Abstract
Eutectic high-entropy alloys (EHEAs) have unique properties, making them a significant sub-branch of HEAs. Researchers are interested in their high strength, good castability, and ductility. However, due to the lack of a complete phase diagram database and previous research results, the traditional trial-and-error method will greatly reduce the research efficiency, and the composition design of EHEAs faces many difficulties. This paper summarizes the recent proposals of the thermo-dynamic factors that have a decisive role in the design of EHEAs. Regarding previous research, we discussed the methods for designing the main eutectic compositions currently used in research. These methods aid in developing novel approaches to fabricating EHEAs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In 2004, Yeh and Cantor et al. proposed a new class of alloy system, namely high-entropy alloys (HEAs) [1]. Unlike traditional alloy systems, high-entropy alloys are usually composed of at least four principal elements, so they have extremely high mixing entropy. According to the second law of thermodynamics, the mixing configuration entropy per mole can be expressed as, \({\Delta S}_{mix}=-R{\sum }_{i=1}^{n}{c}_{i}{\text{ln}}{c}_{i}\) [2], where R is the gas constant, \({c}_{i}\) is the mole fraction of the i-th element, and n is the total number of constituent elements. According to the "high entropy" effect, when multiple principal elements are mixed in equimolar fractions, random solid solutions with stable compounds are formed [3, 4]. It is noted from previous research that mixing multiple elements in HEAs results in high entropy, lattice distortion, sluggish diffusion, and cocktail effects. Specifically, the presence of a high entropy plays an important role in simplifying the micro-structures so that they principally consist of a simple solid solution with structures like body-centered cubic (bcc)/face-centered cubic (fcc). The distortion of the lattice has a significant impact on the mechanical, physical, and chemical properties of alloys. This can cause sluggish diffusion, resulting in the development of nanocrystalline or amorphous structures. Additionally, the combination of different elements can create a composite effect on properties, with interactions between the elements playing a crucial role [2]. However, the strength and ductility of traditional high-entropy alloys are difficult to balance at the same time. In previous studies, high-strength alloys show compromised ductility and vice-versa [5,6,7]. In order to balance the shortcomings of this traditional high-entropy alloy, the concept of eutectic high-entropy alloys (EHEAs) was proposed by Lu et al. in 2014, which has become the most important sub-branch in the field of high-entropy alloy research [8,9,10]. The fcc phase provides enough plasticity for the alloy, and the bcc phase makes the alloy have excellent strength, and EHEAs that consist of both soft fcc and hard bcc or intermetallic phase provide ideal strength and ductility (usually showing a layered or rod-like structure). Eutectic alloys offer several advantages including: (1) a microstructure that is resistant to changes at high temperatures, (2) low-energy phase boundaries, (3) the ability to control the microstructure, (4) high fracture strength, (5) a stable defect structure, (6) good resistance to creep at high temperatures, and (7) a regularly layered or rod-like eutectic structure that forms an in-situ composite material [11].
In recent years, due to the presence of an excellent two-phase equilibrium system of EHEAs, it has attracted recent attention. However, the traditional trial-and-error and experimental methods seem to be unable to meet the current needs. During the design and development of EHEAs, the influence of many thermodynamic parameters on the formation of the eutectic phase must first be considered [12]. Therefore, it is very important to determine reasonable parameters to generate a stable eutectic phase, such as mixing entropy (ΔSmix), mixing enthalpy (ΔHmix) and atomic size difference (δr), and valence electron concentration (VEC) are the factors that form multiple key influencing factors for the phase formation [13,14,15].
Recently, researchers have designed some methods to explore the components of EHEAs, including the simple mixing method [16, 17], mixing enthalpy method [12], CALPHAD-assisted method [20,21,22,23,24,25], and solidification process simulation [26,27,28,29,30,31]. These methods are mainly based on the phase diagram and solidification process simulation mainly with the help of Thermo-calc, JmatPro, and Pandat software. Furthermore, experimental experiences are often used in compositional design, especially for the design and fabrication of Co-free EHEA [32,33,34,35]. Apart from the above said conventional design methods, a new infinite solid-solution strategy was proposed by Ye et al., [36] and four EHEAs with excellent compressive mechanical properties were successfully designed, namely NiAl–20V–17Cr, NiAl–30V–5Mo, NiAl–30Cr–Mo and NiAl–20V–10Cr–5Mo. An EHEA with seven components was designed by Shah et al. using an integrated computational materials engineering (ICME)-based framework [37]. A method combining machine learning (ML) and thermodynamic calculations to quickly locate the eutectic composition in the Ni–Co–Cr–Al system was proposed by Liu et al. [38]. A Co-free and cost-effective EHEA based on the valance electron concentration (VEC) criterion was designed by Wu et al. [39]. Three EHEAs of Al20.45Co10Cr10Ni59.55, Al17.5Co20Cr20Ni52.5, and Al16.3Co25Cr25Ni33.7 were designed by Liu et al. based on the pseudo-ternary phase diagram and microscopic observation [40]. This paper mainly reviews the main components of the EHEA phases fabricated in recent years and the physical factors affecting phase formation and discusses and analyzes the proposed alloy design methods.
