Abstract
The effect of Zn or Zn + Cu addition on the precipitation in Al–Mg–Si alloys is summarized and analyzed by analyzing DSC curves, aging strengthening curves, and precipitates, which is very helpful for Al–Mg–Si alloys industrial application. With Zn addition, the hardness of Al–Mg–Si alloys increased, and the peak aging time might shorten or increase, which might be related to the Mg/Si atomic ratio. With Zn + Cu addition, the effects on the age-hardening curves of Al–Mg–Si alloys depended on the Zn + Cu content and aging system. With Zn addition, the precipitation of Al–Mg–Si alloy changed, such as the appearance of η′ and the composition and structure of β″ and β′. With Zn + Cu addition, the precipitation of Al–Mg–Si alloys changed, such as the composition of Mg–Si phases and the activation energy of β″. Moreover, items that require attention in future investigations were pointed out, including activation energy, location of Zn and Cu atoms in precipitation, etc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Al–Mg–Si alloys are widely used in the aerospace, automobile manufacturing and construction industries [1,1,2,3]. Al–Mg–Si alloys can be strengthened by heat treatment. During the aging process, many precipitates appear in the alloy, and the mechanical properties of the alloy increase. A systematic and in-depth understanding of the aging precipitation process and precipitates of Al–Mg–Si alloys is very helpful for their industrial application. Moreover, it is a common method to improve the properties of Al–Mg–Si alloys and broaden their industrial application by adding other microalloying elements (such as Zn, Cu and Ag) to change the original precipitate phase types, number density, morphology and size. Zn and Cu are very common microalloying elements, but there is no review of the effect of Zn or Zn + Cu addition on the precipitation in Al–Mg–Si alloys. A review about the effect of Zn or Zn + Cu addition on the precipitation in Al–Mg–Si alloys is essential for studying the effect systematically and deeply in future. Meanwhile, there is always a certain amount of Zn and Cu in many waste Al. Zn and Cu are important elements commonly accumulated during recycling in the industry. The review is beneficial to the recovery and utilization of Zn and Cu. Hence, referring to the literature of past 20 years, this work summarizes and analyzes the effects of Zn or Zn + Cu on the precipitation of Al–Mg–Si alloys by analyzing DSC curves, age-strengthening curves and precipitates.
2 Precipitates in Al–Mg–Si Alloys
Referring to the literature in the past 20 years, the precipitates in Al–Mg–Si alloys are summarized in Tables 1 and 2. As shown in Table 1, with different Mg/Si atomic ratios, the precipitate sequence is different. As shown in Table 2, with different Mg/Si atomic ratios or different aging systems, the space group, lattice parameter, composition, etc., of the same precipitate might be different. Hence, it is necessary to study the relationship among Mg content, Si content, Mg/Si atomic ratio and precipitates in Al–Mg–Si alloy.
3 Effects of Zn Addition
3.1 Introduction
To achieve better properties and more applications of Al–Mg–Si alloys, adding Zn to the alloy is a common method in research and industrial applications. Many studies have shown that with the addition of Zn, the precipitation of Al–Mg–Si alloys changes. Hence, in this section, the changes in precipitation of Al–Mg–Si alloys with added Zn are summarized and analyzed. We hope that this part promotes the industrial application of Zn in Al–Mg–Si alloys and becomes a reference for future research.
3.2 Changes in the Precipitation of Al–Mg–Si Alloys with Added Zn
The DSC curves of Al–Mg–Si alloys are shown in Fig. 1 with various Mg/Si ratios and different Zn contents [27]. The DSC curves indicate that the precipitate sequence of the present Al–Mg–Si alloy does not change with the addition of Zn. With a low Mg/Si ratio and excess Si, the transformation of GP zones into β″ is promoted, and the formation temperature of β″ decreases. The results indicate that the effects of Zn on the precipitation are related to Mg/Si ratios.
