Abstract
Green synthesis of silver nanoparticles (AgNPs) is an alternative to the conventional synthesis procedures which includes physical and chemical methods mostly requiring toxic chemicals, energy, high temperature, and pressure. This study involves biosynthesis of AgNPs using mangroves, a salt-tolerant tidal vegetation with very unique morphology and unusual physiological processes. Our study reports the first ever use of three mangrove plants from Indian Sundarban for bioreduction, namely Avicennia alba, Sonneratia caseolaris, and Sonneratia apetela. The biosynthesized AgNPs were characterized by UV–vis spectroscope, particle size analyzer, scanning electron microscope, transmission electron microscope, energy dispersive X-ray spectrometer, and atomic force microscope. Antimicrobial activities of these AgNPs were assessed against Escherichia coli, Agrobacterium tumefaciens, Streptococcus mutans, Staphylococcus aureus, Tricophyton rubrum, and Aspergillus flavus. Biosynthesized AgNPs showed absorption maxima between 419 and 448 nm which corresponds to their respective surface plasmon resonance. Previous biosynthesis of AgNPs using mangrove plants have reported 60–110 nm average particle size, whereas in our study, S. caseolaris was the most potent bioreductant which synthesized AgNPs with average diameter (D90%) of 18.3 nm. The particles exhibited considerable antimicrobial activities against all six microorganisms. AgNPs synthesized by S. caseolaris using 5 ppm AgNO3 showed the most significant activity with maximum zone of inhibition (13.5 ± 0.8 mm) against E. coli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanoparticles of noble metals are being used widely nowadays because of their unique applications in the field of textile, medicine, energy saving, environment, cosmetics, electronics, and many more [1]. Moreover, increasing need of research in this field has emphasized on different synthesis procedure, characterization, and applicability of those nanoparticles.
Silver nanoparticles (AgNPs) are now under extensive study because of their wide range of applications along with various way of synthesis. The usual synthesis procedure includes physical and chemical methods which mostly require toxic chemicals, energy, high temperature, and pressure [2]. Application of the green chemistry technology instead of using these chemical processes can be one substitute where generation of hazardous substances is reduced or eliminated [3]. Recently, one of the major focuses of nano-researchers is to develop efficient green chemistry methods for synthesis of metal nanoparticles [4]. The alternative green synthesis of AgNPs involves use of non-hazardous chemicals, environment friendly solvents, and renewable materials [5], which have potentiality to produce biocompatible metal nanoparticles economically [6, 7]. The basic approach behind these methods is to use several plant extracts and powder [8–16] plant latex [17], polysaccharides and phytochemicals [18], microbes (both bacteria and fungi) [19–23], and yeasts [24] as reducing agents for the formation of noble metal nanoparticles. Use of various plant products in these economic and alternative methods can synthesize AgNPs both intracellularly and extracellularly [25].
Mangroves are the intertidal salt-tolerant group of plants which have wide applications in folk medicine since ages due to their anthelmintic, anti-inflammatory and other medicinal properties. They are found mainly in the tropical and subtropical intertidal zones of the world largely confined to the region between 30° north to 30° south of the equator. Their ability to survive under very stressful condition has led them to unique morphology and unusual physiological adaptations [26]. Due to their exclusive characteristics, many researchers have shown interest to investigate various bioprospects of mangroves, as well as these plants are used for green synthesis of AgNPs [2, 27, 28].
India shares a part of the largest estuarine mangrove forest in the world, Sundarban, (which covers ∼350 km in width) with Bangladesh [29]. Bioprospecting of mangroves from this unique habitat is still a much unexplored domain of research. Our aim of this study was to employ and establish a novel green synthesis procedure of AgNPs induced by mangrove leaves by assessing their comparative potential as bioreductant. The mangrove plants used in this study has been already reported to have various remarkable medicinal and antimicrobial properties [26, 30]. Using this unique vegetation for the novel green synthesis of metal nanoparticles will add a new dimension to their numerous applications and bioprospects.