2 Thermodynamic Parameter(s) for Multicomponent Alloy Formation
During the solidification process of the eutectic phase, in order to ensure the stability of the eutectic phase formation, some empirical physical parameters are proposed to design the alloy composition, such as mixing entropy ΔS mix, mixing enthalpy ΔH mix, atomic size difference δr and valence electron concentration VEC [13, 14]. Table 1 lists the alloys with fully eutectic composition and the above empirical physical parameters discovered in recent years. We have also carried out computational verification on them. Below we will discuss their calculation methods and the references provided for the design of eutectic phase formation.
2.1 Mixing Entropy
According to Boltzmann's hypothesis, the formula for calculating the configurational entropy change per mole (ΔSmix) during solid solution formation from n elements with equimolar fractions is [1, 13]:
The formula for calculating the mole percent of a component, \({C}_{i}\), involves adding up the values of C subscript i from i equals 1 to n. In this equation, the equipment control is equal to 4, and the gas constant, R, is 8.314 J·K−1 mol−1. When dealing with equiatomic ratio alloys, the entropy of mixing reaches its maximum. This means that multi-component HEAs with equal or nearly equal atomic ratios have a significantly higher entropy of mixing compared to conventional alloys. According to Zhang et al., when 12 ≤ \({\Delta S}_{mix}\)≤17.5 J/mol, it is easy to form a solid solution [14]. Thus at high values of ΔSmix at sufficiently high temperatures, a particular solid solution phase is stable [12].
2.2 Mixing Enthalpy
The equation for determining the enthalpy of mixing in a multi-component alloy system with n elements is:
where Ωij (= 4\({\Delta H}_{AB}^{mix}\)) is the regular solution interaction parameter between the i-th and j-th elements, ci or cj is the atomic percentage of the i-th or j-th component, \({\Delta H}_{AB}^{mix}\) is the binary alloy mixing enthalpy [13]. In the calculation, the mixing enthalpy value \({\Delta H}_{AB}^{mix}\) is based on the Miedema macroscopic model of the binary liquid alloy, which can be obtained elsewhere [41]. Intermetallic phases form when the enthalpy of mixing between constituent elements is highly negative. Conversely, phases separate when enthalpy of mixing is positive or less negative [42]. Therefore, a reasonable mixing enthalpy value can ensure the formation of a stable eutectic phase. For the formation of solid solution and intermetallic phases in EHEAs, negative mixing enthalpy and high mixing entropy are required. [43].
2.3 Atomic Size Difference
When there are significant variations in the sizes of atoms present, it can result in significant distortion of the lattice and can decrease the stability of solid solutions. This can also intensify the lattice hysteresis effect of high-entropy alloys and increase the likelihood of a segregation effect occurring. To describe the size difference of atoms inside an n element alloy, the introduction of the atomic size difference factor (δ) is expressed as follows:
where \({c}_{i}\) is the atomic percentage of the ith component, \(\overline{r }={\sum }_{i=1}^{n}{c}_{i}{r}_{i}\) is the average atomic radius, and \({c}_{i}\) values may be obtained from elsewhere [13, 44]. The parameter δ is also crucial for predicting the phase formation, a smaller value of δ is preferable for the creation of solid solutions with a single phase. Since EHEAs have a dual-phase structure, the value of δ should not be small, and a eutectic dual-phase structure is formed when δ > 3% [12].
2.4 Valence Electron Concentration
The phase stability of alloy systems depends on electron concentration, which can be determined by VEC or valence electrons (e/a), the average number of itinerant electrons per atom. The valence electron concentration (VEC) of multi-component alloys can be calculated as the e/a ratio of the constituent components or the weighted average of VEC. Since HEAs mainly contain transition metals, and the e/a of transition metals is controversial, follow-up studies are all based on VEC [45]. In order to study the effect of VEC on the phase equilibrium of HEA, the VEC of multi-element HEA can be determined by the following formula:
where VECi is the VEC of a single element, this can be obtained by looking up the periodic table of elements. According to the research of Chanda et al., the VEC of EHEAs should be kept in the range of 6 ≤ VEC ≤ 8.5, which facilitates the formation of a two-phased eutectic structure (fcc + bcc phase) [12].
