surface pressure. The velocity of the barriers was 30 cm2/min. The Teflon trough and barriers were cleaned with chloroform and ultrapure water. Experiments were conducted at room temperature of 23 °C. Water was used as subphase because in previous studies with polar lipids, see for instance ref. [18], no significant influence of a 0.9% NaCl phosphate buffered subphase was observed. For the spreading of the tear components onto the water, the SCH strip was placed in contact with water during 30 s.
Previously to the full study, a test for the Langmuir technique was done with the SCH strip applied to the healthy eyes of a young person (that means values of the SCH test SCH ≥ 10 mm, and values of the TFBUT test TFBUT ≥ 10 s, according to the grading scheme explained in section 3.1), and a Brewster angle microscope, model μBAM (from NIMA-Nanofilm, Coventry, UK), was also used. The SCH strip was placed in contact with water during 30 s in a small round trough (area of 64 cm2, volume of 64 mL, and without barriers) and the surface was observed by BAM. The first time this person was not advised not to use eye cosmetics. The BAM images obtained (see Fig. 1a, b) revealed the presence of thick lipid films which were attributed to the transfer of lipid cosmetics to the strip. The second time we repeated the same but with the absence of eye cosmetics, and then different BAM images were obtained (see Fig. 1c, d). These images show the lipid transfer but without the characteristics appearing in Fig. 1a, b. After that, the criteria of not using eye cosmetics on the day of tests were implemented. In another experiment, another SCH strip from the same person was used for the Langmuir experiments, and the isotherm obtained is shown in Fig. 2.
2.3 Sample and Statistical Analysis
Tested eyes were selected from an adult population, and the inclusion criteria for the study was that these people do not present apparent ocular pathology, do not wear contact lenses and have not had any previous surgery. People were asked not to use eye cosmetics on the day the tests were performed. The data were analysed with the Minitab 17 statistical program. The Pearson correlation coefficient was applied to test the correlation between two variables. The Pearson chi-square statistical test was applied to contingency tables, imposing the significance criteria that values of probability (P values) lower than 0.05 indicate that the hypothesis of independence between variables can be discarded.
3 Results and Discussion
The study was conducted in an adult population (ages in the range 45–92), and 44 eyes were studied.
3.1 Clinical Tests
Table S1 (see Supplementary data) presents the results of the SCH and TFBUT tests. The calculated mean values and standard deviations are, respectively, 9.14 and 7.48 mm for the SCH, and 4.31 and 1.79 s for the TFBUT.
The SCH and the TFBUT tests are traditional tests for the diagnosis of dry eye [17]. Cut-off values of the clinical tests have been proposed enabling a distinction to be made between healthy and affected eyes, and to classify the severity of the dry eye syndrome [16]. In the case of SCH test, the cut-off values are healthy eye (SCH ≥ 10 mm), moderate dry eye (5 mm ≤ SCH < 10 mm) and severe dry eye (SCH < 5 mm). For the TFBUT test, the cut-off values are healthy eye (TFBUT ≥ 10 s), moderate dry eye (5 s ≤ TFBUT < 10 s) and severe dry eye (TFBUT < 5 s). According to this grading scheme, the eyes (Table S1) are distributed; thus, 34.1% healthy eyes, 39.0% moderate dry eyes and 26.9% severe dry eyes for the SCH test, and 40.6% moderate dry eyes and 59.4% severe dry eyes for the TFBUT test. In this work, we are going to consider each clinical test separately in order to correlate each one with the Langmuir results.
3.2 Langmuir Experiments
Figure 3 shows typical π-A isotherms obtained with the method described in section 2.2. In most of the cases, a good isotherm (G) is obtained, but in some cases, the increase in the surface pressure is low or very low, with a maximum value of surface pressure (πlimit) less than 4 mN/m, and is called a weak isotherm (W). A good isotherm allows calculation of the elastic modulus, as is presented in the inset in Fig. 3. The global of all π-A isotherms is presented in Supplementary data (Fig. S1 and S2). Usually, the elastic modulus plot reaches a maximum, which is characterised by a pair of values corresponding to the surface pressure, πmax, and the elastic modulus, Cs−1max.