Several studies reveal that specially formulated AgNPs have good antibacterial activity [31, 32]. AgNPs are potent antimicrobials because of their unique ability of targeting cell walls, cytoplasmic membranes, and proteins of microorganisms [33]. The antimicrobial and antiviral activities of AgNPs along with silver ions and compounds have been methodically investigated by some previous researchers [34–36]. Here, this is the first report where leaves of mangrove plants from Indian Sundarban were used to synthesize AgNPs which further accompanied by their characterization and antimicrobial properties.
2 Materials and Methods
2.1 Plant Material
In our present study, fresh leaves of three mangrove species namely Kala bain (Avicennia alba), Chak Keora (Sonneratia caseolaris), and Keora (Sonneratia apetela) were collected from Jharkhali Ecological Park (N 22°01′22.9″ E 88°41′10.2″), Sundarban, India. Leaves were taken to the laboratory, identified [37] and washed thrice thoroughly with distilled water to remove soil and dirt.
2.2 Chemicals
All analytical reagents and media components (AgNO3, culture media, etc.) were purchased from Merck (Mumbai, India) and Hi-Media (Mumbai, India).
2.3 Bioreduction Using Leaf Extracts
About 10 g of each plant leaves was finely cut and boiled separately with 100 ml sterilized double distilled water for 5 min. All the three leaf extracts were filtered with Whatman no. 1 filter paper and used for bioreduction. Three different concentrations of silver nitrate (AgNO3) namely 1, 5, and 10 mM were prepared. Eighteen milliliter of each AgNO3 concentration was reduced by 2 ml of each filtered leaf extract at room temperature.
After that, about 100 μl (diluted with 1:30 v/v Milli Q water) of biosynthesized AgNP solution was monitored periodically in PerkinElmer Lambda 35 UV–vis spectrophotometer (between 300 and 800 nm with 1 nm intervals). After 6 h of incubation, the solution was centrifuged with 12,000 rpm for 20 min, and their pellets were re-dispersed in sterilized double distilled water. The centrifugation and redispersion was repeated three times to ensure the complete separation of nanoparticles.
2.4 Characterization of the Biosynthesized AgNPs
2.4.1 Particle Size Analyzer
The AgNP solutions were directly transferred to the cuvette and the hydrodynamic radius was measured in the particle analyzer (Beckman Coulter Particle Size Analyzer Delsa Nano C). The volume distribution and number distribution results along with the D(90 %) values were tabulated and analyzed. The effect of AgNO3 concentration on the diameters of biosynthesized AgNPs was also investigated.
2.4.2 Scanning Electron Microscope (SEM)
Thin films of aqueous suspension of AgNPs were prepared by placing 100 μl of the suspension on glass coverslips which were then allowed to air dry. They were kept in a desiccator to avoid moisture prior to measurement and later analyzed with SEM (Carl Zeiss EVO-MA 10 SEM) operated at 15–20 kV voltage.
2.4.3 Transmission Electron Microscope (TEM)
Three to 4 μl of the sample solution was placed on carbon-coated copper grids and kept in a desiccator after drying. The grids were observed in JEOL JEM 2100 HR with EELS equipped with energy dispersive X-ray analysis (EDX).
2.4.4 Energy Dispersive X-Ray Analysis (EDX)
One hundred microliters of each centrifuged solution was used to form a thin film of AgNPs on glass coverslip which were stored in a desiccator and later analyzed with field emission scanning electron microscopy (FESEM; JEOL JSM-7600f FESEM) at 15 kV voltage, equipped with EDX (Oxford Instruments, INCA PENTA FET X3).
2.4.5 Atomic Force Microscopy (AFM)
The thin films of AgNPs in different dilutions dried on glass coverslips were analyzed in AFM (Bruker AXS, Veeco di Innova) in tapping mode.