DSC curves of the Al–Mg–Si–Zn alloy with different Mg/Si ratios [27]
An overview of the effects of Zn on age-hardening curves of Al–Mg–Si alloys is shown in Figs. 2 and 3 [28, 29], and the effects are as follows:
-
1.
Hardness With the addition of Zn, regardless of the Mg/Si ratio, the hardness of the Al–Mg–Si alloys increases during the whole aging process.
-
2.
Peak aging time With the addition of Zn, the peak aging time of the Al–Mg–Si alloys get shortened (as shown in Fig. 2b). This result means that the transformation of GP zones into β″ is promoted, which is consistent with the result in Fig. 1. However, other results show that with the addition of Zn, the peak aging time of Al–Mg–Si alloys increases (as shown in Fig. 3). The different results of Figs. 2 and 3 may be due to the different Mg/Si ratios, which needs further study.
Age-hardening curves at different temperatures: a 150 °C and b 190 °C [28]
Age-hardening curves at 180 °C for different times [29]
The related studies about the effects of Zn on the type, number, composition and structure of precipitates in the Al–Mg–Si alloys are summarized in Table 3. As shown in Table 3, the effects of Zn on precipitation are summarized as follows:
(1) Precipitation sequence Some studies have shown that with the addition of Zn, only Mg–Si phases (such as β″) appear in Al–Mg–Si alloys. Other studies show that with the addition of Zn, Mg–Si phases and Mg–Zn phases (such as η′) appear at the same time. These results show that with the addition of Zn, the precipitate sequence of the Al–Mg–Si alloy may change, and the changes depend on the Zn content. When the Zn content is high, the Mg–Si phases and Mg–Zn phases appear at the same time, and the precipitation sequence of the Al–Mg–Si alloys change.
(2) Changes in the Mg–Si phases (such as β″ and β′) With the addition of Zn, the number density of precipitates in the Al–Mg–Si alloys increases, which means that the hardness of the Al–Mg–Si alloys with the addition of Zn is higher (as shown in Figs. 2 and 3). The composition and structure of the β″ and β′ phases change because the Zn atoms enter into the phases.
(3) It is worth noting that the effects of Zn on each precipitate in the same sample may be different. A study shows that with the addition of Zn, most precipitates have disordered cross sections, but some β″ with no disordered cross sections still exist.
3.3 Items that Require Attention in Future Investigations
-
1.
Activation energy DSC curves are always used to study the activation energy [38]. As shown in Fig. 1, the changes in the activation energy of Al–Mg–Si alloys with added Zn have not been studied. However, the results of DSC curves are easily affected by other factors, such as the sample composition, heating rate, sample size and instrument model. When comparing the results of different studies quantitatively, the conclusions may not be accurate. First-principles calculations are also always used to study the activation energy [39]. However, the number of precipitates from first-principles calculations is limited, and the experimental conditions that can be considered are limited, which means that the results of first-principles calculations cannot always reflect the actual experimental process. Hence, we think that an improved way to investigate the activation energy is to combine DSC curves and first-principles calculations.
-
2.
Disordered cross sections of precipitates As shown in Fig. 4, with the addition of Zn, precipitates with disordered cross sections appear because Zn atoms enter into the phases. The whole aging process includes an under aging stage, a peak aging stage and an overaging stage, and the precipitates of the Al–Mg–Si alloys are different at different aging stages. Whether the effect mechanism of Zn on the structural changes of precipitates is the same at different aging stages needs further study. Moreover, the reasons why the effects of Zn on each precipitate in the same sample may be different also need further study.
-
3.
Mg–Zn precipitates and Mg–Si precipitates As shown in Table 3, whether Mg–Si phases and Mg–Zn phases appear at the same time depends on the Zn content, and when the Zn content is high, Mg–Si phases and Mg–Zn phases appear at the same time. With the addition of Zn, the appearance of the dual-phase strengthening effects of Zn can be fully exerted in the Al–Mg–Si alloys. Mg–Zn precipitates appear in the Al–Mg–Zn alloy, and the aging temperature is approximately 120 °C [28, 30]. In the Al–Mg–Si alloy, Mg–Si precipitates appear, and the aging temperature is higher than 120 °C. Therefore, the Mg–Zn precipitates and the Mg–Si precipitates do not appear at the same time in all studied Al–Mg–Si–Zn alloys. In addition to increasing the Zn content, we also need to develop a new aging system for Al–Mg–Si alloys with added Zn to facilitate Mg–Zn precipitates and Mg–Si precipitates. Moreover, the interaction between Mg–Zn precipitates and Mg–Si precipitates during the aging process also needs further study.
