Abstract
Magnesium metal matrix composites are now been used in large number of structural applications. An attempt is made to synthesise pure Mg and Mg/5 wt% MgO (~ 10 µm) composite through powder metallurgy route assisted by rapid microwave sintering. The microwave sintering temperatures are varied at 450 °C, 500 °C and 550 °C, and different mechanical and physical characterisations of the composites are studied. The composites show improved properties with the addition of magnesium oxide as reinforcement. XRD investigations show that no reaction has taken place between magnesium and magnesium oxide at all sintering temperatures. At higher sintering temperature, few magnesium particles oxidise to form MgO. Microstructure shows well-distributed MgO particles in magnesium matrix. The addition of 5 wt% hard MgO particles improve hardness and increase 0.2% compressive yield strength (CYS) and ultimate compressive strength (UCS) than pure magnesium at all sintering temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Magnesium is one of the lightest metals used in different structural applications. It is sixth most abundant element in earth’s crust and requires less energy than aluminium for its production[1]. Other than the low density, it has high damping capacity, fairly good electromagnetic shielding and is easily machinable. However, low strength and ductility, poor creep and wear resistance, high temperature instability and corrosion limit its application. In the past two decades, new magnesium alloys and composites have circumvented their limitations to a large extent [1, 2]. This led to widespread use of magnesium and its alloys in the field of aerospace, automotive, sports, electronics, biomedical fields [1, 3,4,5,6,7]. Reinforcing magnesium with metal and ceramics particles has allowed researchers to improve mechanical, wear and high temperature properties considerably [2, 8, 9].
A wide range of oxides, carbides, borides, nitrides, metals, etc., have been used as particulate reinforcements in magnesium or its alloy matrix [1]. The study on magnesium or its alloy reinforced with MgO is limited though MgO has high hardness, high temperature stability, and good corrosion resistance. Liquid-based techniques have been mainly used for fabricating Mg/MgO composites. Goh et al. successfully fabricated Mg/MgO nanocomposites through disintegrated melt deposition technique and reported improved thermal stability, microhardness, yield strength and modulus compared to conventional Mg/SiC composites [10]. Rogal and Dobrzynska fabricated in situ AZ91 Mg alloy/MgO nanocomposite by reacting CO2 with AZ91 alloy at 595 °C using thixomolding technology. The composites showed hardness of 103 ± 2 HV, compressive yield strength of 220 MPa, and compressive strength of 460 MPa [11]. Xuecheng Cai et al. synthesised an Mg/MgO nanocomposite by in situ reaction of magnesium with oxygen and then high-pressure consolidation of cryomilled powders at 6 GPa. The composites showed significant improvement of high temperature hardness and compressive strength [12]. Very few or no study of ex situ Mg/MgO composite processed through powder metallurgy assisted by microwave sintering has been reported in the literature. Fabrication of ex situ Mg/MgO composites through powder metallurgy route is challenging due to hygroscopic nature of MgO. The fine MgO powder adsorbs a layer of water vapour which leads to agglomeration of MgO powders while mixing with matrix powder. It also creates problems during sintering stage as vapour layers vaporise due to heat which increases porosity and may develop cracks. All these problems are minimised in liquid fabrication routes as preheated MgO powders are added to molten magnesium.
Therefore, an attempt is made in the present study to synthesise pure magnesium and Mg- 5(wt%) MgO composite through powder metallurgy route. MgO reinforcement particles are selected for their excellent mechanical and thermal properties. Rapid microwave sintering of both pure Mg and Mg/MgO composite is done at varying sintering temperature of 450 °C, 500 °C and 550 °C to optimise the sintering temperature. Post-sintering observations have been reported. Microstructural and phase analysis of pure magnesium and Mg/5MgO with varying sintering temperature is investigated. Hardness and compressive behaviour with sintering temperature variation are also studied.
2 Experimental Procedure
Elemental magnesium powder of more than 99% purity (supplied by Alfa Aesar) having APS ≅ 40 µm was used as matrix material, and magnesium oxide powder (supplied by Alfa Aesar) of APS ≅ 10 µm was used as reinforcing phase.
