Abstract
The objective of this study is to analyse the anti-corrosion characteristics of NiCrMoFeNbTa powder particles cladded on the Inconel 625 alloy. The microstructure and hardness of the NiCrMoFeNbTa cladded layer were evaluated by means of FESEM and nanoindentation techniques. At various time intervals (0, 18, 42 and 70 h), the anti-corrosion behaviour was investigated on IN 625 substrate and cladded specimens. In addition, the average surface roughness (Ra) value was obtained for the substrate and clad specimens to interrogate their resistant behaviour. The FESEM results proved the presence of cellular structure with interdendritic phases in the cladded region, and these phases improved the hardness at the clad layer compared to the substrate. The passive film formed on the NiCrMoFeNbTa cladded layer was reported to be compact and stable which resisted corrosion to a greater extent than the IN 625 substrate samples. On comparing the substrate and cladded samples, the unexposed clad specimen provided maximum corrosion resistance in terms of lower current density, higher polarization resistance and lower surface roughness values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Surface engineering has changed the traditional ways of repairing the surface of the components. It involves many techniques that can rebuild or treat damaged surfaces by depositing the materials on the surfaces. This deposited film will enhance the mechanical characteristics, thereby aids in improving the durability of components. These methods eliminate the necessity for the new parts since the new parts are very expensive and thus can save money and time. Also, these methods are more preferred in applications employed in hostile environments [1,2,3,4]. Some of the widely used traditional techniques are thermal spraying, chemical vapour deposition (CVD), electrodeposition, physical vapour deposition (PVD), hardfacing, etc. These conventional techniques have their limitations. The controllability and repeatability of the electro and vapour deposition process are tricky [5, 6]. On the other hand, the thermal spray process can have a better bonding behaviour and repeatability compared with the deposition process. But this process is not effective for smaller and curved substrates due to the low degree of adhesion. In some applications, because of the heat-affected zone (HAZ), a series of problems arise after long-term usage [7]. The hardfacing technique involves repairing worn-out surfaces by recreating them and thereby improving the working life. However, the distortion effect produced by the high-temperature arc greatly limits the usage of this technique [8]. The drawbacks of the traditional techniques have been overcome by the laser surface treatments.
Laser surface treatments (LST) involve a high-energy laser that melts the substrate’s surface. LST can be applied to materials with any geometry, being able to produce excellent bonding behaviour with the substrates, rapid, eco-friendly, highly efficient and capable of creating coatings with high precision [9,10,11,12]. These advantages have paved the way for implementing LST to improve the tribological properties of components that are employed in severe aggressive environments [13, 14]. Among the various methods of LST, the laser cladding process is mostly preferred for treating worn-out surfaces with greater accuracy. The coating material is directly deposited on the metal pool at a uniform rate, thereby producing an even film of the coated layer. It can create effective thick coatings and is majorly employed in repairing the components of nuclear reactors, turbine blades, boiler tubes, pressurized water reactors, steam turbines, etc., which operate in harsh environments [15,16,17].
Nickel-based superalloys are designed for high-temperature applications since these alloys resist heat even near to their melting point. It is a combination of nickel with chromium, molybdenum, iron and other refractory elements which makes it resistant to high temperatures. Considering the design aspects, these alloys exhibit better values of thermal conductivity, low density, elastic modulus and are cost effective [18,19,20]. NiCrMoFeNbTa alloy (Inconel 625) is a nickel-based superalloy that is obtained due to the solid solution strengthening effect of Mo and Nb in the Ni–Cr matrix. In addition, these alloys exhibit better fatigue strength, higher hardness, greater tensile properties and ductility, and provide resistance against local corrosion attacks in intrusive environments [21, 22]. The adequate composition of Mo (~ 10%) resists crevice and pitting type of corrosion [23]. These features made the IN 625 alloy get well adapted for a wide range of applications in furnace hardware, chemical plants, space shuttle engines, heat exchangers, control rods, gas turbines, seawater equipments, etc. [24]. Surface treatment using the laser cladding process will be a better option for improving the anti-corrosion behaviour and other mechanical properties of IN 625 components. The choice of the coating material is important as it is required to sustain in those environments and also it has to aid in improving durability. Since higher Cr content provides stable passive film during corrosion, the substrate material is preferred as the cladding material (IN 625).