-
4.
Alloys with a high Si content and very low Mg/Si ratio Due to good casting properties and excellent mechanical properties [40–42], alloys with a high Si content and very low Mg/Si ratio are very important for industrial application in the aerospace and automobile manufacturing industries, such as A357 (Mg: 0.5–0.7 wt%; Si: 6.5–7.5 wt%; Mg/Si atomic ratio ≈ 0.1:1). With different Mg/Si ratios and Si contents, the effects of Zn on the precipitates of Al–Mg–Si alloys are different (as shown in Figs. 1 and 2). Hence, with the addition of Zn, the precipitation changes in the Al–Mg–Si alloys with a high Si content and very low Mg/Si ratio need further study. Moreover, an excess addition of Zn is harmful to the casting properties of an alloy [43], including its fluidity and hot cracking resistance, which needs to be considered before use in industrial applications.
-
5.
The reasons why the effects of Zn on peak aging time are different in different studies also need further study (as shown in Figs. 1 and 2).
Disordered cross sections of precipitates: a FFT filtered image and b suggested atomic overlay [[30]]
4 Effects of the Addition of Zn + Cu
4.1 Introduction
Like Zn, Cu is also a widely used microalloying element in Al–Mg–Si alloys. The changes in the precipitation of Al–Mg–Si alloys with added Cu have been summarized and analyzed in our other paper. Some research results showed that with the addition of Cu, the precipitation sequence of the Al–Mg–Si alloy changed, which was SSS → atomic clusters → GP zones → β″, L, QP, QC → β′ and Q′ → Q [44–46]. In this section, the changes in precipitation of the Al–Mg–Si alloys with added Zn + Cu are summarized and analyzed. We hope that this part promotes the industrial application of Zn + Cu in Al–Mg–Si alloys and becomes a reference for future research.
4.2 Effects of Zn + Cu on Precipitation Behaviors
Figures 5 and 6 show the DSC curves of Al–Mg–Si alloys with added Zn + Cu. Compared with the Al–Mg–Si alloy with added Cu, the precipitation sequence of Al–Mg–Si alloys with added Zn + Cu has not changed. As shown in Fig. 5, compared with the Al–Mg–Si alloy with added Cu, the GP zones of the Al–Mg–Si alloy with added Zn + Cu appear at a higher temperature; GP, β″, β′ (Q′), and Q appear at a lower temperature; and the β-phase appears at the same temperature. These results mean that with added Zn, the activation energy of GP zones increase; the activation energy of GP, β″, β′ (Q′) and Q decrease; and the activation energy of β is the same. These results are the same as those in reference 47. As shown in Fig. 6a [49], the activation energy E for GP zone dissolution in the Zn-free alloy is 87.8 kJ/mol, the E in the 0.5Zn alloy is 90.4 kJ/mol, the E in the 1.5Zn alloy is 79.7 kJ/mol, and the E in the 3.0Zn alloy is 109.4 kJ/mol. The effects are different with different Zn contents. Comparing Fig. 6a and b, which show the different Zn contents and aging systems, respectively, the effects on the precipitates of Al–Mg–Si alloys are different.
DSC curves of the as-quenched alloy [48]
DSC curves of the alloy: a natural aged alloy and b preaged alloy [49]
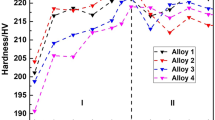
An overview of the effects of Zn + Cu on age-hardening curves of Al–Mg–Si alloys is shown in Figs. 7 and 8 [49, 50], and the effects are as follows:
-
1.