2.5 Antimicrobial Activity
Pathogenic bacteria namely Escherichia coli (MTCC 3221), Agrobacterium tumefaciens (MTCC 609), Streptococcus mutans (MTCC 497), Staphylococcus aureus (MTCC 7405) and fungi namely Tricophyton rubrum (MTCC 296) and Aspergillus flavus (MTCC 1884) were obtained from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology, Chandigarh, India. Active cultures were maintained according to MTCC directions. Antimicrobial activity of the biosynthesized AgNPs were tested against these bacteria.
Briefly, a small disc of sterile filter paper (diameter 5 mm) was aseptically soaked in biosynthesized AgNPs and placed on the surface of the seeded medium. Disc soaked with respective plant extracts were used as positive control, whereas sterilized distilled water impregnated discs acted as negative controls. After 24–48 h incubation at 37 °C, the zones of inhibition including the disc diameter were measured using Hi-Media Antibiotic Zone Scale-C (PW297).
3 Results and Discussion
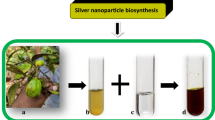
The detailed characterization study on extracellular biosynthesis of AgNPs employing three mangrove leaf extracts was carried out in this work. Addition of leaf extracts to AgNO3 solution resulted in distinct change in color of the reaction mixture. The transparent AgNO3 solution turned into pale yellow and then dark brownish which indicates the gradual reduction of AgNO3 by the leaf extracts (Fig. 1). The color intensity of the solution varies proportionately with the concentration of the AgNO3, i.e., the higher the concentration, the higher the color intensity. The reaction mixture with 1 ppm AgNO3 showed yellowish color, whereas the same containing 5 ppm AgNO3 produced dark brown color at the end of reaction. This color change arises because of the excitation of surface plasmon resonance (SPR) in the AgNPs [5].
In our study, the visual change in color of the solutions was different for each leaf extracts. This may be due to the different bioactive components present in three different leaf extracts which causes a varied range of bioreduction. Variation in the availability of bioreducing molecules in three different plant extracts is responsible for this difference in the absorbance values. Presence of a wide variety of biomolecules like enzymes, proteins, amino acids and polysaccharides in the leaf extracts may act as the reducing and capping agents for the bioreduction process, among which the enzyme “nitrate reductase” plays the most vital role as reported in the earlier works [38, 39]. In our study, the leaf extracts were prepared by boiling them at around 100 °C which probably destroys the active enzymes present in the leaves. Therefore, the bioreduction process reveals the presence of some reducing agents in the leaf extracts which are thermostable in nature.
After 6 h of incubation, no change in the intensity of color was observed in the reaction mixture, indicating the complete reduction of silver ions. The reaction mixtures of three leaves showed similar behavior with maximum absorption peak ranging between 419 and 448 nm which corresponds to SPR of silver nanoparticles established at 420 nm [40]. AgNPs synthesized using A. alba leaf extract had absorption maxima at 448 nm (Fig. 2a), whereas S. caseolaris and S. apetela showed distinct peaks at 424 and 419 nm (Fig. 2b, c). These absorption peaks can be directly co-related with particle size where smaller AgNPs will have an absorbance maximum around 400 nm, which increases with size and disappears when particle size falls outside nanodimensions [41]. Thus, the results from our study confirm the synthesis of nano ranged silver particles.
The particle analyzer results were represented in two distribution patterns: volume distribution and number distribution, respectively. The cumulative frequency given as D(90 %) in the distribution table found in the particle analyzer showed varied results. Both the volume and number distribution results which stand for the average particle size of the biosynthesized AgNPs are depicted in Table 1. As a whole, S. caseolaris leaf extract was found to be the most potent bioreductant in case of 5 % mM AgNO3 concentration, which was able to produce nanoparticle with average diameter (D90%) of 18.3 nm (Table 1). The maximum range of particle diameter reached up to 65.4 nm. The average AgNP diameters (number distribution) are graphically represented in the Fig. 3a which clearly indicates S. caseolaris to be the most potent bioreductant.