3 Summary
To accurately obtain the dual-phase structure of EHEAs, the physical thermodynamic method is used as the guiding tool. Therefore, determining a reasonable mixing entropy, mixing enthalpy, atomic size difference, and VEC is crucial for the formation of the eutectic phase. According to previous reports, higher ΔSmix and negative ΔHmix are necessary for the formation of the solid solution and intermetallic phases in EHEAs. However, the value of δ should be relatively large, and higher δ can aggravate lattice distortion and lead to the destabilization of simple phases in EHEAs. Specifically, the eutectic structures in various HEAs are formed under the following conditions: − 18 ≤ ΔHmix ≤ − 6, 6 ≤ VEC ≤ 8.5, and δr > 3 [12, 43]. According to Eqs. (1–4), we calculated these physical parameters for EHEAs with all-eutectic composition from four to seven components proposed in recent years, as shown in Table 1. To view the influence of EHEAs thermodynamic parameters on phase transition more clearly and intuitively, Fig. 1 lists the relationship between two typical thermodynamic parameters, atomic size difference and mixing enthalpy. Clearly, the ingredients that form a typical two-phase eutectic high-entropy alloy are located in the red marked area. For fcc + Laves/bcc phased EHEAs, the mixing enthalpy is in line with the general situation, lower than − 7 kJ/mol and greater than − 18 kJ/mol. When the mixing enthalpy is too large, lower than − 18 kJ/mol, it is difficult to form a fcc solid solution, and it can also be understood that it is easy to form an ordered intermetallic compound with low mixing entropy and high mixing enthalpy, as shown in the green area in Fig. 1 [66].
4 Design Methods for Eutectic High Entropy Alloys
Since EHEAs exhibit an excellent balance of plasticity and ductility, the development of EHEAs has attracted attention in recent years. So far thermodynamic parameters-based methods (discussed above) have been extensively utilized, in addition to phase diagram calculations, etc. The applicable scenarios using the existing composition design methods and their limitations will be discussed in detail in this short review.
4.1 Simple Mixing Method
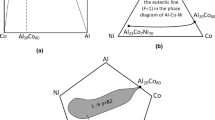
Jiang et al. [16] proposed the simple mixing method, a new design strategy that uses mixing enthalpy and binary eutectic composition to locate eutectic composition in HEA. According to previous studies, it is known that CoCrFeNi alloys have a simple fcc solid solution structure in the cast state, and is called CoCrFeNi high-entropy base elements (HEBE), and elements with larger enthalpy mixed with CoCrFeNi (such as ZrNbTaHfAl) are called eutectic forming elements (EFE) [67]. By consulting the binary mixing enthalpy table, it is shown that the mixing enthalpy (ΔHmix) between Nb, Ta, Zr, and Hf elements and Co, Cr, Fe, and Ni elements is very negative. In addition, by finding the elements contributing to the formation of the eutectic phase are identified. Finally, by combining binary eutectic compositions in a 1:1 equimolar ratio, four new EHEAs, namely CoCrFeNiNb0.6, CoCrFeNiTa0.47, CoCrFeNiZr0.51, and CoCrFeNiHf0.49, were designed. The structure on display is a combination of fcc and Laves phases, forming a eutectic composition. Thereafter, based on this idea, Xie et al. [68, 69] proposed an improved simple hybrid method to design some five-element CoaCrbFecNid-M (M is the EFE elements) EHEAs. Unlike before, the EFE elements are in non-equiatomic ratios. It first determines the eutectic point of each binary system, determines the parameters based on the simple mixing method, and finally obtains the full eutectic structure by fine-tuning the x value, the design idea is shown in Fig. 2. These studies showed good utility in the compositional design of EHEAs, but they were limited to the development of five-component EHEAs. To overcome this limitation, Jiao et al. [17] proposed a simple hybrid method for designing EHEAs with more than five elements. They created four new EHEAs, consisting of fcc and Laves phases: CoCrFeNiNb0.25Ta0.20, CoCrFeNi–Ta0.25Hf0.25, CoCrFeNiNb0.15Zr0.15–Hf0.15 and CoCrFeNiZr0.17Hf0.16Ta0.16.
Here is a diagram detailing the modified simple mixing method [68]
4.2 Mixed Enthalpy Method
Lu et al. [18] proposed a new strategy to design EHEAs using mixing enthalpy, also known as the mixing enthalpy method. Due to the very negative mixing enthalpy of both Al and Ni in AlCoCrFeNi2.1EHEA, Al is substituted with elements such as Zr, Nb, Hf, and Ta that have similar negative mixing enthalpy as Ni. Finally, the molar ratio of the elements is obtained through the inverse relationship between the element content and the mixing enthalpy, and finally, the new EHEAs composition is designed through fine-tuning. Four novel EHEAs with fully eutectic compositions of Zr0.6CoCrFeNi2.0, Nb0.74CoCrFeNi2.0, Hf0.55CoCrFeNi2.0, and Ta0.65CoCrFeNi2.0 were designed. They displayed a lamellar eutectic morphology consisting of fcc and laves dual phases, as depicted in Fig. 3. This method is highly effective and offers accurate results, and it was relatively easy to locate new EHEA components. However, it is only applicable to modify a few elements in the existing EHEA to obtain a new eutectic composition, and the scope of application is relatively narrow.
