Abstract
Here, we report a scalable and rapid method to fabricate magnetically responsive agarose microgels doped with microbial cells. Low-temperature melting agarose and food-grade sunflower oil were used to fabricate microbeads during emulsification and gel setting. Microscopic algae and fungi cells were doped into ∼100-μm-sized beads as single culture or mixed. Magnetic nanoparticles were deposited either on cell walls or on bead walls. We found that the cells encapsulated in magnetically responsive microbeads were viable and able for germination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Currently, cell encapsulation in various organic matrixes is regarded as one of the efficient methods in biotechnology [1]. Cell encapsulation allows for protecting the cells from harsh environments and facilitation of enzymatic activity; as a result, encapsulated cells can be utilised in biological reactors and whole-cell biosensors [2]. Another fascinating area where encapsulated cells may find applications is the fabrication of artificial multicellular assemblies [3]. Currently, a number of approaches have been successfully employed to fabricate microparticles carrying microbial cells [4]. Among many other challenges, the development of scalable technologies of cell-doped microscopic capsules is responsive to external stimuli. Particularly, fabrication of magnetically responsive cell aggregates functionalised using magnetic nanoparticles is an important area in current biotechnology [5]. In this communication, we report a simple yet efficient approach to fabricate magnetic agarose microbeads doped with microbial cells.
2 Materials and Methods
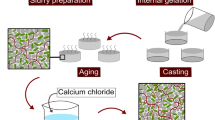
All chemicals were purchased from Sigma-Aldrich and used without further purification. Magnetic nanoparticles (MNPs) were synthesized as described elsewhere [6]. We used the following microorganisms: microalga Chlorella pyrenoidosa and fungi Saccharomyces cerevisiae and Trichoderma asperellum, cultivated according to established protocols. Agarose microbeads were prepared using food-grade sunflower (5 mL) mixed with 1 % aqueous low-temperature agarose (250 μL). Both liquids were heated to 60 °C, then cells and poly(allylamine) (PAH)-stabilised MNPs were added to agarose solution. After mixing, the resulting water in oil emulsion was formed after intensive vortexing. To set agarose gel, the emulsion temperature was reduced rapidly using an ice bath. Then, the microbeads were separated by centrifugation and washed with 20 % aqueous ethanol. Imaging and viability tests were performed using optical microscopy (Carl Zeiss Axio Scope A1 instrument equipped with MRC5 CCD camera and appropriate fluorescence excitation\emission light source). Magnetic separation of the agarose microbeads was performed using a permanent magnet.
3 Results and Discussion
Here, we report a versatile and convenient approach to fabricate cell-doped magnetic agarose microbeads en masse. Our technology is based on previously reported approaches of cell encapsulation in agarose [7]. We employed a simple emulsification process by mixing heated sunflower oil and low-temperature agarose followed by vortexing and gel setting. We were able to obtain polydisperse ∼ 100-μm-diameter agarose microgels doped with live microbial cells. Both single culture and mixed culture (i.e. fungi\algae cells) were effectively encapsulated in agarose microbead, as shown in Fig. 1a. Any other particles can be used to dope agarose beads, i.e. magnetic calcium carbonite microcrystals [8] (Fig. 1b). We also used polymer-stabilised magnetic nanoparticles (PAH-MNPs) to coat the cells via the direct nanoparticle deposition [6] before encapsulation into agarose microbeads. This resulted in fabrication of magnetically responsive cell-doped agarose microbeads, as shown in Fig. 1c, d, demonstrating magnetic yeast. Varying the concentration and\or surface modification of magnetic nanoparticles, the higher loading might be obtained. These microbeads can be effectively manipulated by a strong permanent magnet, including temporary immobilisation on planar surfaces and rotation (data not shown). Alternatively, magnetic nanoparticles can be deposited on non-magnetic cell-doped agarose microcapsules, as shown in Fig. 1e, where a thin cut through a single PAH-MNPs-coated microbead is shown. As one can see, the brown layer of magnetite is located at the distal edges of the microbead, without deep penetration into the capsule.
Encapsulation of live microbial cells into agarose microbeads (optical microscopy images). a Yeast\microalgae carrying agarose microbead. b Agarose microbead doped with magnetic calcium carbonite microcrystals.c, d Magnetically responsive cell-doped agarose microbeads. e Histological image of a single PAH-MNP-coated microbead. f Fluorescence microscopy image demonstrating live microbial cells in agarose microbeads. g Germination of the encapsulated Trichoderma asperellum conidia (formation of fungi hyphae) inside the microbead
The approach we report here is highly biocompatible, as shown in Fig. 1f, g. First, we employed live\dead stain to evaluate the distribution of viable yeast cells inside the agarose microbeads. In addition, we managed to cultivate the encapsulated Trichoderma asperellum conidia yielding formation of fungi hyphae, strongly indicating that the MNP-doped agarose microcapsule provides the cells with a safe environment.
4 Conclusions
Here, we demonstrate a rapid and scalable approach to fabricate magnetically responsive cell-doped agarose microbeads. The complete preservation of cellular viability was confirmed.
References
Benita, S. (1996). Microencapsulation: methods and industrial applications. New York: Marcel Dekker.
Kailasapathy, K. (2002). Microencapsulation of probiotic bacteria: technology and potential applications. Current Issues in Intestinal Microbiology, 3, 39–48.
Konnova, S. A., Kahraman, M., Zamaleeva, A. I., Culha, M., Paunov, V. N., Fakhrullin, R. F. (2011). Functional artificial free-standing yeast biofilms. Colloid Surface B, 88, 656–663.
de Vos, P., Lazarjani, H. A., Poncelet, D., Faas, M. M. (2014). Polymers in cell encapsulation from an enveloped cell perspective. Advanced Drug Delivery Reviews, 67–68, 15–34.
Safarik, I., Pospiskova, K., Horska, K., Safarikova, M. (2012). Potential of magnetically responsive (nano)biocomposites. Soft Matter, 8, 5407–5413.
Zamaleeva, A. I., Sharipova, I. R., Shamagsumova, R. V., Ivanov, A. N., Evtugyn, G. A., Ishmuchametova, D. G., et al. (2011). A whole-cell amperometric herbicide biosensor based on magnetically functionalised microalgae and screen-printed electrodes. Analytical Methods, 23, 509–513.
Orive, G. R., Hernández, M., Gascón, A. R., Igartua, M., Pedraz, J. L. (2003). Survival of different cell lines in alginate-agarose microcapsules. European Journal of Pharmaceutical Sciences, 18, 23–30.
Fakhrullin, R. F., Bikmullin, A. G., Nurgaliev, D. K. (2009). Magnetically responsive calcium carbonate microcrystals. ACS Applied Materials and Interfaces, 1(9), 1847–1851.
Acknowledgments
The work is performed according to the Russian Government Program of Competitive Growth of Kazan Federal University and funded by the subsidy allocated to Kazan Federal University for the state assignment in the sphere of scientific activities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Konnova, S., Fakhrullin, R. Fabrication of Magnetically Responsive Agarose Microbeads Doped with Live Microbial Cells. BioNanoSci. 7, 75–77 (2017). https://doi.org/10.1007/s12668-016-0301-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-016-0301-2