The effect of AgNO3 concentration on the size of the biosynthesized AgNPs can be understood from Fig. 3b, where a linear relationship between the AgNO3 concentration and the particle size was observed in case of A. alba leaf extract. On the contrary, S. caseolaris leaf extract synthesized least sized particles (18.3 and 23 nm, respectively) with 5 and 10 mM AgNO3. AgNPs with smaller diameter was synthesized using 5 mM AgNO3 concentration, whereas the larger diameter was found in case of 1 mM AgNO3 concentration. Previous biosynthesis of AgNPs using mangrove plants have reported the average particle sizes 71–110 nm using Avicennia marina [2] and 60–95 nm using Rhizophora mucronata [27]. In our study, S. caseolaris leaf extract was able to synthesize AgNPs up to 18.3 nm diameter. The objective of using three concentrations of AgNO3 was to optimize the precursor concentration which would be most productive for least sized AgNP synthesis.
Here, from the results of the number distribution pattern, it can be said that 5 and 10 mM AgNO3 solutions are far more effective precursor than 1 mM. Further studies are being carried out to establish the exact desired AgNO3 concentrations needed for the green synthesis of AgNP using mangrove plant extracts. As a whole, the color intensity and the shapes of the biosynthesized AgNPs showed direct dependence on the concentration of the Ag+ ion in solution.
Scanning electron microscopy was utilized to characterize the particles and their sizes and distribution by taking images from drop-coated films of the silver nanoparticles synthesized by the treatment of AgNO3 solution with mangrove leaf extract for 6 h. Topology and dispersion of biosynthesized AgNPs were studied. Majority of the AgNPs observed from SEM images found to be spherical and cuboidal and ranged in nanometer size. The images broadly revealed two to three types of particle size, one with diameter of 20–40 nm and another with diameter 50 nm or more than that (Fig. 4a–c). Presence of this heterogeneous size distribution data can be complemented with the UV–vis analysis (Fig. 2a–c) where the broad SPR band ranges between 419 and 448 nm. The AgNPs were mostly not in physical contact with each other as revealed from the images.
The EDX spectrum (Fig. 5) recorded from silver nanoparticle-coated glass coverslip showed strong signal of silver. The signal of gold was also observed due to the gold coating on the sample. The optical absorption peak of the biosynthesized AgNPs was typically observed at approximately 3 keV (Fig. 5) which is characteristic for typical metallic silver nanocrystals due to surface plasmon resonance [17].
3D representation of the AFM images of the nanoparticles revealed their topology, and the average diameter was found to be in between 15 to 50 nm (Fig. 6a–c). TEM images (Fig. 7a–c) of the sample solutions along with the elemental mapping using EDX (Fig. 8) re-confirm the formation of nano-sized sliver particles.
Along with the synthesis and characterization of nanomaterials, a branch of nanotechnology also deals with their various applications which serves several pharmaceutical and drug delivery fields. Silver is known as an established antimicrobial agent since ages. Though silver ion and silver-based compounds are highly toxic to microorganisms, showing strong biocidal effect against microbial species, still they have only limitation as antimicrobial agents for several reasons like interfering effects of salts and discontinuous release of inadequate concentration of silver ions from the metal. In contrast, these kinds of limitation can be overcome using silver nanoparticles as these are highly reactive species because of large surface area [12]. Toxic and antimicrobial activity of biosynthesized AgNPs have been reported by many [1, 8, 15, 20, 21, 42, 43]. A study showed antibacterial activity of AgNPs at very low dosage against E. coli is advantageous compared to two common disinfectants due to its negligible environmental waste or hazardous by-product [33].