A good (G) and a weak (W) π-A isotherm corresponding to eyes number 4 and 33, respectively. Inset: The corresponding elastic modulus from the G isotherm, obtained applying Eq. (1)
The shape of the π-A isotherms is that of typical lipids, even though tears collected with the SCH strip contain proteins and other tear components, as well as lipids. The surface pressure after the SCH strip contacts water remains at zero with open barriers. This fact can be explained, even though proteins are surface active, by the low amount of the tear film collected, which is around a few microliters, in the same order of the tear film volume. After contacting water with the strip, these soluble proteins diffuse to the whole water subphase, and the protein concentration attained is very low and does not interfere appreciably with the lipids extended on the surface. We have estimated that this protein concentration is around 0.0001–0.0002 g/L. Thus, we have also done tests with a lysozyme solution at a greater concentration of 0.001 g/L and the lysozyme isotherm always present a zero value in the surface pressure during compression (Fig. 4). Only at higher lysozyme concentrations, i.e. 0.1 g/L, an isotherm is obtained (Fig. 4). This behaviour is maintained after 140 min indicating no significant changes in lysozyme adsorption at the air-water interface at this level of protein concentration. We have also tested the isotherm of the phospholipid DPPC (dipalmitoylphosphatidylcholine) on this 0.001 g/L lysozyme subphase and we have not observed any change with respect to the DPPC isotherm on water subphase.
In Table 1, the main characteristics obtained from the π-A isotherms and elastic modulus plots are presented. Only those isotherms classified as G allows us to calculate the values of π and Cs−1 at the maximum, πmax and Cs−1max, respectively. The calculated mean value and standard deviation for πlimit are, respectively, 7.28 and 8.59 mN/m. On the other hand, and only for the isotherms classified as G, we obtain as mean value and standard deviation, respectively, 30.51 and 3.40 mN/m for the Cs−1max, and 9.16 and 1.36 mN/m for the πmax.
Analysing the isotherms and the elastic modulus plots of those classified as G (Fig. S1 and S2 in Supplementary material), it is observed that even though notable differences have been obtained between the values of πlimit in the isotherms, all of them reach a maximum in the elastic modulus plots that is located around 8–9.5 mN/m of surface pressure (X axis) and around 29–31 mN/m of elastic modulus (Y axis). Analysing the values of the elastic modulus, and its maximum values, the physical states of the film can be obtained [19, 20]. As for Cs−1 between 12.5 and 100 mN/m, it corresponds to the liquid expanded state (LE); the studied films show an LE state, which indicates the presence of unsaturated lipids and non-polar lipids.
The results reported in our study are not directly comparable with those of the literature since the lipids are collected in a different way. Hagedorn et al. [3], Petrov et al. [4] and Mudgil and Millar [5] collected Meibomian lipids from eyelids or from contact lenses, while our lipids came from the tear film. Nevertheless, the isotherms obtained in our study are comparable with those reported in the other cited studies, and in all cases, the isotherms do not collapse. These can be explained because the amount of lipid spread is not enough and also because the lipid composition contains non-polar lipids which tend to form multilayers instead of a compact monolayer. An estimation of the elastic modulus (Cs−1) at π = 10 mN/m for the Meibomian lipids reported in [6] gives a value around 24 mN/m, while for the phospholipids used in that study, higher values were obtained. Comparing these values with our values in Fig. S1 and S2, it is seen that at π = 10 mN/m, our values range between those of Meibomian lipids and phospholipids. We think that this indicates we have a mixture of Meibomian lipids and phospholipids, which are always present in tears [8].
3.3 Discussion
We analyse if there is dependence between the number of good (G) and weak (W) isotherms and both the SCH and the TFBUT tests results. The results of Tables S1 and 1 are distributed in the contingency Table 2. The rows in Table 2 show the eyes classified in healthy eyes, moderate dry eyes and severe dry eyes, according to the cut-off values explained in section 3.1, and the columns show the eyes classified according to the type of isotherm. The Pearson chi-square statistical test is applied to the sets in Table 2, to assess the dependence or independence between them. The chi-square test gives a value of P < 0.001 between the SCH test and the type of isotherms, indicating dependence between them with statistical significance, while between the TFBUT test and the type of isotherms, the value of P = 0.447 indicates that the independence between both variables cannot be discarded with the significance criteria imposed.
