Abstract
The influences of microgravity lead to development of the muscular atrophy and reduction of the volume of muscle fibers, both slow and fast types, changes of the muscles structure, decrease in a muscular tone, and also endurance and general muscles functionality. This study was designed to investigate the changes of the titin’s structure in m. soleus and m. extensor digitorum longus in rat after 7 days of gravitational unloading. Electrophysiological and immunological analysis of both muscles was performed after the rats were maintained in antiortostatic position for 7 days. Results suggest that 7 days of gravitational unloading decrease the titin’s intact N2A-isoform and increase titin’s T2 fragment in m. soleus. At the same time no influence of gravitational unloading was found on titin’s N2A-izoform in m. extensor digitorum longus. Titin reduction in m. soleus and not in m. extensor digitorum longus can suggest different mechanisms of adaptation to the gravitational changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Functional unloading or hypogravity can be consequence of multiple conditions like a prolong bed rest and spinal cord injury and may lead to the serious physiological changes in skeletal muscles [1]. These changes commonly appear as decrease of the muscle size and volume [2, 3]. Titin plays an important role in maintaining the muscle sacromere structure and its decrease affects the muscles contractile properties [4]. Understanding the role of titin in molecular mechanisms of the morfo-functional changes in muscles can lead to development of new rehabilitation therapies.
2 Material and Methods
All experiments were conducted on adult laboratory rats (180–200 g) according to the rules of the laboratory animals’ care and bioethical standards. As a model of gravitational unloading, we used a model of anti-orthostatic support described by Morey-Holton E.R et al. (2002) [5].
After 7 days of gravitational unloading, m. soleus (SM) (n = 12) and m. extensor digitorum longus (EDL) (n = 8) were prepared, and titin isomers were evaluated using DSN-gel electrophoresis according to Tatsumi R. and Hattori A. (1995) [6]. The quantity of a titin was estimated in relation to the heavy chains of a myosin (MHC) [7]. Immunoblotting of titin was conducted by method [8]. Statistical analysis was performed with t student criterion.
3 Results and Discussion
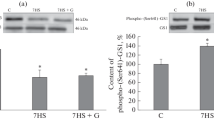
Decrease of N2A-izoform of a titin in comparison to MHC in 25 % (p < 0.05) was observed in SM after 7 days of gravitational unloading. Three times increase of T2 fragment (p < 0.05) was followed by reducing the relative amount of N2A-izoform of a titin that demonstrates change of a proteolysis of intact N2A-izoform of a titin in the conditions of gravitational unloading. Our results show that one to two molecules of intact N2A-izoform were disrupted in SM after unloading for 7 days (Fig. 1a).
Changes of isoform structure of a titin in m. soleus (a) and m. extensor digitorum longus (b) after 7 days of gravitational unloading. On an axis of ordinates the following are designated: the relations of number of N2A-izoform of a titin and T2 fragment in relation to heavy chains of a myosin by results of a densitometry of proteinaceous strips on the elekroforegram expressed in the relative units. The x-axis: the white columns—“control” and gray—“anti-orthostatic hanging.” The level of significance p < 0.05
Decreasing of a titin of abundance of MHC in N2A-izoform was not observed in EDL after 7-day unloading (Fig. 1b).
We also found that content of a titin in SM was reduced during the first 7 days after unloading and did not change in EDL. Our findings were supported by the data of the other studies. It was shown that development of atrophic changes in skeletal muscles in animals and human was accompanied by decrease in titin and a nebulin, during hypogravity conditions [4, 9, 10], and during actual microgravity [11, 12]. It was confirmed that 7-day gravitational unloading led to an essential atrophy of postural, anti-gravitational SM, but not the fast EDL. Similar results were described in experiments conducted during the space flight when the atrophy was not observed in sural and forward tibial muscles at rats after 14 daily flights [13]. In model experiments, a 14-day hanging caused atrophy of soleus, but not sural and forward tibial muscles [14, 15]. It is shown that decrease in a sectional area of fiber in soleus muscle after a 14-day hanging was more in slow, than in fast fibers [16].
4 Conclusions
The reduction of titin in m. soleus after gravitational unloading, along with other changes observed in muscle, may contribute to the development of “hypogravitational muscle syndrome.” These data show that muscle atrophy can be related to the muscles activity, so the less used muscles, such as EDL, are less sensitive to gravitational changes while anti-gravitational muscles, such as SM, quickly develop atrophic changes.