Pure magnesium and Mg/5wt % MgO composites were fabricated through powder metallurgy technique. The Mg and MgO powders were weighed and mixed in a steel jar on a roller mixer at 80 rpm for 3 h. No balls were used during mixing of powders. About 1% stearic acid was added to reduce the agglomeration of powders particles to each other and to the wall of the container. Ball milling of powders was not preferred as powders may get oxidised due to increase in temperature inside the container.
The mixed powders were carefully collected and immediately cold compacted by using 20-tonne manual hand-operated hydraulic press. Compaction was done at 450 MPa to obtain a (ϕ12 × 10) mm cylindrical green compacts. Graphite was used as a die-wall lubricant. The green compacts of both pure magnesium and Mg/5wt. %MgO were sintered at 450 °C, 500 °C, and 550 °C for 25 min with a heating rate of 10 °C/min in a microwave furnace (Make—Nano Tec, Chennai, India 4.4KW, 2.45 GHz). Two directional rapid microwave sintering was done in protective atmosphere of argon.
2.1 Density and Porosity
Archimedes principle was used to measure the density of samples. The liquid medium used was ethanol (ρ = 0.789 g/cc) having purity of 99.9%. Five samples of each composition were measured to get average density. The theoretical density was measured using rule of mixture. Then, porosity was estimated according to the following formula[13].
2.2 Phase Identification and Analysis
Phase identification of samples was done using X-ray diffractometer (Made – Rigaku Corp. Model: Rigaku Smart Lab 9 kW Powder type) by exposing them to Cu Kα (λ = 1.540 Å). The phases were identified by matching the obtained Bragg angles value and interplanar spacing d, with the standard data of magnesium and magnesium oxide. Further, relative intensity method was used for quantitative analysis of different phases as given by Eq. (2) [14, 15]. The relative intensity fractions of MgO were calculated based on Mg (0 1 1), MgO (0 0 2) planes in pure Mg and Mg/MgO composites.
IMgO = Intensity of MgO phase and IMg = Intensity of Mg phase.
2.3 Microstructural Examination
Samples for microstructural analysis were prepared by standard polishing technique and etched with acetic picral solution. The samples were observed on optical microscope, and grain size was measured using line intercept method. Samples were also observed on scanning electron microscope (CARL ZEISS MICROSCOPY LTD, Model: EVO—Scanning Electron Microscope MA15 / 18) coupled with energy dispersive spectroscopy (EDS).
2.4 Mechanical Properties
Vickers microhardness test was done on Vickers microhardness tester (Micro Mech Technologies, Pune, India) at room temperature at 25 g load and 15 s dwell time on a flat and polished specimen according to ASTM standard E384 – 08. Five readings were taken for each sample. Room temperature compression test was performed on a Universal Testing Machine (100 kN, INSTRON 5982) with a strain rate of 0.5 mm/min in accordance with ASTM test standard E9-89a. Three samples of diameter 10 mm and length-to-diameter ratio (l/d) ~ 1 were tested for each composition.
3 Results and Discussion
Pure Mg and Mg/5wt. %MgO composites were successfully fabricated, and following post-processing observations are shown in Table 1.
Pure Mg and Mg/MgO composite was successfully synthesised through powder metallurgy route. It is observed that pure Mg samples at all temperatures shows no visible cracks. However, thin oxide layer appears in pure Mg samples at 550 °C. In case of composites sample, no visible cracks or oxide layer is observed at 450 °C. However, as the temperature increases to 500 °C, the composite shows thin oxide layer and very fine cracks. As the temperature is further increased to 550 °C, the composite gets burned completely. The composite could not be successfully made at 550 °C in spite of several attempts. The appearance of cracks in composite sample is due to large mismatch of coefficients of thermal expansion of Mg and MgO particles. Moreover, high heating rate can be achieved using microwaves as powder compacts absorb the microwaves and gets heated from centre towards surface [16]. They also reported in another work that above 350 °C, centre of compacts shows higher temperature than its surface due to absorption of microwave energy by compacts[17]. This lead to high thermal stress near Mg-MgO interface. As the temperature is increased from 450 °C to 500 °C, thermal stresses become pronounced and fine cracks appear. As temperature is further increased to 550 °C, the cracks become thick and samples are oxidised completely.