D. Virdi et al. [25] analysed the hardness of Inconel 625 coating produced using laser cladding. Tai-Cheng Chen et al. [26] studied the wear behaviour of thermally sprayed IN 625 films and reported that the formed coating was very stable and provided better wear resistance compared with the carbon steel. Z. Liu et al. [27] and H.Y.Al-Fadhli et al. [28] examined the corrosion and erosion-corrosion behaviour of High Velocity Oxygen Fuel (HVOF) sprayed IN 625 coating and reported that IN 625 exhibits better resistance towards erosion and corrosion than the base samples. Tai-Cheng Chen et al. [29] compared the tribological behaviour of IN 625 coatings and found that the IN 625 coatings experienced reduced wear rate, low coefficient of friction, better hardness and corrosion-resistant property. Thus, from the literature, it is evident that IN 625 will be an appropriate choice of cladding material for improving the tribological properties of the critical components. This study is aimed to evaluate the anti-corrosion of the Inconel 625 coating on the IN 625 substrate. The microstructure and hardness of the IN 625 coatings were investigated. At various time periods (0, 18, 42 and 70 h), the anti-corrosion behaviour was investigated on the IN 625 and cladded specimens in an electrochemical workstation for interrogating the resistance they offered against corrosion. After the corrosion analysis, the corroded morphology of the specimens was analysed with the aid of FESEM and EDS analysis. Moreover, the polarization resistance values were compared to further evaluate the resistance offered. Surface roughness was evaluated on the substrate and clad samples in response to the corrosion resistance. The results of all analyses have been compared, presented and the values tabulated.
2 Experimentation
2.1 Materials and Laser Cladding
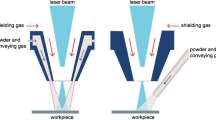
Figure 1a represents the morphology of the as-received NiCrMoFeNbTa (IN 625) powder particles. From this figure, the average size of the IN 625 alloy particles is found to be 100 ± 25 µm. The elemental composition of the IN 625 material is tabulated in Table 1. Inconel 625 is selected as the substrate material. IN 625 substrate’s surface was rinsed by cleaning agents like acetone and then sandblasted. The sandblasting process was performed on the rinsed samples. The surface roughness of the sand-blasted samples was found to be ~ 6 µm. After these pre-processing steps, the cladding process was executed as depicted in Fig. 1b. The process parameters like gas flow rate (shield and carrier gas), laser power, scanning speed, powder feed rate, etc. will decide the cladding powder distribution and the thickness of the cladded layer. The optimized process parameter used during cladding is tabulated in Table 2 [30]. This laser beam heated the surface, thereby creating the molten metal pool [31]. The NiCrMoFeNbTa alloy particles reached this pool via a coaxial feeder system. These particles got settled in the metal pool which tended to the formation of a finite layer of IN 625 particles on IN 625 substrate [32]. The cladded samples were collected, and some post-processing procedures were carried out for investigating the morphology of the clad layer. The chemical etching process was performed on the diamond polished cladded surface to evaluate the microstructure at the cladded layer.
2.2 Corrosion Test
After the cladding process, the substrate and clad samples underwent corrosion tests at 0, 18, 42 and 70 h to evaluate the resistance they offered against corrosion. The corrosion test was performed in an electrochemical workstation. This workstation consisted of a three-electrode assembly namely the Pt, AgCl and the working electrode (sample). The electrolytic solution was prepared by dissolving 3.5% wt of sodium chloride crystals in 100 ml of DI water. This solution was the same as the chloride content in ocean water and was able to induce severe corrosion attacks on the surface [33]. As per the ASTM G44-99 standards, the test samples were prepared and pre-processed before the corrosion test. First, the test samples were cleaned with acetone to remove the surface dust and with the help of SiC sheets, the rinsed samples were abraded and again cleaned with acetone for performing the corrosion test. The substrate and clad samples were immersed in the NaCl solution for the total duration of 70 h, and the corrosion test was performed at 0, 18, 42 and 70 h. After stabilizing the potential and changing the scan rate, the corrosion process was performed on the samples. The Tafel plots were obtained for the IN 625 substrate and clad samples and the corresponding current density and electrode potential values were tabulated. The corroded morphology of the samples was analysed to reveal the passive film formation along with the corresponding elemental mapping using the EDS spectra. Moreover, impedance analysis was also performed to further interrogate the sample’s corrosion resistance. The polarization resistance values were calculated using the equilibrium circuit, tabulated and compared. The surface roughness of the samples before and after the corrosion test was evaluated by means of images captured using the Scanning Probe Microscope (SPM) and laser microscope.