Hardness As shown in Fig. 7, with the addition of Zn + Cu, the hardness of Al–Mg–Si alloys increase during the whole aging process. The result is the same as those in references 41 and 52. As shown in Fig. 8, the hardness of the Al–Mg–Si alloys with added Cu is not necessarily higher with increasing Zn content.
-
2.
Peak aging time As shown in Fig. 7, with the addition of Zn + Cu, the peak aging time of Al–Mg–Si alloys does not change. As shown in Fig. 8, the peak aging time of Al–Mg–Si alloys may shorten. These different results may be due to the different Zn contents or different aging systems.
Evolution of Vickers hardness during aging process [50]
Evolution of Vickers hardness during aging process [49]
Hence, with different Zn contents or different aging systems, the effects on the age-hardening curves of Al–Mg–Si alloys are different during the whole aging process.
The related studies about the effects of Zn + Cu on the type, number, composition and structure of precipitates in Al–Mg–Si alloys are summarized in Table 4. As shown in Table 4, the effects of Zn on the precipitates are summarized as follows:
-
1.
Precipitation sequence Some studies have shown that with the addition of Zn, only Mg–Si phases appear in Al–Mg–Si–Cu alloys. Other studies showed that with the addition of Zn + Cu, Mg–Si phases, Al–Cu phases (such as Q) and Mg–Zn phases (such as η′) appeared at the same time. These results show that with the addition of Zn + Cu, the precipitate sequence of the Al–Mg–Si alloy may change, which depends on the Zn content, Cu content, aging system and Mg/Si atomic ratio.
-
2.
Mg–Si phases (such as GP zones and β″) The number density of GP zones decrease, and the number density of β″ increases. These results are the same as the DSC curves (as shown in Fig. 5). The Cu and Zn atoms are partitioned into clusters, and the precipitate composition changes, but the structure of the Mg–Si precipitates does not change.
-
3.
Al–Cu phases (such as Q) Zn become enriched in the Q grain boundary precipitates, and the Zn/Cu ratio in the Q phase is different with different Zn and Cu contents.
4.3 Items that Require Attention in Future Investigations
(1) Zn content, Cu content and Zn/Cu ratio With different Zn/Cu ratios and different Zn and Cu contents, the effects on the precipitation behaviors of Al–Mg–Si alloys are different. In most previous studies, the Zn content was the main variable. There is a lack of research on the variation in Cu content and Zn/Cu ratio. Hence, the relationship among the Cu content, Zn content, Zn/Cu ratio and the precipitation behaviors of Al–Mg–Si alloys needs further study. Moreover, in this kind of research, we should pay attention to the influence of the different aging systems on the experimental results.
(2) Mg–Si precipitates, Mg–Zn precipitates and Al–Cu precipitates: With the addition of Zn + Cu, Mg–Si precipitates, Mg–Zn precipitates and Al–Cu precipitates may appear in the Al–Mg–Si alloys, which depend on the Zn content, Cu content, aging system and Mg/Si atomic ratio. Three-phase strengthening is the best way to improve the strength of Al–Mg–Si alloys. However, the optimum precipitation temperatures of the Mg–Si strengthening phases, Mg–Zn strengthening phases and Al–Cu strengthening phases are different. In the existing studies, with the addition of Zn + Cu, the aging system is the same as the original system. Therefore, the Mg–Si strengthening phases, Mg–Zn strengthening phases and Al–Cu strengthening phases can not completely precipitate, and the strengthening efficiency of Zn + Cu is not fully utilized. Hence, it is imperative to design a new aging system for Al–Mg–Si alloys with Zn + Cu, such as a two-stage aging system and a multistage aging system. Additionally, the interaction between Mg–Si precipitates, Mg–Zn precipitates and Al–Cu precipitates during the aging process also needs further study. Moreover, the main strengthening phases with different contents of Mg, Si, Zn and Cu and different heat treatments should be more clearly described in future.