3.1 Density Measurement
Density measurement results of pure Mg and Mg/MgO composites are shown in Table 1. The density of both pure Mg and Mg/5MgO composites increases with increase in sintering temperature. The wettability of matrix surrounding the reinforcement particle increases due to increase in sintering temperature. This facilitates better powder flow through diffusion, and hence, neck formation among particles occurs easily. This lead to denser product [18]. In addition, denser MgO (ρ = 3.54 g/cc) reinforcements further increases density. However, porosity increases in composites with increase in sintering temperatures. This is because at 500 °C, some Mg particles oxidise to form MgO. So, the amount of matrix surrounding the reinforcements relatively decreases which reduces wettability. This decreases powder flow through diffusion. Furthermore, increase in MgO particles usually increases clustering and agglomeration which increases the porosity in composites [19].
3.2 Phase Identification and Analysis
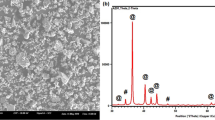
XRD patterns of pure Mg and Mg/5MgO composites sintered at different sintering temperatures can be seen in Fig. 1. It is observed that Mg and MgO peaks are only dominant peaks in the patterns suggesting that no other reaction has taken place during sintering at all temperatures. However, as the sintering temperature is increased, the intensity of diffraction peaks of MgO phase increases in all pure magnesium and Mg/5MgO samples. This indicates that some of the Mg particles oxidise to form MgO phase. The oxygen contained in green preforms or that adsorbed on MgO surface can be the source of oxygen. Stearic acid addition is another source of oxygen, but it can be ignored as its content is very less.
Table 1 shows the volume fraction of MgO phase formed at different sintering temperatures. It can be observed that as the temperature is increased more MgO phase is formed in both pure Mg and Mg/MgO composites. Pure Mg sample at 550 °C sintering temperature shows 8.2% MgO phase formation and Mg/5MgO at 500 °C shows 10.6% MgO phase formation indicating the threshold microwave sintering temperature for both the samples.
3.3 Microstructure
The optical micrograph and SEM micrographs of pure Mg and Mg/MgO composites at different temperatures are shown in Figs. 2, 3, 4, respectively. Grain size calculation from Fig. 2 is shown in Table 1. It is observed that grain refinement of Mg matrix occurs due to addition of MgO reinforcements. Figure 4 shows that smaller MgO particles get settled on grain boundary causing pinning effect of hard particles which leads to refinement of grains. It is also observed that few larger MgO particles are settled near to interface which are responsible for further strengthening. Figure 3 shows uniform distribution of MgO reinforcements in the Mg matrix in composites samples of 450 °C and 500 °C. Agglomeration of MgO particles is also observed at few places. Porosity is seen in all the samples. However, porosity is more in composite samples than the pure Mg samples due to the presence MgO particles. Though porosity is inherent defect in almost all powder metallurgy samples, but here the adsorption of water vapour in MgO surface further contributes to the generation of porosity.
EDS mapping of Mg/5MgO composites at 450 °C and 500 °C is shown in Figs. 5 and 6, respectively. Peaks of Mg and O are clearly observed. The formation of large MgO phase in composites can be seen in EDS map. The concentration of oxygen surrounding the magnesium grains can also be confirmed in EDS area mapping.
3.4 Microhardness
The microhardness measurement of pure Mg and Mg/5MgO composite at different sintering temperatures is shown in Fig. 7. The addition of MgO particles improves the hardness value of composites by ~ 19% compared to pure magnesium. High hardness value is due to the presence of hard MgO particles which restricts the local deformation of Mg matrix during indentation [20, 21]. The reduction in grain size also contributes to increased hardness in composites. However, the increase in hardness value is less as sintering temperature is increased in both pure Mg and Mg/MgO composites.