References
Fitts, R. H., Riley, D. R., Widrick, J. J. (2001). Functional and structural adaptations of skeletal muscle to microgravity. The Journal of Experimental Biology, 204(18), 3201–3208.
Tesch, P. A., Berg, H. E., Bring, D., et al. (2005). Effects of 17-day spaceflight on knee extensor muscle function and size. European Journal of Applied Physiology, 93(4), 463–468.
Akima, H., Kawakami, Y., Kubo, K., et al. (2000). Effect of short-duration spaceflight on thigh and leg muscle volume. Medicine and Science in Sports and Exercise, 32(10), 1743–1747.
Shenkman, B. S., Podlubnaya, Z. A., Vikhlyantsev, I. M., et al. (2004). Contractile characteristics and sarcomeric cytoskeletal proteins of human soleus fibers in muscle unloading: role of mechanical stimulation from the support surface [Article in Russian]. Biophysics, 49(5), 807–815.
Morey-Holton, E. R., & Globus, R. K. (2002). Hindlimb unloading rodent model: technical aspects. Journal of Applied Physiology, 92(4), 1367–1377.
Tatsumi, R., & Hattori, A. (1995). Detection of giant myofibrillar proteins connectin and nebulin by electrophoresis in 2 % polyacrylamide slab gels strengthened with agarose. Analytical Biochemistry, 224(1), 28–31.
Vikhliantsev, I. M., & Podlubnaia, Z. A. (2008). Composition of the titin isoforms in muscles in pathology [Article in Russian]. Biofizika, 53(6), 1058–1065.
Towbin, H., Staehlin, T., Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some application. Proceedings of the National Academy of Sciences of the United States of America, 76(9), 4350–4354.
Toursel, T., Stevens, L., Granzier, H., Mounier, Y. (2002). Passive tension of rat skeletal soleus muscle fibers: effects of unloading conditions. Journal of Applied Physiology, 92(4), 1465–1472.
Udaka, J., Ohmori, S., Terui, T., et al. (2008). Disuse-induced preferential loss of the giant protein titin depresses muscle performance via abnormal sarcomeric organization. The Journal of General Physiology, 131(1), 33–41.
Vikhliantsev, I. M., Terent’eva, A. V., Baltina, T. V., Podlubnaia, Z. A. (2010). Effect of vibrostimulation on support zones of rat’s feet, and support loading on titin N2A-isoform and T2-fragment in m. soleus under the conditions of simulated microgravity [Article in Russian]. Aviakosmicheskaia i Ekologicheskaia Meditsina, 44(2), 45–49.
Okuneva, A. D., Vikhlyantsev, I. M., Shpagina, M. D., et al. (2012). Changes in titin and myosin heavy chain isoform composition in skeletal muscles of Mongolian Gerbil (Meriones unguiculatus) after 12-day spaceflight [Article in Russian]. Biophysics, 57(5), 581–586.
Hansen, G., Martinuk, K. J. B., Bell, G. J., et al. (2004). Effects of spaceflight on myosin heavy-chain content, fibre morphology and succinate dehydrogenase activity in rat diaphragm. Pflügers Archiv / European Journal of Physiology, 448(2), 239–247.
Schuenke, M. D., Reed, D. W., Kraemer, W. J., et al. (2009). Effects of 14 days of microgravity on fast hindlimb and diaphragm muscles of the rat. European Journal of Applied Physiology, 106(6), 885–892.
Kuznetsov, M. V., Baltin, M. É., Fedianin, A. O., Eremeev, A. A., Baltina, T. V. (2014). Effect of vibrostimulation of foot and supporting afferentation on functional state of shin muscles in rats during hindlimb unloading [Article in Russian]. Biofizika, 59(5), 990–994.
Ohira, Y., Jiang, B., Roy, R. R., Oganov, V., Ilyina-Kakueva, E., et al. (1992). Rat soleus muscle fiber responses to 14 days of spaceflight and hindlimb suspension. Journal of Applied Physiology, 73(2 Suppl), 51S–57S.
Acknowledgments
This work was supported by RSF, research project no. 15-15-20036, partially supported by RFBR, research project no. 15-04-05951a.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were conducted on adult laboratory rats (180–200 g) according to the rules of the laboratory animals’ care and bioethical standards.
Rights and permissions
About this article
Cite this article
Fedyanin, A., Eremeev, A., Baltina, T. et al. Influence of Gravitational Unloading on Titin’s Structure in the Rat Hind Limb Muscles. BioNanoSci. 7, 349–351 (2017). https://doi.org/10.1007/s12668-016-0360-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-016-0360-4