(3) Location of the Zn and Cu atoms in Al–Mg–Si alloys: With the addition of Zn + Cu, the Cu and Zn atoms may be partitioned into clusters in the Al–Mg–Si alloys. In Al–Mg–Si alloys with added Zn + Cu, the location of the Zn and Cu atoms (such as substituting Mg atoms or Si atoms) and Zn/Cu ratio in Mg–Si precipitates, the location of Si and Cu atoms (such as substituting Mg atoms or Zn atoms) and Cu/Si ratio in Mg–Zn precipitates and the location of Mg and Zn atoms (such as substituting Cu atoms) and Zn/Mg ratio in Al–Cu precipitates need further study. It is worth noting that Mg–Si precipitates, Mg–Zn precipitates and Al–Cu precipitates change with aging, and Mg–Si precipitates, Mg–Zn precipitates and Al–Cu precipitates are different at different aging stages. The results of Weng et al. showed that during aging, Ag atoms underwent the following process: segregation at the GP zone/α-Al and β″/α-Al interfaces → incorporation into the interior of β″ → release into the α-Al matrix [63]. Hence, further study is needed to see whether the location of the Zn and Cu atoms in Al–Mg–Si alloys would change.
(4) The reasons for the different results in some studies need to be further studied, such as hardness and peak aging time.
5 Conclusion
This work summarizes and analyzes the effects of Zn or Zn + Cu on the precipitation of Al–Mg–Si alloys by analyzing DSC curves, age-strengthening curves, and precipitates.
-
1.
With the addition of Zn, the hardness of Al–Mg–Si alloy increased, and the peak aging time might shorten or increase, which might be related to Mg/Si atomic ratios. With the addition of Zn + Cu, the effects on age-hardening curves of Al–Mg–Si alloys depended on the Zn + Cu content and aging system.
-
2.
With the addition of Zn, the precipitation of Al–Mg–Si alloy changed, such as the appearance of η′, the composition and structure of β″ and β′ phase. With the addition of Zn + Cu, the precipitation of Al–Mg–Si alloy changed, such as the composition of Mg–Si phases and the activation energy of β″.
Moreover, items that require attention in future investigations were pointed out. With the addition of Zn, items that require attention in future investigations in Al–Mg–Si alloy include activation energy, disordered cross sections of precipitates, Mg–Zn precipitates and Mg–Si precipitates, alloy with high Si content and very low Mg/Si ratio. With the addition of Zn + Cu, items that require attention in future investigations in Al–Mg–Si alloy include Zn content, Cu content and Zn/Cu ratio, Mg–Si precipitates, Mg–Zn precipitates and Al–Cu precipitates, location of Zn and Cu atoms in Al–Mg–Si alloys, etc.
References
Guo H, Yue X, Hot Working Technol, 45 (2016) 139. (in Chinese)
Mao G, Yan H, Zhu C, Wu Z, and Gao W, J Alloys Compd 806 (2019) 909.
Zhang X M, Zhou Z L, Tang J G, Bin K, Hu J L, J Mater Eng 12 (2013) 49.
Matsuda K, Sakaguchi Y, Miyata Y, Uetani Y, Sato T, Kamio A, and Ikeno S, J Mater Sci 35 (2000) 179.
Fang X, Song M, Li K, and Du Y, J Min Metall B 46 (2010) 171.
Edwards G A, Stiller K, Dunlop G L, Acta Mater 46 (1998) 3893.
Marioara C D, Andersen S J, Jansen J, and Zandbergen H W, Acta Mater 49 (2001) 321.
Miao W F, Laughlin D E. Scr Mater 40 (1999) 873.
Matsuda K, Ikeno S, Terayama K, Matsui H, Sato T, and Uetani Y, Metall Mater Trans A Phys Metall Mater Sci 36 (2005) 2007.
Matsuda K, Gamada H, Fujii K, Uetani Y, Sato T, and Kamio A, Metall Mater Trans A Phys Metall Mater Sci 29 (1998) 1161.