Scanning electron microscopy images of four new EHEAs: a Zr0.6CoCrFeNi2.0, b Nb0.74CoCrFeNi2.0, c Hf0.55CoCrFeNi2.0, and d Ta0.65CoCrFeNi2.0, respectively [18]
4.3 CALPHD Assisted Method
To design the eutectic alloy composition more accurately, He and his colleagues [20] proposed a method for designing the eutectic composition using the pseudo-binary phase diagram. First, it is necessary to find out the elements that form eutectic structures with single-phase HEA matrix elements, mainly through the existing binary or ternary phase diagram. Thermo-Calc software database is then utilized to calculate the pseudo-binary phase diagram, determine the phase formation process during solidification, obtain the eutectic composition, and finally verify the accuracy of the theoretical results through experiments. According to this idea, the hypoeutectic structure of CoCrFeNiNb0.5 was successfully designed. The results obtained matched well with the pseudo-binary phase diagram. In addition, Gasan and Ozcan used thermodynamic and computational methods to design new EHEAs [25]. To find the eutectic point, they considered 324 equilibrium phase diagrams and verified their thermodynamic factors. Finally, (Co40Cr10Fe5Mo5Ni40)82.2Al17.8 EHEAs with fcc and B2 phase compositions were designed. Subsequently, Wu et al. [22] proposed a method for designing EHEA using CALPHAD. The method involved calculating pseudo-binary diagrams based on the eutectic group phase and establishing a pseudo-ternary phase diagram to guide alloy design. Pandat 2016.1 software and the PanHEA2017 database were used to create the CoCrNi–NiAl pseudo-binary and NiCo–Cr–NiAl pseudo-ternary phase diagrams. AlCoCrNi EHEAs with both fcc and B2 phases were prepared using thermodynamic guidance. Layered and rod-like mixed eutectic microstructures were characterized. However, the results were inaccurate due to the imperfect database of CALPHAD. Hence, perfecting the database is crucial for the effective utilization of this design method.
The concept of alloy design with the assistance of CALPAHD is being developed. Mukarram et al. [23, 70] calculated the CoCrFeNi–Ta pseudo-binary phase diagram using Thermocalc software and the TCHEA database, and successfully developed eutectic and hypereutectic alloys with fcc and laves phase compositions. In addition, they evaluated the influence of Mo addition to CoCrFeNi and the resultant formation of a eutectic microstructure through pseudo-binary phase diagram calculations and successfully developed a eutectic consisting of fcc (A2) and intermetallic phases (σ and μ) through experiments (CoCrFeNiMo0.5 and CoCrFeNiMo1.0). Modifications in the CoCrFeNi–Ta pseudo binary phase diagram calculated with the help of Thermocalc software were made in view of the obtained experimental results, as shown in Fig. 4. Using the same database, Vikram et al. [24] designed and developed a Co-based EHEA using Thermo-Calc reproduced analytical calculations. Finally, using this method, CALPHAD is employed to verify or guide the EHEA composition design. Due to the relative imperfection of the database, as the number of constituent elements increases, the difficulty of establishing phase diagrams also increases. Therefore, this method cannot be directly used in the composition design of EHEAs like traditional binary and ternary alloys. In the process of pseudo-binary phase diagram simulation and needs fine-tuning. Therefore, for the design of EHEAs by the CALPHD-assisted method, the establishment of a comprehensive database and a considerable number of experimental conclusions are the key directions in the future.
Pseudo-binary Phase Diagram Adjustment of CoCrFeNi–Ta [23]
4.4 Solidification Process Simulation
Wang et al. [31]. proposed a new method to design EHEA by calculating the solidification path (using JMatPro software), which mainly predicts the eutectic composition through solidification path analysis, and then verifies the accuracy of the results through experiments. Specifically, as shown in Fig. 5, The solidification paths of NiAl, bcc, and liquid are represented by black, orange, and blue curves, respectively. There was no inflection point in the solidification cooling curves of the three components. Three EHEAs were successfully designed: NiAl–Mo8.7Cr8.7V8.7, NiAl–Mo14.5Cr14.5Fe14.5, and NiAl–Mo10Cr10V10Fe10. They consist of eutectic dendrites with B2 and bcc layered structures. No primary phase appears, but two phases (eutectic) solidify simultaneously.