3 Results and Discussion
3.1 Microstructural Examination
Figure 2a represents the optical microscopic image captured at the cladded layer. The captured image shows that the NiCrMoFeNbTa alloy was coated evenly on the IN 625 substrate and the clad layer is free from voids and other defects. This fine deposition is because of the optimized process parameters during the cladding process [30, 34]. In addition, the optical micrographs show that the cladding layer consists of cellular dendritic microstructures. Figure 2b shows that the cladded layer is said to be rich in cellular/dendritic structures with interdendritic phases. These phases are distributed in the γ-nickel matrix [35]. This fine formation of cellular microstructure in the cladded region is due to the rapid solidification effect after the cladding process [36]. The magnified view of Fig. 2b is shown in Fig. 2c. From this, it is evident that there is a segregation of interdendritic secondary phases in the γ-nickel matrix. Since the NiCrMoFeNbTa alloy contains notable percentages of niobium and molybdenum, during solidification, the γ phase (dendritic) is formed first, followed by the precipitation of interdendritic phases, due to the effect of niobium and molybdenum. Mostly, these secondary phases are rich in carbides of Nb, Cr and Mo throughout their microstructure [37,38,39]. Figure 2d shows the point scan image (inset) used for EDS analysis along with the elemental graph obtained at the cladded layer. From this elemental graph, it is found that the highest peak is obtained for nickel followed by chromium and other elements. Since, the substrate and the cladding material are nickel-based superalloys which constitute for higher elemental peaks for the nickel element. The Nb and Mo elemental peaks are observed in this graph which proves the existence of secondary interdendritic phases. Moreover, the elemental concentrations (wt%) of Ni, Cr, Mo, Nb, and Fe were measured and the values are 69.27, 18.85, 6.40, 3.73 and 1.75, respectively.
3.2 Nanohardness Evaluation
The material with greater hardness is reported to provide better wear resistance [40, 41]. A constant load of 1500 μN was applied by the diamond probe followed by the unloading process, leaving the plastic deformation [42]. Figure 3a represents the optical microscopic image used for the nanoindentation test. It shows the substrate, interface and cladded regions after the cladding process. In Fig. 3b, the indentation depth is plotted against the applied load. In general, the indentation depth is in inverse relationship with the hardness offered. Greater indentation depth will result in lower hardness [43]. From Fig. 3b, it can be inferred that the substrate has a greater value of indentation depth (~ 83 nm) compared to the values obtained in the interface (~ 60 nm) and the cladded (~ 41 nm) layer. The depth value at the clad layer is one-half of the substrate’s indentation depth value. The nanohardness value of the substrate, interface and the cladded layer are 2.57, 3.02 and 4.39 GPa, respectively. On comparing the hardness values, the cladded layer exhibits higher hardness than the interface and the substrate layers due to its lower indentation depth. The hardness value obtained in the clad layer is nearly twice the substrate’s hardness value. This is because of the precipitation of Nb- and Cr-rich carbides as the secondary phases in the cladded region [44,45,46]. These precipitates are responsible for improving the hardness at the cladded layer.
3.3 Corrosion Test
The major application of IN 625 alloy is in hostile environments experiencing severe corrosion attacks. The fuel tanks of the biomass reactors are reported to contain precipitates of chloride and sulphide. These precipitates readily react with the IN 625 alloy resulting in the initiation of corrosion attacks, thereby reducing the corrosion-resistant behaviour [47]. In the case of supercritical boilers, pitting corrosion was observed on the Inconel 625 surface due to the salt deposits which may turn into corrosive acids after long-term use [48]. Mostly at elevated temperatures, the chromium present in the IN 625 alloy is oxidized to dichromate which can readily react with the formed passive film and thus minimize the resistance [49]. The reaction that accounts for the corrosion in Inconel 625 alloy is represented in Eqs. (1–7) [50].