An interesting similarity was found between the average particle size data and the antimicrobial activity of the AgNPs. The plant and the concentration which was able to synthesize the least size particles (18.3 nm) showed the highest antibacterial activity against E. coli. Water extracts of the leaves used in this study were used as positive control and sterilized water was used as negative control. The results of disc diffusion assay from Table 2 showed the maximum zone of inhibition with AgNPs synthesized by S. caseolaris and 5 ppm AgNO3 against E. coli (13.5 ± 0.8 mm). The antibacterial and antifungal effects of the three plants used in this study were also tested in our laboratory and found positive though the data has not been shown here [30].
4 Conclusion
Nowadays, nanotechnology research has grabbed considerable attention because of their unique ability to control the matter in atomic and molecular scale [20]. With the growing awareness and environmental concerns, there is a constant need to make the inorganic nanoparticles biocompatible and environmentally benign using green chemistry [6]. Therefore, different ways are being developed to find new, greener, safer, economical, easy-to-use, and highly effective rapid synthesis methods for the preparation of metal nanoparticles. Metals especially silver nanoparticles are presently getting significant attention because of their various applications in the field of catalysis, plasmonics, optoelectronics, biological sensor, and pharmaceutical applications. Their performance depends critically on their size, shape, and composition. In our study, the biosynthesized AgNPs showed potent antimicrobial activity which on one hand represents the novel green synthesis of nanomaterials and their medicinal application; and on another hand it explores huge bioprospecting potential of an important bioresource derived from Indian Sundarban. Additionally, this study exhibits the formation of nanoparticle from the interaction of an inorganic compound and a significant biological resource, mangrove. From the comparative potential study of three mangrove plants as reducing agents, S. caseolaris is proved to be the most potent bioreductant with the ability of producing AgNPs with antimicrobial activity. Further study is being carried out to establish whether the antimicrobial activity of the mangrove plants used for bioreduction in this study is influencing the antimicrobial activity of the biosynthesized AgNPs.
References
Ramteke, C., Chakraborty, T., Sarangi, B. K., Pandey, R. A. (2013). Journal Chemical. doi:10.1155/2013/278925.
Gnanadesigan, M., Anand, M., Ravikumar, S., Maruthupandy, M., Ali, M. S., Vijayakumar, V., et al. (2012). Antibacterial potential of biosynthesised silver nanoparticles using Avicennia marina mangrove plant. Applied Nanoscience, 2, 143–147.
Vigneshwaran, N. (2006). A novel one-pot ‘green’ synthesis of stable silver nanoparticles using soluble starch. Carbohydrate Research, 341, 2012–2018.
Iravani, S. (2011). Green synthesis of metal nanoparticles using plants. Green Chemistry, 13, 2638–2650.
Mani, U., Dhanasingh, S., Arunachalam, R., Paul, E., Shanmugam, P., Rose, C., et al. (2013). A simple and green method for the synthesis of silver nanoparticles using Ricinus communis leaf extract. Progress in Nanotechnology and Nanomaterials, 2, 21–25.
Govindaraju, K., Tamilselvan, S., Kiruthiga, V., Singaravelu, G. J. (2010). Biogenic silver nanoparticles by Solanum torvum and their promising antimicrobial activity. Biopesticides, 3, 394–399.
Roy, N., & Barik, A. (2000). Green synthesis of silver nanoparticles from the unexploited weed resources. International Journal of Nanotechnology and Applications, 4, 95–101.
Parashar, U. K., Saxenaa, P. S., Srivastava, A. (2009). Bioinspired synthesis of silver nanoparticles. Digest Journal of Nanomatter and Biostructures, 4, 159–166.
Annavaram, V., Posa, V. R., Uppara, V. G., Jorepalli, S., Somala, A. R. (2015). Facile green synthesis of silver nanoparticles using Limonia acidissima leaf extract and its antibacterial activity. Bionanoscience. doi:10.1007/s12668-015-0168-7.
Kulkarni, A. P., Srivastava, A. A., Harpale, P. M., Zunjarrao, R. S. (2011). Plant mediated synthesis of silver nanoparticles—tapping the unexploited sources. Journal of Natural Product and Plant Resources, 1, 100–107.