Similarly, Ai et al. [26] designed CoCrFeNiTax type EHEAs by analyzing binary phase diagrams and thermodynamic calculations (JMatPro). It is verified by experiments that the obtained CoCrFeNiTa0.43 alloy has a layered eutectic phase composed of fcc/laves phase. In addition, Ali Shafiei proposed a simple method to predict the composition of the EHEA in the Al–Co–Cr–Fe–Ni system [30]. To verify the design process, the solidification process was simulated with JMatPro® software version 7.0.0. To determine the phase composition, Wen et al. [28] simulated the solidification process of the coating material (JmatPro). By adjusting the composition content, the Ni1.5CrCoFe0.5Mo0.1Nb0.68 EHEA with fcc phase and laves phase structure was designed.
Solidification process simulation accelerates composition design of EHEAs by quantitatively and visually determining eutectic and phase compositions of alloys. However, it cannot be used independently at present. Like the CALPHAD method, it is mostly used to verify the composition of known EHEAs alloy systems, and it still relies on past research results for newly developed alloys. Moreover, the obtained results also need to be fine-tuned due to the limitations of the thermodynamic database, as well as constraints such as the instability of the solidification process of the metal and the solid solubility of the metal elements.
4.5 Through Experimental Experience
In the composition design of EHEAs and to ensure their industrial applications, it is also necessary to consider saving materials cost and considering excellent performance. Approaches to designing new EHEAs using experience gained by adjusting elements of known alloys are also commonly used. Due to the high cost of Co, the engineering application of some EHEAs is greatly limited. Therefore, Jin et al. [32] proposed a cost-effective method to create more than 10 potential Co-free EHEAs. The main idea is to replace Cobalt with Nickel. Nickel promotes the formation of the fcc phase, while Aluminum promotes the formation of the bcc phase [45]. The intermetallic phase-forming elements were modified. Multiple experiments were carried out to locate the eutectic point. As a result, a CrFeNi2.2Al0.8 eutectic HEA was successfully designed, composed of fcc and ordered bcc (B2) phases. Similarly, Dong et al. [33] designed AlCrFeNi3 EHEAs with FeCrNi-type fcc and NiAl-type B2 phases, maintaining good mechanical properties while reducing the materials’ cost. In addition, Yin et al. [34] designed a new cost-effective Fe35Ni25Cr25Mo15 EHEA by avoiding the use of expensive Co and increasing the content of Fe. Jiao et al. [35] designed Fe2Ni2CrMoxEHEAs by equiatomic substitution while considering cost reduction and oxidation/corrosion resistance, showing a eutectic dual-phase structure when x > 0.25. Therefore, this method enables the design of specific alloy systems and is also instructive for the development of EHEA.
5 Summary
The above commonly used EHEAs design methods have their own scope of application and advantages and disadvantages in the process of actual component exploration, as shown in Table 2. In addition to the above design methods, researchers have begun to explore new design methods, mainly by optimizing and using the original design methods. Naishalkumar Shah et al. [37] developed an integrated computational materials engineering (ICME) framework for an EHEA consisting of seven components. The framework includes thermodynamic prediction using CALPHAD, phase field simulation for microstructure, and experimental validation. The predictions can be used for the design of HEA fabricated by various manufacturing processes. The alloy that was designed exhibits a eutectic structure comprising of fcc and laves phases. Based on ML methods and elemental classification results, Liu et al. [38] calculated and predicted the eutectic composition through CALPHAD, and obtained two new types of EHEA Ni49Co16Cr16Al19 and Ni46.7Co15Cr20Al18.3 through experimental verification. Ye et al. [36] proposed a new strategy for designing EHEAs with B2 (NiAl phase) and bcc structures using infinite solid solution, which mainly generate phases by analyzing binary phase diagrams, four with seaweed eutectic dendritic microstructures. Several EHEAs were designed, including NiAl–20V–17Cr, NiAl–30V–5Mo, NiAl–30Cr–Mo and NiAl–20V–10Cr–5Mo using similar methods.
Recently, Wu et al. [39] maintained the VEC of the alloy by substituting Ni and Fe in equal proportions for Co in AlCoCrFeNi2.1. A Co-free AlCrFe1.5Ni2.6 EHEA composed of L12 and B2 phases was designed according to this VEC criterion. According to the idea of a simple mixing method, Fang et al. [71] categorized the EHEA elements into two groups: A and B. Group A contains elements with similar atomic radii and chemical properties, resulting in a mixing enthalpy that is nearly zero, these elements have a tendency to form stable solid solutions [72]. On the contrary, the mixing enthalpy of group B elements is relatively negative, and the difference in atomic radius is relatively large, so it is easy to form a stable phase. Then, by finding the composition of the eutectic point in the binary phase diagram, a new type of cost-effective FeNi-based EHEAs was successfully proposed, and [FeNi]65Cr15Mn10Nb10 has a complete eutectic composition experimentally. Similarly, Li et al. [72] designed a new EHEA by replacing similar elements. Mainly based on a known EHEA, it causes lattice distortion through partial element substitution to form a new EHEA. The main idea is shown in Fig. 6.