The polarization curves obtained from the corrosion test of the substrate and the cladded specimens were expressed in terms of the Tafel plot as in Fig. 4a and b. The current density (I) is plotted against the electrode potential (V) and the current remains constant even after increasing the potential. No active to passive transitions have been reported, which proves that there is a continuity in the formation of oxide film on the samples. The measured V and I value for the substrate and clad samples at 0, 18, 42 and 70 h are tabulated in Table 3. In general, lower the value of I, higher the corrosion resistance offered [51, 52]. From this table, among the IN 625 base samples, the 70 h sample has the minimum value of I (2.363 × 10–7 A/cm2). An increasing trend is observed in the I value of 0, 42, and 18 h substrate samples, respectively. Hence, the oxide layer developed on the 70 h sample is said to be stable and provides better corrosion resistance. The clad samples have been reported to exhibit lower current densities and potential values than the IN 625 base samples. The precipitation of the interdendritic phases in the γ-Ni matrix accounts for the compact and stable passive film formation at the clad surface. These precipitates resist corrosion to a better extent, thereby improving the corrosion resistance property. On comparing the current density values from Table 3, the unexposed clad sample has the lowest value (6.145 × 10–8 A/cm2) and offers greater corrosion resistance than the other cladded and substrate samples.
The corroded morphologies and the elemental graphs of the IN 625 substrate samples at different intervals are shown in Fig. 5a–h. From the FESEM image of the substrate samples, the oxide film formed on the 70 h sample is more stable than the films of other substrate samples. The other sample’s passive film is greatly affected by the Cl− ions present in the test solution which react with the film and form pits during the corrosion process. Figure 6a–h represents the FESEM images and EDS maps of the clad samples at 0, 18, 42, and 70 h, respectively. It can be seen that all the cladded samples have better film morphology due to the existence of cellular structures containing interdendritic carbide phases distributed in the γ-Ni matrix. These precipitates will withstand the corrosion attacks forming a strong passive film. Among the cladded samples, the unexposed clad sample has compact and stable film formation and thus offers maximum resistance against corrosion than other cladded and substrate samples. The elemental compositions obtained from the elemental graphs of the substrate and cladded samples are listed in Table 4. Figure 7 depicts the passive film formation on the NiCrMoFeNbTa clad layer.
Mechanism of passivation layer formation a NiCrMoFeNbTa clad layer, b Reaction of Ni, Cr ions in the clad layer with the O ions during the corrosion process, c Formation of stable passive film on the clad layer due to the effect of secondary phase and d As time increases, the fragile patches start to leave the surface
3.4 Electrochemical Impedance Spectroscopy Analysis
The polarization curves show that the cladded samples have better anti-corrosion behaviour due to their low I values. To further confirm these results, impedance analysis was carried out and the results were plotted as the Nyquist and Bode plots, respectively. The Nyquist and Bode plot gives information about the real and imaginary components of impedance and the phase shift within the applied frequency range [53, 54]. Figure 8a and c represents the Nyquist plot of the substrate and clad samples at various time intervals. These plots represent a capacitive loop which proves that the electrochemical reaction is dominant during the EIS analysis [55]. Generally, the semicircular arc radius of the Nyquist plot determines the resistance against corrosion. Greater arc radius results in maximum corrosion resistance [56]. From Fig. 8a, the 70 h substrate sample has the maximum semicircular arc radius. Other substrate samples experience a decreasing trend in their arc radius. Hence, the 70 h substrate sample offers maximum resistance to corrosion among the substrate samples. On the other hand, the cladded samples exhibit a higher arc radius (Fig. 8c) than the base samples. This is due to the formation of a strong passive layer containing carbide precipitates. Particularly, the unexposed cladded sample provides significant resistance against corrosion since this sample has a maximum arc radius than all samples.
