Abstract
Fast neuronal network oscillations in an in vitro model are a challenging task. Here, we report that sustained gamma (31–35 Hz) oscillations can be induced by kainate (50 nM) in submerged rat hippocampal slices using modified Hájos’s type recording chamber with a superfusion inlet positioned close to the CA3 pyramidal cell layer. The general features of these kainate-induced gamma oscillations were similar to those previously reported in the hippocampal slices using the interface-type chamber and superfused hippocampus in vivo. We suggest that close positioning of the superfusion inlet improves oxygen supply and temperature control of the oscillation-generating network and that this modification could be useful in studies of the gamma rhythmogenesis in the submerged slices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Brain slices in vitro importantly contribute to understanding of the cellular basis of brain function [1]. However, there are notable differences between brain slices and the intact brain in relation to the neuronal network activity as reflected in the electroencephalogram [2]. To study cellular mechanisms underlying neuronal network activity under more realistic conditions, an interface-type slice chamber was introduced, in which the brain slice is held at the interface between artificial cerebrospinal fluid and humidified gas [3]. Hippocampal slices placed in the interface-type chamber display physiologically realistic gamma oscillations in the presence of various agents including cholinergic agonists and kainate [3, 4]. The features of these oscillations are close to those induced by kainate in the superfused hippocampus in vivo [5]. Yet submerged slices offer experimental advantages, including fast exchange of pharmacological agents, visually guided patch-clamp recording, and imaging techniques. Previously, synchronous network activity in submerged slices has been recorded only transiently [6]. Recently, however, sustained network oscillations at beta frequencies were successfully recorded in submerged hippocampal slices at increased flow rates of solution with a superfusion from both slice surfaces in Hájos’s chamber to improve slice oxygen supply [7]. Here, we propose a modification in Hájos’s chamber, in which the superfusion inlet is positioned very close to the CA3 pyramidal cell layer. With this modification, we could evoke sustained kainate-induced oscillations in the gamma frequency range that better approaches the interface-type slice chamber and superfused hippocampus in vivo.
2 Material and Methods
This work has been carried out in accordance with EU Directive 2010/63/EU for animal experiments and all animal-use protocols were approved by Kazan Federal University on the use of laboratory animals (ethical approval by the Institutional Animal Care and Use Committee of Kazan State Medical University N9-2013). Acute horizontal brain slices were prepared from postnatal day (P)15–31 Wistar rats. The animals were anesthetized with isoflurane and decapitated, and the brain was rapidly removed to oxygenated (95 % O2, 5 % CO2), ice-cold (2–4 °C) artificial cerebrospinal fluid (ACSF) of the following composition (mM): NaCl 126, KCl 3.5, CaCl2 2, MgCl2 1.3, NaHCO3 25, NaH2PO4 1.2, and glucose 11 (pH 7.4). Five hundred-micrometer-thick horizontal slices were cut using a vibratome (Microm HM 650 V, Thermo Scientific, Germany). Slices were kept in oxygenated ACSF at room temperature (20–22 °C) for at least 1 h before use. Experiments were performed in the same solution at 32–33 °C. Extracellular recordings of the local field potentials (LFP) and multiple unit activity (MUA) were performed from the CA3 pyramidal layer of the hippocampal slices using Menendez de la Prida’s 16-shank silicone probe with 100 μm separation distance between the shanks (Neuronexus Technologies, USA). The signals were amplified and filtered (×1000; 0.1 Hz–10 kHz) using a custom-made 16-channel amplifier, digitized at 33 kHz using analog-to-digital converter (Digidata 1440A, Molecular Devices, USA), and saved on a PC for post hoc analysis using custom-written functions in MATLAB (MathWorks, USA). Spectral analysis was carried out using the Matlab Chronux toolbox. Spectral power was estimated using direct multi-taper estimators (5 Hz bandwidth, 3 tapers, 200 ms spectral window).