Schematic diagram of new EHEA designed by partial similarity substitution method [72]
6 Conclusion and Outlook
In this paper, we review the factors influencing the phase formation thermodynamics of EHEAs and computationally verify their current guiding role. The alloy design strategies and research progress that have been reported in recent years are reviewed. In the process of designing the composition of EHEAs, in addition to considering the internal factors of thermodynamics and the solidification process, external factors such as its cost-effectiveness should also be considered. Therefore, in addition to maintaining its excellent performance in mechanical properties, it is also necessary to consider its cost control. The lack of a complete database and the amount of experimental data bring challenges to the composition design of EHEAs.
References
Yeh J W, Chen S K, Lin S J, Gan J Y, Chin T S, Shun T T, Tsau C H, and Chang S Y, Adv Eng Mater 6 (2004) 299. https://doi.org/10.1002/adem.200300567
Yeh J W, Ann Chim Sci Matér 31 (2006) 633. https://doi.org/10.3166/acsm.31.633-648
Ye Y F, Wang Q, Lu J, Liu C T, and Yang Y, Mater Today 19 (2016) 349. https://doi.org/10.1016/j.mattod.2015.11.026
Murty B S, Yeh J W, Ranganathan S, and Bhattacharjee P, High-Entropy Alloys. Elsevier, Amsterdam (2019).
Lu Y, Dong Y, Jiang H, Wang Z, Cao Z, Guo S, Wang T, Li T, and Liaw P K, Scr Mater 187 (2020) 202. https://doi.org/10.1016/j.scriptamat.2020.06.022
Zhang Z, Ma P, Fang Y, Yang Z, Zhang N, Prashanth K G, and Jia Y, J Alloys Compd 947 (2023) 169417. https://doi.org/10.1016/j.jallcom.2023.169417
Ma P, Fang Y, Wei S, Zhang Z, Yang H, Wan S, Prashanth K G, and Jia Y, J Mater Res Technol 25 (2023) 7090. https://doi.org/10.1016/j.jmrt.2023.07.124
Lu Y, Dong Y, Guo S, Jiang L, Kang H, Wang T, Wen B, Wang Z, Jie J, Cao Z, Ruan H, and Li T, Sci Rep 4 (2014) 6200. https://doi.org/10.1038/srep06200
Maity T, Prashanth K G, Balcı Ö, Kim J T, Schöberl T, Wang Z, and Eckert J, Int J Plast 109 (2018) 121. https://doi.org/10.1016/j.ijplas.2018.05.012
Maity T, Prashanth K G, Balçi Ö, Wang Z, Jia Y D, and Eckert J, Compos Part B Eng 150 (2018) 7. https://doi.org/10.1016/j.compositesb.2018.05.033
Glicksman M E, Principles of Solidification: an Introduction to Modern Casting and Crystal Growth Concepts, Springer Science & Business Media, New York (2010).
Chanda B, and Das J, J Alloys Compd 798 (2019) 167. https://doi.org/10.1016/j.jallcom.2019.05.241
Yang X, and Zhang Y, Mater Chem Phys 132 (2012) 233. https://doi.org/10.1016/j.matchemphys.2011.11.021
Zhang Y, Zhou Y J, Lin J P, Chen G L, and Liaw P K, Adv Eng Mater 10 (2008) 534.