The Bode plots will evaluate the resistance characteristic via the area covered by the curves of this plot. A larger covered area results in better anti-corrosion behaviour [57]. Figure 8b represents the Bode plots of the substrate samples. It can be seen that the 70 h sample has the maximum area covered than the 0, 18 and 42 h samples. Thus, the 70 h sample provides better resistance towards corrosion. On the other hand, all the cladded samples have a better area covered than their respective substrate samples (Fig. 8d). In particular, the unexposed clad sample has a wider area coverage and offers maximum resistance against corrosion than other cladded and substrate samples. In addition, with the aid of an equilibrium circuit [Inset in Fig. 8d], the polarization resistance value (Rp) was calculated because higher Rp values result in greater corrosion resistance [58]. Table 5 lists the equilibrium circuit parameters obtained for the substrate and clad samples. Higher Rp values were noticed in the clad samples than the IN 625 substrates. Notably, the unexposed cladded sample has the maximum Rp value (185,900) and provides better anti-corrosion behaviour during the corrosion process. Thus, the electrochemical studies show the existence of the larger semicircular arc radius (Nyquist plot), wider area coverage (Bode plot) and greater Rp values in the clad samples thereby proves that the cladding process providing better resistance against corrosion.
3.5 Surface Roughness Examination
Surface roughness is a way of measuring the irregularities present on the surface which influences the wear resistance and corrosion rate because rough surfaces have many irregularities (interfacial sites) that are prone to corrosion attacks and reduce their resistance [59, 60]. Figure 9a–d shows the surface plot and the 2D images captured at the substrate and cladded region before performing the corrosion test. The average surface roughness value (Ra) in these regions is obtained from these images. The base region experiences a surface roughness of 29.3 nm, whereas the surface roughness at the cladded region has been found to be 68.3 nm. The cladded surface has the highest value because of the deposition of NiCrMoFeNbTa alloy particles. On comparing the 2D images of base and cladded samples, it is evident that the alloy particles distribution has increased the surface roughness in the cladded sample. Figure 10a–d shows the 3D images captured from the substrate samples at various time intervals after the corrosion test. These figures show that the surface irregularities present in the 70 h sample are lower than that of 0, 42 and 18 h samples. In the case of clad samples, their surface seems to be rougher (Fig. 11a–d) due to the presence of precipitates of Nd- and Cr-rich carbides. In addition, the average surface roughness value (Ra) has been calculated for the substrate and clad samples and is listed in Table 6. The lower surface roughness value results in greater resistance towards corrosion [61]. From this table, the roughness value increases as the sample’s exposure time increases (18 and 42 h) and then decreases (70 h). This is due to the stableness of passive film at 18 and 42 h intervals of the substrate and clad samples. At higher intervals (70 h), there will be fragile oxide patches on the film which pave the way for a reduction in the surface roughness value. And also from Table 6, it is seen that the 70 h substrate and the unexposed clad samples have the lowest surface roughness values (2.908 µm and 6.678 µm) and offer maximum resistance against corrosion than the other substrate and cladded samples. And also, all the cladded samples have higher surface roughness than the substrate. This is due to the precipitation of interdendritic secondary phases in the cladded region. Hence, the cladded samples offer greater corrosion resistance with increased surface roughness values.
4 Conclusion
In this work, the laser cladding of NiCrMoFeNbTa alloy particles on the IN 625 substrate was performed. The characteristics of cladded samples like corrosion resistance, hardness, surface roughness and the cladded microstructure were analysed and the results are summarized as given as follows:
The optical and FESEM images captured at the clad layer show the presence of cellular/dendritic structure with interdendritic secondary phases that are distributed in the γ-nickel matrix. Moreover, the secondary phases are rich in the carbides of niobium and chromium which alter the mechanical properties like hardness and surface roughness. The cladded layer experiences a higher hardness (4.39 GPa) than the interface and substrate region. The cladded samples have better passive film, and in particular, the unexposed clad sample provides maximum resistance as it has the minimum current density value. In addition, the cladded samples have a comparatively higher Rp and Ra value and provide better corrosion resistance than the substrate samples. Thus, laser cladding of NiCrMoFeNbTa alloy particles shows improved corrosion resistance and hardness property. Hence, it is well suited for improving the working life of the components in hostile environments.