Shankar, S. S., Rai, A., Ahmad, A., Sastry, M. (2004). Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. Journal of Colloid and Interface Science, 27, 496–502.
Nabikhan, A., Kandasamy, K., Raj, A., Alikunhi, N. M. (2010). Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum, L. Colloids and Surfaces B: Biointerfaces, 79, 488–493.
Song, J. Y., Eun-Yeong, K., Kim, B. S. (2010). Biological synthesis of platinum nanoparticles using Diospyros kaki leaf extract. Bioprocess and Biosystems Engineering, 33, 159–164.
Ponarulselvam, S., Panneerselvam, C., Murugan, K., Aarthi, N., Kalimuthu, K., Thangamani, S. (2012). Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pacific Journal Tropical Biomedicine, 2, 574–580.
Sathishkumar, M., Sneha, K., Yun, Y. S. (2010). Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Bioresource Technology, 101, 7958–7965.
Kumar, T. S., Rahuman, A., Rajakumar, G., Marimuthu, S., Bagavan, A., Jayaseelan, C., et al. (2011). Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitology Research, 108, 693–702.
Bar, H., Bhui, D. K., Sahoo, G. P., Sarkar, P., De, S. P., Misra, A. (2009). A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids and Surfaces A, 339, 134–139.
Park, Y., Hong, Y. N., Weyers, A., Kim, Y. S., Linhardt, R. J. (2011). Polysaccharides and phytochemicals: a natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnology, 5, 69–78.
Metuku, R. P., Pabba, S., Burra, S., SVSSSL Hima Bindu, N., Gudikandula, K., Singara Charya, M. A. (2014) Biosynthesis of silver nanoparticles from Schizophyllum radiatum HE 863742.1: their characterization and antimicrobial activity. 3 Biotech, 4, 227–234.
Nanda, A., & Saravanan, M. (2009). Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine: Nanotechnology, Biology and Medicine, 5, 452–456.
Pourali, P., Baserisalehi, M., Afsharnezhad, S., Behravan, J., Ganjali, R., Bahador, N., et al. (2013). The effect of temperature on antibacterial activity of biosynthesized silver nanoparticles. Biometals, 26, 189–196.
Kathiresan, K., Manivannan, S., Nabeel, M. A., Dhivya, B. (2009). Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids and Surfaces B: Biointerfaces, 71, 133–137.
Balaji, S. D., Basavaraja, S., Deshpande, R., Mahesh, B. D., Prabhakar, K. B., Venkataraman, A. (2009). Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids and Surfaces B: Biointerfaces, 68, 88–92.
Subramanian, M., Alikunhi, N. M., Kathiresan, K. (2010). In vitro synthesis of silver nanoparticles by marine yeasts from coastal mangrove sediment. Advanced Science Letters, 3, 428–433.
Ahmad, A., Mukherjee, P., Senapati, S., Mandal, D., Khan, M. I., Kumar, R., et al. (2003). Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids and Surfaces B: Biointerfaces, 27, 313–318.
Bandaranayake, W. M. (2002). Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetlands Ecology and Management, 10, 421–452.
Gnanadesigan, M., Anand, M., Ravikumar, S., Maruthupandy, M., Vijayakumar, V., Selvam, S., et al. (2011). Biosynthesis of silver nanoparticles by using mangrove plant extract and their potential mosquito larvicidal property. Asian Pacific Journal Tropical Medicine, 4, 799–803.
Singh, M., Kumar, M., Kalaivani, R., Manikandan, S., Kumaraguru, A. K. (2013). Metallic silver nanoparticle: a therapeutic agent in combination with antifungal drug against human fungal pathogen. Bioprocess and Biosystems Engineering, 36, 407–415.
Gopal, B., & Chauhan, M. (2006). Biodiversity and its conservation in the Sundarban Mangrove Ecosystem. Aquatic Sciences, 68, 338–354.