3 Results and Discussion
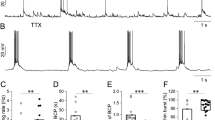
Oscillatory neuronal network activity in submerged hippocampal slices were evoked by the agonist of glutamate receptor kainate [4]. When the standard submerged slice chamber (RC-27LD, Warner Instruments, USA; Fig. 1a, b) was used, only transient (1–2 min of duration) oscillations were evoked in CA3 pyramidal layer of the rat hippocampal slices in spite of kainate (300 nM) presence in superfusion solution (Fig. 2a, b). This chamber characterized by the space volume of 2 ml and the large distance (2–3 cm depending on the slice position in the chamber) between superfusion inlet and the slice recording site (Fig. 1a, b). Power spectrum density analysis revealed that in the standard chamber, kainate-induced oscillations are characterized by the peak frequency in the range of 23–27 Hz with a mean value of 25.9 ± 0.9 Hz (n = 4 slices from P15–21; Fig. 2b, c). The power of oscillations in this conditions was 9.6 ± 3.7 μV2 (n = 4 slices from P15–21; Fig. 2b, c).
Standard and modified submerged slice chambers construction. a Photograph of the standard submerged slice chamber (RC-27LD, Warner Instruments, USA) installed in the electrophysiological setup. Numbers indicate the following: 1 superfusion inlet, 2 superfusion outlet, 3 sensor of temperature controller (TC-344B, Warner Instruments, USA), 4 reference electrode, 5 thermistors of the temperature controller. b Scaled drawings of the top view and the cross-section of the standard submerged slice chamber. Arrows on the top view indicate the superfusion inlet and outlet positions. c Photograph of the modified custom-made submerged slice chamber installed in the electrophysiological setup. d Scaled drawings of the top view and the cross-section of the modified submerged slice chamber
Kainate-induced oscillations in the rat hippocampal slices in standard and modified submerged slice chambers. a Example of field recording traces from CA3 pyramidal layer in P20 rat hippocampal slice before, at 2 and 5 min of kainate (300 nM) in the standard submerged chamber. b Power spectrum plot of oscillations induced in CA3 pyramidal layer in P20 rat hippocampal slice by kainate (300 nM) in the standard submerged chamber. Note the transient oscillations at a beta range 20–30 Hz. c Power spectrum density plot of oscillations induced in CA3 pyramidal layer in P20 rat hippocampal slice by kainate (300 nM) in the standard submerged chamber. Note the peak power density at 27 Hz. d Example of field recording traces from CA3 pyramidal layer in P31 rat hippocampal slice at 3, 5, and 10 min of kainate (50 nM) in the modified submerged chamber. e Power spectrum plot of oscillations induced in CA3 pyramidal layer in P31 rat hippocampal slice by kainate (50 nM) in the modified submerged chamber. Note the permanent oscillations at a gamma range 30–40 Hz. f Power spectrum density plot of oscillations induced in CA3 pyramidal layer in P31 rat hippocampal slice by kainate (50 nM) in the modified submerged chamber. Note the peak power density at 34 Hz
Previously, it has been demonstrated that cholinergically induced oscillations can be reliably evoked in a modified custom-made submerged slice chamber [7]. The authors indicate that the local oxygen level is critical for generation of fast oscillations and propose three ways of oxygen supply improving under submerged conditions: (i) optimizing a chamber design for laminar flow of superfusion solution, (ii) increasing the flow rate of superfusion fluid, and (iii) superfusing both surfaces of the slice. They found that sustained cholinergically induced oscillations can be detected at a superfusion flow rate of about 6 ml/min while at a rate less than 3 ml/min, the power of oscillations decreases dramatically. Moreover, the authors proposed a custom-made chamber with diminished volume of space and laminar flow of superfusion solution from two inlet tubes superfusing the upper and bottom surfaces of the slice placed onto nylon mesh at a high of 2 mm from chamber bottom.
Here, we have constructed the modified custom-made submerged slice chamber (Fig. 1c, d) with similar design to Hájos’s chamber [7]. This chamber characterized by the space volume of 550 μl instead of 2 ml for a standard chamber. Moreover, the nylon mesh was at a high of 2 mm from the chamber bottom instead of 500 μm for a standard chamber. The superfusion solution flow rate in our experiments was 10 ml/min. However, we have found that to evoke the reliable fast oscillations, the distance from the superfusion inlet to the slice recording site was critical. Thus, we have choose to use only one inlet tube but with movable magnetic fixation (Fig. 1c, d). When the superfusion inlet in the modified slice chamber was placed above the slice at a distance of ∼1 mm from the recording site instead of 2–3 cm for a standard chamber, the sustained oscillations in CA3 pyramidal layer of the rat hippocampal slices were induced by only 50 nM kainate (Fig. 2d, e). Oscillations characterized by the power of 9.9 ± 2.2 μV2 (n = 5 slices from P16–31; Fig. 2e, f) similar to those in a standard chamber (P > 0.05; ANOVA test). However, the peak frequency of kainate-induced oscillations in the modified chamber was in the range of 31–35 Hz with a mean value of 32.8 ± 0.9 Hz (n = 5 slices from P16–31; Fig. 2e, f) that was higher than those in a standard chamber (P < 0.01; ANOVA test). While the power of the oscillations strongly depends from oxygen supply [7] and the frequency of the oscillations—from temperature [8], we suggest that close positioning the superfusion inlet improves oxygen supply and temperature control of the oscillation-generating network. When the superfusion inlet was placed above the slice close to the recording site in a standard slice chamber again, the transient oscillations were induced by 50 nM kainate but at a gamma frequency range (Suppl. Fig. 1).