Data Availability
The experimental datasets obtained from this research work and then the analysed results during the current study are available from the corresponding author on reasonable request.
References
Dong H, editor, Surface Engineering of Light Alloys: Aluminium, Magnesium and Titanium Alloys.
Holmberg K and Matthews A, Coatings Tribology: Properties, Mechanisms, Techniques and Applications in Surface Engineering. Elsevier, (2009).
Davis JR, editor. Surface Engineering for Corrosion and Wear Resistance, ASM international (2001).
Zhecheva A, Sha W, Malinov S, and Long A, Surf Coat Technol 200 (2005) 2192.
Mehran Q M, Fazal M A, Bushroa A R, and Rubaiee S, Crit Rev Solid State Mater Sci 43 (2018) 158.
Carlsson J O, and Martin P M, in Handbook of Deposition Technologies for films and coatings. William Andrew Publishing (2010), p. 314. https://doi.org/10.1016/B978-0-8155-2031-3.00007-7
Siddiqui A A, and Dubey A K, Opt Laser Technol. 134 (2021) 106619.
Ahn D G, Int J Precis Eng Manuf 14 (2013) 1271.
Earl C, Castrejón-Pita J R, Hilton P A, and O’Neill W, J Manuf Process 21 (2016) 214.
Singh A, and Harimkar S P, JOM 64 (2012) 716.
Mao B, Siddaiah A, Liao Y, and Menezes P L, J Manuf Process 53 (2020) 153.
Kusiński J, Kac S, Kopia A, Radziszewska A, Rozmus-Górnikowska M, Major B, Major L, Marczak J, and Lisiecki A, Tech Sci 60 (2012) 711.
Wahab J A, Ghazali M J, Yusoff W M, and Sajuri Z, Trans IMF 94 (2016) 193.
Ferreira M G, and Li R, in Organic and Inorganic Coatings for Corrosion Prevention–Research and Experiences, CRC Press (2019) p 194.
Liu Y, Ding Y, Yang L, Sun R, Zhang T, and Yang X, J Manuf Proces 66 (2021) 341.
Zhong M, and Liu W, Proc Inst Mech Eng Part C: J Mech Eng Sci 224 (2010) 1041.
Jeyaprakash N, and Yang C H. Mater Manuf Process 35 (2020) 1383.
Darolia R, Int Mater Rev 64 (2019) 355.
Pollock TM, and Tin S, J Propuls Power 22 (2006) 361.
Prakash F P, Jeyaprakash N, Duraiselvam M, Prabu G, and Yang C H, Surf Coat Technol 397 (2020) 126055.
Leary M, Mazur M, Williams H, Yang E, Alghamdi A, Lozanovski B, Zhang X, Shidid D, Farahbod-Sternahl L, Witt G, and Kelbassa I, Mater Des 157 (2018) 179.
Lotfi M, Jahanbakhsh M, and Farid A A, Tribol Int 99 (2016) 107.
Abioye T E, McCartney D G, and Clare A T, J Mater Process Technol 217 (2015) 232.
Abioye T E, Folkes J, and Clare A T, J Mater Process Technol 213 (2013) 2145.
Verdi D, Garrido M A, Múnez C J, and Poza P, Mater Sci Eng A 598 (2014) 15.
Chen T C, Chou C C, Yung T Y, Tsai K C, and Huang J Y, Surf Coat Technol 303 (2016) 78.
Liu Z, Cabrero J, Niang S, and Al-Taha Z Y, Surf Coat Technol 201 (2007) 7149.
Al-Fadhli H Y, Stokes J, Hashmi M S, and Yilbas B S, Surf Coat Technol 200 (2006) 5782.
Chen T C, Chou C C, Yung T Y, Cai R F, Huang J Y, and Yang Y C, Surf Coat Technol 385 (2020) 125442.
Jeyaprakash N, Yang C H, and Ramkumar K R, Appl Phys A 126 (2020) 1.
Norhafzan B, Khairil C M, and Aqida S N, in IOP Conference Series: Materials Science and Engineering Vol. 1078, No. 1. IOP Publishing (2021), p 012037.