Bakshi, M., & Chaudhuri, P. (2014). Antimicrobial potential of leaf extracts of ten mangrove species from Indian Sundarban. International Journal Pharmaceutics Biology Science, 5, 294–304.
Ahmad, M. B., Shameli, K., Darroudi, M., Yunus, W. M. Z. W., Ibrahim, N. A., Hamid, A. A., et al. (2009). Antibacterial activity of silver/clay/chitosan bionanocomposites. Research Journal Biological Sciences, 4, 1156–1161.
Abou El-Nour, K. M. M., Eftaiha, A., Al-Warthan, A., Ammar, R. A. A. (2010). Synthesis and applications of silver nanoparticles. Arabian Journal of Chemistry, 3, 135–140.
Chamakura, K., Perez-Ballestero, R., Luo, Z. P., Bashir, S., Liu, J. (2011). Comparison of bactericidal activities of silver nanoparticles with common chemical disinfectants. Colloids and Surfaces B: Biointerfaces, 84, 88–96.
Magana, S. M., Quintana, P., Aguilar, D. H., Toledo, J. A., Angeles-Chavez, C., Cortes, M. A., et al. (2008). Antibacterial activity of montmorillonites modified with silver. Journal of Molecular Catalysis A: Chemical, 281, 192–199.
Nithya, R., & Ragunathan, R. (2009). Synthesis of silver nanoparticle using Pleurotus sajor caju and its antimicrobial study. Digest Journal of Nanomater and Biostructures, 4, 623–629.
Lara, H. H., Nuñez, N. V. A., Turrent, L. I., Rodriguez-Padilla, C. (2010) Mode of antiviral action of silver nanoparticles against HIV-1. Journal of Nanobiotechnology, 8, 1–10.
Banerjee, L. K, Sastry, A. R. K, & Nayar, M. P. (1989) Mangroves in India: identification manual. Calcutta: Botanical Survey of India.
Anil Kumar, S., Abyaneh, M. K., Gosavi Sulabha, S. W., Ahmad, A., Khan, M. I. (2007). Nitrate reductase mediated synthesis of silver nanoparticles from AgNO3. Biotechnology Letters, 29, 439–445.
Kalimuthu, K., Babu, R. S., Venkataraman, D., Mohd, B., Gurunathan, S. (2008). Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids and Surfaces B: Biointerfaces, 65, 150–153.
Mulvaney, P. (1996). Surface plasmon spectroscopy of nanosized metal particles. Langmuir, 12, 788–800.
Brause, R., Moeltgen, H., Kleinermanns, K. (2002). Characterization of laser-ablated and chemically reduced silver colloids in aqueous solution by UV–vis spectroscopy and STM/SEM microscopy. Applied Physics B, 75, 711–716.
Zargar, M., Hamid, A. A., Bakar, F. A., Shamsudin, M. N., Shameli, K., Jahanshiri, F., et al. (2011). Green synthesis and antibacterial effect of silver nanoparticles using Vitex negundo L. Molecules, 16, 6667–6676.
Krishnaraj, C., Jagan, E. G., Rajasekar, S., Selvakumar, P., Kalaichelvan, P. T., Mohan, N. (2010). Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids and Surfaces B: Biointerfaces, 76, 50–56.
Acknowledgments
The authors are thankful to University Grants Commission (UGC), India; Centre for Nanoscience and Nanotechnology (CRNN), University of Calcutta; and DBT-CU IPLS Programme, University of Calcutta, for financial and infrastructural support.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bakshi, M., Ghosh, S. & Chaudhuri, P. Green Synthesis, Characterization and Antimicrobial Potential of Sliver Nanoparticles Using Three Mangrove Plants from Indian Sundarban. BioNanoSci. 5, 162–170 (2015). https://doi.org/10.1007/s12668-015-0175-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-015-0175-8