Jin X, Zhou Y, Zhang L, Du X, and Li B, Mater Des 143 (2018) 49. https://doi.org/10.1016/j.matdes.2018.01.057
Jiang H, Han K, Gao X, Lu Y, Cao Z, Gao M C, Hawk J A, and Li T, Mater Des 142 (2018) 101. https://doi.org/10.1016/j.matdes.2018.01.025
Jiao W, Miao J, Lu Y, Chen X, Ren Z, Yin G, and Li T, J Alloys Compd 941 (2023) 168975. https://doi.org/10.1016/j.jallcom.2023.168975
Lu Y, Jiang H, Guo S, Wang T, Cao Z, and Li T, Intermetallics 91 (2017) 124. https://doi.org/10.1016/j.intermet.2017.09.001
Tan Y, Li J, Wang J, and Kou H, Intermetallics 85 (2017) 74. https://doi.org/10.1016/j.intermet.2017.02.004
He F, Wang Z, Cheng P, Wang Q, Li J, Dang Y, Wang J, and Liu C T, J Alloys Compd 656 (2016) 284. https://doi.org/10.1016/j.jallcom.2015.09.153
Rahul M R, and Phanikumar G, Metall Mater Trans A 50 (2019) 2594. https://doi.org/10.1007/s11661-019-05210-3
Wu M, Wang S, Huang H, Shu D, and Sun B, Mater Lett 262 (2020) 127175. https://doi.org/10.1016/j.matlet.2019.127175
Mukarram M, Mujahid M, and Yaqoob K, J Mater Res Technol 10 (2021) 1243. https://doi.org/10.1016/j.jmrt.2020.12.042
Vikram R J, Gupta K, and Suwas S, Scr Mater 202 (2021) 113993. https://doi.org/10.1016/j.scriptamat.2021.113993
Gasan H, and Ozcan A, Met Mater Int 26 (2020) 1152. https://doi.org/10.1007/s12540-019-00515-9
Ai C, He F, Guo M, Zhou J, Wang Z, Yuan Z, Guo Y, Liu Y, and Liu L, J Alloys Compd 735 (2018) 2653. https://doi.org/10.1016/j.jallcom.2017.12.015
Jain R, Dewangan S K, Kumar V, and Samal S, Mater Sci Eng A 797 (2020) 140059. https://doi.org/10.1016/j.msea.2020.140059
Wen X, Cui X, Jin G, Liu Y, Zhang Y, and Fang Y, Surf Coat Technol 405 (2021) 126728. https://doi.org/10.1016/j.surfcoat.2020.126728
Yurchenko N, Panina E, Zherebtsov S, and Stepanov N, Materialia 16 (2021) 101057. https://doi.org/10.1016/j.mtla.2021.101057
Ali Shafiei A, Met Mater Int 27 (2021) 127. https://doi.org/10.1007/s12540-020-00655-3
Wang L, Yao C, Shen J, Zhang Y, Liu G, Wu X, and Zhang G, Mater Sci Eng A 830 (2022) 142325. https://doi.org/10.1016/j.msea.2021.142325
Jin X, Bi J, Zhang L, Zhou Y, Du X, Liang Y, and Li B, J Alloys Compd 770 (2019) 655. https://doi.org/10.1016/j.jallcom.2018.08.176
Dong Y, Yao Z, Huang X, Du F, Li C, Chen A, Wu F, Cheng Y, and Zhang Z, J Alloys Compd 823 (2020) 153886. https://doi.org/10.1016/j.jallcom.2020.153886
Yin Y, Kent D, Tan Q, Bermingham M, and Zhang M-X, J Mater Sci Technol 51 (2020) 173. https://doi.org/10.1016/j.jmst.2020.01.066
Jiao W, Jiang H, Qiao D, He J, Zhao H, Lu Y, and Li T, Mater Chem Phys 260 (2021) 124175. https://doi.org/10.1016/j.matchemphys.2020.124175
Ye X, Xiong J, Wu X, Liu C, Xu D, Zhang W, Fang D, and Li B, Scr Mater 199 (2021) 113886. https://doi.org/10.1016/j.scriptamat.2021.113886
Shah N, Rahul M R, and Phanikumar G, Metall Mater Trans A 52 (2021) 1574. https://doi.org/10.1007/s11661-021-06218-4
Liu F, Xiao X, Huang L, Tan L, and Liu Y, Mater Today Commun 30 (2022) 103172. https://doi.org/10.1016/j.mtcomm.2022.103172
Wu H, Xie J, Yang H, Shu D, Hou G, Li J, Zhou Y, and Sun X, J Mater Res Technol 19 (2022) 1759. https://doi.org/10.1016/j.jmrt.2022.05.165
Liu Q, Liu X, Fan X, Li R, Tong X, Yu P, and Li G, J Alloys Compd 904 (2022) 163775. https://doi.org/10.1016/j.jallcom.2022.163775
Takeuchi A, and Inoue A, Mater Trans 46 (2005) 2817. https://doi.org/10.2320/matertrans.46.2817
He F, Wang Z, Ai C, Li J, Wang J, and Kai J J, Mater Chem Phys 221 (2019) 138. https://doi.org/10.1016/j.matchemphys.2018.09.044
Kim M J, Kang G C, Hong S H, Park H J, Mun S C, Song G, and Kim K B, J Mater Sci Technol 57 (2020) 131. https://doi.org/10.1016/j.jmst.2020.03.045
Kittel C, Introduction to Solid State Physics, 8th edn. Wiley, Hoboken (2005).