4 Conclusions
When the previously reported sustained network oscillations in submerged hippocampal slices were rather in the beta frequency range [7], using the modified submerged electrophysiological chamber with a movable superfusion inlet enable to induce reliable and fast oscillations in the gamma frequency range similar to those observed in the interface-type slice chamber in vitro [4, 9] and in the superfused rat hippocampus in vivo [5]. This approach can be useful for the in vitro analysis of the cellular mechanisms underlying neuronal network activity in different brain structures.
References
Yamamoto, C., & McIlwain, H. (1966). Potentials evoked in vitro in preparations from the mammalian brain. Nature, 210(5040), 1055–1056.
Steriade, M. (2001). The intact and sliced brain. Cambridge: MIT Press.
Fisahn, A., Pike, F. G., Buhl, E. H., Paulsen, O. (1998). Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature, 394(6689), 186–189. doi:10.1038/28179.
Hájos, N., Katona, I., Naiem, S. S., Mackie, K., Ledent, C., Mody, I., et al. (2000). Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. European Journal of Neuroscience, 12(9), 3239–3249. doi:10.1046/j.1460-9568.2000.00217.x.
Khazipov, R., & Holmes, G. L. (2003). Synchronization of kainate-induced epileptic activity via GABAergic inhibition in the superfused rat hippocampus in vivo. The Journal of Neuroscience, 23(12), 5337–5341.
Mcmahon, L. L., Williams, J. H., Kauer, J. A. (1998). Functionally distinct groups of interneurons identified during rhythmic carbachol oscillations in hippocampus in vitro. The Journal of Neuroscience, 18(15), 5640–5651.
Hájos, N., Ellender, T. J., Zemankovics, R., Mann, E. O., Exley, R., Cragg, S. J., et al. (2009). Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. European Journal of Neuroscience, 29(2), 319–327. doi:10.1111/j.1460-9568.2008.06577.x.
Gulyás, A. I., Szabó, G. G., Ulbert, I., Holderith, N., Monyer, H., Erdélyi, F., et al. (2010). Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. The Journal of Neuroscience, 30(45), 15134–15145. doi:10.1523/JNEUROSCI.4104-10.2010.
Tsintsadze, V., Minlebaev, M., Suchkov, D., Cunningham, M. O., Khazipov, R. (2015). Ontogeny of kainate-induced gamma oscillations in the rat CA3 hippocampus in vitro. Frontiers in Cellular Neuroscience, 9(195), 1–15. doi:10.3389/fncel.2015.00195.
Acknowledgments
The authors wish to thank Dr. Khazipov R. for the helpful discussion and critical reading of the manuscript. This work was supported by the RFBR grant no. 14-04-01457, the program of competitive growth of Kazan Federal University, and the subsidy allocated to Kazan Federal University for the state assignment in the sphere of scientific activities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This work has been carried out in accordance with EU Directive 2010/63/EU for animal experiments and all animal-use protocols were approved by Kazan Federal University on the use of laboratory animals (ethical approval by the Institutional Animal Care and Use Committee of Kazan State Medical University N9-2013).
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 176 kb)
Rights and permissions
About this article
Cite this article
Juzekaeva, E., Nasretdinov, A. & Mukhtarov, M. Modified Recording Chamber for Sustained Kainate-Induced Gamma Oscillations in Submerged Rat Hippocampal Slices. BioNanoSci. 7, 115–118 (2017). https://doi.org/10.1007/s12668-016-0313-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-016-0313-y