Lynch J P, Brown L M, and Jacobs M H, Acta Mater 30 (1982) 1389.
Maruyama N, Uemori R, Hashimoto N, Saga M, and Kikuchi M, Scr Mater 36 (1997) 89.
Andersen S J, Zandbergen H W, Jansen J, TrÆholt C, Tundal U, Reiso O, Acta Mater 46 (1998) 3283.
Hasting H S, Froseth A Q, Andersen S J, Vissers R, Walmsley J C, Marioara C D, Danoix F, Lefebvre W, Holmestad R. J Appl Phys 106 (2009) 691.
Zhao D, Zhou L, Kong Y, Wang A, Wang J, Peng Y, Du Y, Ouyang Y, and Zhang W. J Mater Sci 46 (2011) 7839.
Ramachandran S, Jung K, Narayan J, and Conrad H, Mater Sci Eng A 435 (2006) 693.
Ehlers F J H, Comput Mater Sci 81 (2014) 617.
Ninive P H, LVvik O M, and Strandlie A, Metall Mater Trans A Phys Metall Mater Sci 45 (2014) 2916.
Ninive P H, Strandlie A, Gulbrandsen-Dahl S, Lefebvre W, Marioara C D, Andersen S J, Friis J, Holmestad R, and Løvvik O M, Acta Mater 69 (2014) 126.
Wenner S, and Holmestad R, Scr Mater 118 (2016) 5.
Cayron C, and Buffat P A, Acta Mater 48 (2000) 2639.
Andersen S J, Zandbergen H W, Jansen J, Tráhol C, Tundal U, and Reiso O, Acta Mater 46 (2007) 3283.
Matsuda K, Tada S, Ikeno S, Sato T, and Kamio A, Scr Mater 32 (1995) 1175.
Frøseth A G, Høier R, Derlet P M, Andersen S J, Marioara C D, Phys Rev B 67 (2003) 224106.
Andersen S J, Marioara C D, Vissers R, Frøseth A, and Zandbergen H W, Mater Sci Eng A 444 (2007) 157.
Chen H, Lu J, Kong Y, Li K, Yang T, Meingast A, Yang M, Lu Q, and Du Y, Acta Mater 185 (2020) 193.
Li Y, Gao G, Wang Z, Di H, Li J, Xu G. Materials 11 (2018) 2591.
Chi S, Deng Y, Xu X, and Guo X, Materials 13 (2020) 650.
Jiao N N, Lai Y X, Chen S L, Gao P, and Chen J H, J Mater Sci Technol 70 (2021) 105.
Saito T, Wenner S, Osmundsen E, Marioara C D, Andersen S J, Røyset J, Lefebvre W, and Holmestad R, Philos Mag 94 (2014) 2410.
Saito T, Ehlers F J H, Lefebvre W, Hernandez-Maldonado D, Bjørge R, Marioara C D, Andersen S J, and Holmestad R, Acta Mater 78 (2014) 245.
Ding X P, Cui H, Zhang J X, Li H X, Guo M X, Lin Z, Zhuang L Z, and Zhang J S, Mater Des 65 (2015) 1229.
Yang W, Liu L, Zhang J, and Ji S, Mater Sci Eng A 682 (2017) 85.
Li L, Ji S, Zhu Q, Wang Y, Dong X, Yang W, Midson S, and Kang Y, Metall Mater Trans A Phys Metall Mater Sci 49 (2018) 3247.
Trudonoshyn O, Rehm S, Randelzhofer P, and Körner С, Mater Charact 158 (2019) 109959.
Yang W, Shen W, Zhang R, Cao K, Zhang J, and Liu L, Mater Charact 169 (2020) 110579.
Trudonoshyn O, Randelzhofer P, and Körner C. J Alloys Compd 872 (2021) 159692.
Kim J H, Daniel Marioara C, Holmestad R, Kobayashi E, and Sato T, Mater Sci Eng A 560 (2013) 154.
Hirosawa S, Nakamura F, and Sato T, Mater Sci Forum 561–565 (2007) 283.