Guo S, Ng C, Lu J, and Liu C T, J Appl Phys 109 (2011) 103505. https://doi.org/10.1063/1.3587228
Chen X, Sui Y, Qi J, He Y, Wei F, Meng Q, and Sun Z, J Mater Res 32 (2017) 2109. https://doi.org/10.1557/jmr.2017.10
Jin X, Zhou Y, Zhang L, Du X, and Li B, Mater Lett 216 (2018) 144. https://doi.org/10.1016/j.matlet.2018.01.017
Wang M, Lu Y, Wang T, Zhang C, Cao Z, Li T, and Liaw P K, Scr Mater 204 (2021) 114132. https://doi.org/10.1016/j.scriptamat.2021.114132
Jiang H, Qiao D, Jiao W, Han K, Yiping L, and Liaw P K, J Mater Sci Technol 61 (2021) 119. https://doi.org/10.1016/j.jmst.2020.05.053
Wang M, Lu Y, Lan J, Wang T, Zhang C, Cao Z, Li T, and Liaw P K, Acta Mater 248 (2023) 118806. https://doi.org/10.1016/j.actamat.2023.118806
Gwalani B, Wang T, Jagetia A, Gangireddy S, Muskeri S, Mukherjee S, Lloyd J T, Banerjee R, and Mishra R S, Entropy 22 (2020) 431. https://doi.org/10.3390/e22040431
Dong Y, Jiang L, Jiang H, Lu Y, Wang T, and Li T, Mater Des 82 (2015) 91. https://doi.org/10.1016/j.matdes.2015.05.046
Yu Y, He F, Qiao Z, Wang Z, Liu W, and Yang J, J Alloys Compd 775 (2019) 1376. https://doi.org/10.1016/j.jallcom.2018.10.138
Jiang H, Qiao D, Lu Y, Ren Z, Cao Z, Wang T, and Li T, Scr Mater 165 (2019) 145. https://doi.org/10.1016/j.scriptamat.2019.02.035
Tillmann W, Wojarski L, Stangier L, Manka M, and Timmer C, Weld World 64 (2020) 1597. https://doi.org/10.1007/s40194-020-00944-w
Huo W, Zhou H, Fang F, Xie Z, and Jiang J, Mater Des 134 (2017) 226. https://doi.org/10.1016/j.matdes.2017.08.030
Huo W, Zhou H, Fang F, Zhou X, Xie Z, and Jiang J, J Alloys Compd 735 (2018) 897. https://doi.org/10.1016/j.jallcom.2017.11.075
Ai C, Wang G, Liu L, Guo M, He F, Zhou J, Chen Y, Wang Z, and Gan B, Intermetallics 120 (2020) 106769. https://doi.org/10.1016/j.intermet.2020.106769
Guo Y, Liu L, Zhang Y, Qi J, Wang B, Zhao Z, Shang J, and Xiang J, J Mater Res 33 (2018) 3258. https://doi.org/10.1557/jmr.2018.177
Jiang H, Zhang H, Huang T, Lu Y, Wang T, and Li T, Mater Des 109 (2016) 539. https://doi.org/10.1016/j.matdes.2016.07.113
Samal S, Rahul M R, Kottada R S, and Phanikumar G, Mater Sci Eng A 664 (2016) 227. https://doi.org/10.1016/j.msea.2016.04.006
Jain R, Umre P, Sabat R K, Kumar V, and Samal S, J Mater Eng Perform 31 (2022) 8124. https://doi.org/10.1007/s11665-022-06829-x
Jiang L, Lu Y, Wu W, Cao Z, and Li T, J Mater Sci Technol 32 (2016) 245. https://doi.org/10.1016/j.jmst.2015.08.006
Wu Q, Wang Z, Zheng T, Chen D, Yang Z, Li J, Kai J J, and Wang J, Mater Lett 253 (2019) 268. https://doi.org/10.1016/j.matlet.2019.06.067
Han L, Xu X, Li Z, Liu Z, Liu C T, and Liu Y, Mater Res Lett 8 (2020) 373. https://doi.org/10.1080/21663831.2020.1772395
Zhang Y, Lu Z P, Ma S G, Liaw P K, Tang Z, Cheng Y Q, and Gao M C, MRS Commun 4 (2014) 57. https://doi.org/10.1557/mrc.2014.11
Ding Z, He Q, and Yang Y, Sci China Technol Sci 61 (2018) 159. https://doi.org/10.1007/s11431-017-9051-6
Xie T, Xiong Z, Xu Z, and Cheng X, Mater Sci Eng A 786 (2020) 139420. https://doi.org/10.1016/j.msea.2020.139420
Xie T, Mater Sci 802 (2021) 140634.
Mukarram M, Munir M A, Mujahid M, and Yaqoob K, Metals 11 (2021) 1484.
Fang D, Wu X, Xu W, Yu L, Liu M, Zhang A, Li B, and Ye X, Mater Sci Eng A 870 (2023) 144919. https://doi.org/10.1016/j.msea.2023.144919
Li J H, and Tsai M H, Scr Mater 188 (2020) 80. https://doi.org/10.1016/j.scriptamat.2020.06.064
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wan, S., Ma, P., Yang, H. et al. Research Progress on Composition Design of Multicomponent Eutectic High Entropy Alloys. Trans Indian Inst Met 77, 1455–1465 (2024). https://doi.org/10.1007/s12666-023-03247-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-023-03247-y