Geng Y, Konovalov S V, and Chen X, Usp Fiz Met 21 (2020) 26.
Kwok C T, Cheng F T, and Man H C, Mater Sci Eng A 290 (2000) 145.
Fesharaki M N, Shoja-Razavi R, Mansouri H A, and Jamali H, Surf Coat Technol 353 (2018) 25.
Xu X, Mi G, Chen L, Xiong L, Jiang P, Shao X, and Wang C, J Alloys Compd 715 (2017) 362.
Rombouts M, Maes G, Mertens M, and Hendrix W, J Laser Appl 24 (2012) 052007.
Solecka M, Kopia A, Radziszewska A, and Rutkowski B, J Alloys Compd 751 (2018) 86.
Tian Y, Ouyang B, Gontcharov A, Gauvin R, Lowden P, and Brochu M, J Alloys Compd 694 (2017) 429.
Patel S J, and Smith G D, in International Symposium on Niobium 2001 (2001), p 1081.
Jeyaprakash N, Yang C H, and Sivasankaran S, Mater Manuf Process 35 (2020) 142.
Konyashin I, Ries B, Hlawatschek D, Zhuk Y, Mazilkin A, Straumal B, Dorn F, and Park D, Int J Refract Met Hard Mater 49 (2015) 203.
Pharr G M, and Bolshakov A, J Mater Res 17 (2002) 2660.
Hu H X, Guo X M, and Zheng Y G, Wear 428 (2019) 246.
Badiger R I, Narendranath S, and Srinath M S, J Manuf Process 18 (2015) 117.
Mithilesh P, Varun D, Reddy A R, Ramkumar K D, Arivazhagan N, and Narayanan S, Proc Eng 75 (2014) 66.
Cozar R, and Pineau A, Metallurg Trans 4 (1973) 47.
Fantozzi D, Matikainen V, Uusitalo M, Koivuluoto H, and Vuoristo P, Surf Coat Technol 318 (2017) 233.
Kim H, Mitton D, and Latanision R M, in Corrosion 2010. OnePetro (2010).
Kritzer P, Boukis N, and Dinjus E. Corrosion 54 (1998) 824.
Schmuki P, Virtanen S, Davenport A J, and Vitus C M, J Electrochem Soc 143 (1996) 3997.
Ezhilselvi V, Nithin J, Balaraju J N, and Subramanian S, Surf Coat Technol 288 (2016) 221.
Sidane D, Chicot D, Yala S, Ziani S, Khireddine H, Iost A, and Decoopman X, Thin Solid Films 593 (2015) 71.
Yin Z F, Zhao W Z, Lai W Y, and Zhao X H, Corros Sci 51 (2009) 1702.
Amudha A, Nagaraja H S, and Shashikala H D, in AIP Conference Proceedings Vol. 2247, No. 1. AIP Publishing LLC (2020), p 040012.
Guo L, Zheng H, Liu S, Li Y, Feng C, and Xu X, Int J Electrochem Sci 11 (2016) 5507.
Natarajan J, Yang C H, and Karuppasamy S S, Materials 14 (2021) 6183.
Ahn S H, Choi Y S, Kim J G, and Han J G, Surf Coat Technol 150 (2002) 319.
Chira M, Hegyi A, Vermeşan H, Szilagyi H, and Lăzărescu A, Proc Manuf 46 (2020) 4.
Hagen C M, Hognestad A, Knudsen O Ø, and Sørby K, Prog Organ Coat 130 (2019) 17.
Walter R, and Kannan M B, Mater Des 32 (2011) 2350.
Jeyaprakash N, Yang C H, and Karuppasamy S S. Surf Rev Lett (2022).
Acknowledgements
The authors would like to acknowledge the Ministry of Science and Technology, Taiwan, for granting the funds for the successful completion of this research work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jeyaprakash, N., Yang, CH., Susila, P. et al. Laser Cladding of NiCrMoFeNbTa Particles on Inconel 625 Alloy: Microstructure and Corrosion Resistance. Trans Indian Inst Met 76, 599–612 (2023). https://doi.org/10.1007/s12666-022-02701-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-022-02701-7