Xu C, Xiao W, Zheng R, Hanada S, Yamagata H, and Ma C, Mater Des 88 (2015) 485.
Strihavkova E, Weiss V, and Michna S, Metallurgist 56 (2013) 708.
Mao F, Yan G, Xuan Z, Cao Z, and Wang T, J Alloys Compd 650 (2015) 896.
Kim J M, Seong K D, Jun J H, Kim K T, Jung W J. J Korea Foundry Soc 24 (2004) 138 (in Korean)
Ding L, Jia Z, Liu Y, Weng Y, and Liu Q, J Alloys Compd 688 (2016) 362.
Xiao Q, Liu H, Yi D, Yin D, Chen Y, Zhang Y, and Wang B, J Alloys Compd 695 (2017) 1005.
Cayron C, Buffat P A, Mater Sci Forum 331–337 (2000) 1001.
Bo Y, Mingxing G, Wu Y, Zhang J, Zhuang L, and Lavernia E J, J Alloys Compd 797 (2019) 26.
Guo M X, Zhang X K, Zhang J S, and Zhuang L Z, J Mater Sci 52 (2017) 1390.
Guo M X, Zhang Y, Zhang X K, Zhang J S, and Zhuang L Z, Mater Sci Eng A 669 (2016) 20.
Zhu S, Li Z, Yan L, Li X, Huang S, Yan H, Zhang Y, and Xiong B, Mater Sci Technol (United Kingdom) 35 (2019) 1291.
Zhang M, Wang J, Han J, Sui H, Huang H, Jin K, and Qian F, Calphad Comput Coupling Phase Diagrams Thermochem 67 (2019) 101684.
Zhu S, Li Z, Yan L, Li X, Huang S, Yan H, Zhang Y, and Xiong B, Mater Charact 145 (2018) 258.
Yan L, Zhang Y, Li X, Li Z, Wang F, Liu H, and Xiong B, Prog. Nat. Sci. Mater. Int. 24 (2014) 97.
Biswas A, Siegel D J, and Seidman D N, Acta Mater 75 (2014) 322.
Guo M X, Sha G, Cao L Y, Liu W Q, Zhang J S, and Zhuang L Z, Mater Chem Phys 162 (2015) 15.
Yan L, Li Z, An Zhang Y, Xiong B, Li X, Liu H, Huang S, and Yan H, Prog Nat Sci Mater Int 26 (2016) 398.
Zhu S, Li Z H, Yan L Z, Li X W, Huang S H, Yan H W, Zhang Y A, Xiong B Q, Mater Sci Forum 941 (2018) 961.
Lutz A, Malet L, Dille J, de Almeida L H, Lapeire L, Verbeken K, Godet S, Terryn H, and De Graeve I, J Alloys Compd 794 (2019) 435.
Guo M X, Du J Q, Zheng C H, Zhang J S, and Zhuang L Z, J Alloys Compd 778 (2019) 256.
Zhu S, Li Z, Yan L, Li X, Huang S, Yan H, Zhang Y, and Xiong B, J Alloys Compd 773 (2019) 496.
Guo M X, Li G J, Zhang Y D, Sha G, Zhang J S, Zhuang L Z, and Lavernia E J, Scr Mater 159 (2019) 5.
Guo M X, Zhang Y D, Li G J, Jin S B, Sha G, Zhang J S, Zhuang L Z, and Lavernia E J, J Alloys Compd 774 (2019) 347.
Weng Y, Ding L, Zhang Z, Jia Z, Wen B, Liu Y, Muraishi S, Li Y, and Liu Q, Acta Mater 180 (2019) 301.
Acknowledgements
The authors acknowledge the Equipment Pre-research Project of China (No. 41422060204).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mao, G., Liu, S., Gao, W. et al. Effect of Zn or Zn + Cu Addition on the Precipitation in Al–Mg–Si Alloys: A Review. Trans Indian Inst Met 74, 2925–2938 (2021). https://doi.org/10.1007/s12666-021-02385-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-021-02385-5