Working with the same criteria of significance, we obtain, on one hand, a moderate Pearson correlation coefficient with statistical significance (P < 0.05) between the values of the SCH test and the πlimit (see Table 3). On the other hand, we obtain a low Pearson correlation coefficient with no statistical significance (P > 0.05) between the values of the TFBUT test and the πlimit (see Table 3).
These results indicate that the Langmuir technique works well when the volume of tear secretion is sufficient (acceptable SCH test values), which allows us to obtain acceptable isotherms and values derived from them. But the Langmuir technique detects poor tear secretion, which correlates with the recording of weak isotherms, and that is related to dry eye syndrome. In order to analyse in depth the correlation between the Langmuir results and the clinical tests, we considered only the eyes with good (G) isotherm results (a sample of 19 eyes). The Pearson correlation coefficients and the corresponding P values are also presented in Table 3. Thus, considering only the isotherms classified as G, we obtain, on one hand, moderate or low Pearson correlation coefficients with statistical significance (P < 0.05) between the values of the SCH test and the values of πlimit, but with no statistical significance (P > 0.05) between the values of the SCH test and the values of CS−1max or πmax (see Table 3). On the other hand, we obtain moderate or low Pearson correlation coefficients with statistical significance (P < 0.05) between the values of the TFBUT test and the values of πlimit or πmax, but with no statistical significance (P > 0.05) between the values of the TFBUT test and the values of CS−1max (see Table 3). It seems to indicate that the TFBUT test can only be correlated with the isotherms when the latter are good enough. One of the limits of the present study is that the populations studied present low values of the TFBUT test corresponding to moderate or severe dry eye syndrome, as was shown in section 3.1, and consequently, there is a limited variability in the studied eyes.
Finally, we want to point out that the Langmuir technique, in connection with clinical trials, might be utilised in the future to determine the effects of eye infections, of drug delivery, of eye laser surgery or of the use of eye drops [11, 21,22,23,24], through the influence of them on the characteristics of the tear film.
4 Conclusions
The present work provides information on the Langmuir technique when applied to study the tear film collected through a SCH strip, and when correlating with two common clinical tests, the Schirmer and the TFBUT tests. The Langmuir technique is indicated to study the cases in which the tear secretion is enough to impregnate the SCH strip. In these cases, the correlation between the values of TFBUT test and the values of isotherm parameters could give information about the stability of the film. This kind of information could determine if the lipid film presents different characteristics (different values of CS−1max, πmax or πlimit). In those cases where the tear secretion is poor due to eye dry syndrome, the Langmuir technique can also indicate this in the way of weak isotherms. In future studies, the Langmuir study will be done at the temperature of 32 °C, which is the temperature of the anterior segment of the eye, in order to provide more physiological conclusions.
References
Kaercher, T., Hönig, D., & Möbius, D. (1993). Brewster angle microscopy. A new method of visualizing the spreading of Meibomian lipids. Internat Ophthalmol, 17, 341–348.
Kaercher, T., Hönig, D., & Möbius, D. (1995). Meibomian lipid layers seen through BAM. Orbit, 14, 17–24.
Hagedorn, S., Drolle, E., Lorentz, H., Srinivasan, S., Leonenko, Z., & Jones, L. (2015). Atomic force microscopy and Langmuir-Blodgett monolayer technique to assess contact lens deposits and human meibum extracts. J Optom, 8, 187–199.
Petrov, P. G., Thompson, J. M., Abdul Rahman, I. B., Ellis, R. E., Green, E. M., Miano, F., & Winlove, C. P. (2007). Two-dimensional order in mammalian pre-ocular tear film. Experimental Eye Research, 84, 1140–1146.
Mudgil, P., & Millar, T. J. (2011). Surfactant properties of human Meibomian lipids. Investigative Ophthalmology & Visual Science, 52, 1661–1670.
Mudgil, P., Torres, M., & Millar, T. J. (2006). Adsorption of lysozyme to phospholipid and Meibomian lipid monolayer films. Colloids and Surfaces, B: Biointerfaces, 48, 128–137.