3.5 Compressive Properties
The room temperature compression test results of pure magnesium and Mg/5MgO composites fabricated at different sintering temperatures are shown in Table 2. From stress–strain diagram (Fig. 8), it is observed that the gain in strength of composites with addition of 5% MgO reinforcement is coupled with compromise in failure strain. Due to high compressive strength of magnesium oxide, there is ~ 16% increase in strength with addition of only 5% MgO. Load transfer from matrix to distributed hard reinforcements has always increased strength in composites. Uniform distribution of MgO particles and good bonding between Mg and MgO particles as seen from microstructure facilitate the load-bearing strength. This is consistent with previous works[11]. Hall–Petch effect due to grain size reduction in both pure Mg and Mg/MgO composites also increases the compressive yield strength at all sintering temperatures. Elastic modulus mismatch and coefficient of thermal expansion mismatch of Mg and MgO lead to generation of dislocation which play a pivotal role in strengthening. Orowan strengthening is not dominant in micron particle-reinforced composites. Increase in sintering temperature shows increased strength slightly in pure Mg samples and more in composites samples due to grain refinement effect on addition of MgO particles. The reduction in fracture strain in composites is mainly due to the presence of ceramic particles and porosity in samples.
4 Conclusions
The following conclusions may be drawn from the above results and discussions:
-
1.
Pure magnesium and Mg/MgO composites were successfully fabricated by powder metallurgy route assisted by rapid microwave sintering.
-
2.
The pure magnesium samples and Mg/MgO composites samples showed increased MgO phase formation at 550 °C and 500 °C temperature, respectively.
-
3.
Pure magnesium and Mg/MgO composites were nearly 97% and 96% dense, respectively. No reaction took place between Mg and MgO at all sintering temperatures as observed in XRD analysis.
-
4.
MgO was evenly distributed in Mg matrix as seen in microstructure. Agglomeration of MgO particles was also observed at a few places. The presence of hard MgO particles led to grain size reduction thereby strengthening the composites.
-
5.
Mg/MgO composites showed improved hardness, 0.2% CYS and UCS over pure magnesium at all sintering temperature. However, ductility was low in the composites than pure magnesium.
References
Gupta M, and Ling S N M, Magnesium, Magnesium Alloys, and Magnesium Composites, Wiley (2011).
Ye H Z, and Liu X Y, J Mater Sci 39 (2004) 6153.
Kainer K U, and Mordike B L, Magnesium Alloys and Their Applications, Wiley-VCH Weinheim (2000).
Landkof B, Magnesium Alloys and their Applications (2000) 168.
Monteiro W, Buso S, and Silva L, New Features Magn Alloys (2012).
Mathaudhu S, Luo A, Neelameggham N, Nyberg E, and Sillekens W, Essential Readings in Magnesium Technology, Springer (2016).
Zhou H, Liang B, Jiang H, Deng Z, and Yu K, J Magn Alloys 9 (2021) 779.
Gupta M, and Wong W, Mater Charact 105 (2015) 30.
Lloyd D, Int Mater Rev 39 (1994) 1.
Goh C S, Gupta M, Wei J, and Lee L C, J Compos Mater 41 (2007) 2325.
Rogal L, and Litynska-Dobrzynska L, Mater Sci Technol 35 (2019) 349.
Cai X, Xin S, Sun B, Cui H, Yu H, Peng Q, and Shen T, J Mater Sci 53 (2018) 6613.
Wang H Y, Jiang Q C, Wang Y, Ma B X, and Zhao F, Mater Lett 58 (2004) 3509.
Yue N, Lu L, and Lai M, Compos Struct 47(1999) 691.
Jiang Q C, Wang H Y, Ma B X, Wang Y, and Zhao F, J Alloys Compd 386 (2005) 177.
Wong W L E, and Gupta M, Adv Eng Mater 9 (2007) 902.
Wong W, and Gupta M, Technologies 3 (2015) 1.
Tosun G, and Kurt M, Compos Part B: Eng 174 (2019) 106965.
Baghchesara M A, and Abdizadeh H, J Mech Sci Technol 26 (2012) 367.
Aravindan S, Rao P, and Ponappa K, J Magn Alloys 3 (2015) 52.
Aydin F, Sun Y, Ahlatci H, and Turen Y, Trans Indian Inst Met 71 (2018) 873.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shakil, A., Gautam, R.K. & Rao, U.S. Characterisation of Rapid Microwave-Sintered Mg/MgO Composite. Trans Indian Inst Met 76, 749–756 (2023). https://doi.org/10.1007/s12666-022-02772-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-022-02772-6