Miano, F., Calcara, M., Millar, T. J., & Enea, V. (2005). Insertion of tear proteins in a Meibomian lipids film. Colloids and Surfaces, B: Biointerfaces, 44, 49–55.
Svitova, T. F., & Lin, M. C. (2016). Dynamic interfacial properties of human tear-lipid films and their interactions with model-tear proteins in vitro. Advances in Colloid and Interface Science, 233, 4–24.
Millar, T. J., Mudgil, P., Butovich, I. A., & Palaniappan, C. K. (2009). Adsorption of human tear lipocalin to human Meibomian lipid films. Investigative Ophthalmology & Visual Science, 50, 140–151.
Saad-Bhamla, M., Chai, C., Rabiah, N. I., Frostad, J. M., & Fuller, G. G. (2016). Instability and breakup of model tear films. Investigative Ophthalmology & Visual Science, 57, 949–958.
Torrent-Burgués, J. (2016). Langmuir films study on lipid-containing artificial tears. Colloids and Surfaces, B: Biointerfaces, 140, 185–188.
Korb, D. R. (2002). The tear film: structure, function and clinical examination. Oxford: Butterworth Heinemann.
Bron, A. J., Tiffany, J. M., Gouveia, S. M., Yokoi, N., & Voon, L. W. (2004). Functional aspects of the tear film lipid layer. Experimental Eye Research, 78, 347–360.
DEWS. (2007). Report of the dry eye workshop. The Ocular Surface, 5, 75–92 and 93-107.
Cho, P., & Yap, M. (1993). Schirmer test I. A review. Optometry and Vision Science, 70, 152–156.
Behrens, A., Doyle, J. J., & Chuck, R. S. (2006). Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea, 25, 900–907.
Savini, G., Prabhawasat, P., Kojima, T., Grueterich, M., Espana, E., & Goto, E. (2008). The challenge of dry eye diagnosis. Clinical Ophthalmology, 2, 31–55.
Torrent-Burgués, J. (2011). Oleamide and oleamide-lipid mixed monolayers. BioNanoSci, 1, 202–209.
Davies, J. T., & Rideal, E. K. (1993). Interfacial phenomena. N.Y: Academic Press.
Vitovic, P., Nikolelis, D. P., & Hianik, T. (2006). Study of calix[4]resorcinarene-dopamine complexation in mixed phospholipid monolayers at the air/water interface. Biochimica et Biophysica Acta, 1758, 1852–1861.
Gai, M., Frueh, J., Tao, T., Petrov, A. V., Petrov, V. V., Shesterikov, E. V., Tverdokhlebov, S. I., & Sukhorukov, G. B. (2017). Polylactic acid nano- and microchamber arrays for encapsulation of small hydrophilic molecules featuring drug release via high intensity focused ultrasound. Nanoscale, 9, 7063–7070.
Stuart, M. C., Huck, W. T. S., Genzer, J., Müller, M., Ober, C., Stamm, M., Sukhorukov, G. B., Szleifer, I., Tsukruk, V. V., Urban, M., Winnik, F., Zauscher, S., Luzinov, I., & Minko, S. (2010). Emerging applications of stimuli-responsive polymer materials. Nature Materials, 9, 101–113.
Matteini, P., Ratto, F., Rossi, F., & Pini, R. (2012). Emerging concepts of laser-activated nanoparticles for tissue bonding. Journal of Biomedical Optics, 17, 10701.
Mencucci, R., Boccalini, C., Caputo, R., & Favuzz, E. (2015). Effect of hyaluronic acid and carboxymethylcellulose ophthalmic solution on ocular comfort and tear-film instability after cataract surgery. Journal of Cataract and Refractive Surgery, 41, 1699–1704.
Acknowledgements
The authors thank MSc C. Fàbregas and MSc M. Pérez for providing the clinical results.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guaus, E., Torrent-Burgués, J. The Langmuir Technique Applied to the Study of Natural Tears. BioNanoSci. 8, 559–565 (2018). https://doi.org/10.1007/s12668-018-0509-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-018-0509-4