Abstract
The pelletisation study of magnetite iron ore fines starts with green balling using limestone and bentonite as additives. Heating cycle of iron ore pellets is one of the prime segments of the whole pelletisation process. Drying, pre-heating, firing and cooling processes are collectively named as heating cycle of the process. A temperature profile is maintained throughout the induration process to meet the required specification of the pellet quality. This paper emphasizes on the effect of temperature profile on the mineralogical, physical and chemical properties of fired pellet in the heating cycle. This study includes the green balling and heating cycle at different temperatures from 800 to 1250 °C in batch processes of magnetite iron ore fines. The mineralogical phase conversion mainly from magnetite to hematite at different temperatures is evaluated by XRD study. The microstructural characterisation, compressive strength and chemical analysis of the indurated pellets are used to analyse the effect of increasing temperature during pellet induration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the long term forecast, the increase in iron and steel production is inter-linked with an increase in demand for iron ore. The deficit of high grade iron ore has opened up the thrust of research towards utilisation of low grade iron ore fines/slimes/lean grade ores in combination with beneficiation and pelletisation processes [1,2,3,4]. Pelletisation of ultrafine iron ore concentrate can promote conservation of iron ores by the utilization of lean grade resources.
Iron ore pelletization is a two-steps process consisting of pellet production in balling circuits and pellet induration in straight-grate/grate-kiln/vertical shaft kiln systems. Extensive applied research and plant-scale testing are directed to understand the effect of mineralogy of iron ore towards the induration process and quality of the fired pellet [5]. Induration of magnetite ore pellet is an oxidation process. During the pelletisation process, magnetite phase changes to hematite phase as given in Eq. (1) [6]. This chemical reaction is highly exothermic and releases a good amount of energy [7, 8]. It reduces the total energy consumption in comparison with pelletisation of hematite ore. The heat due to exothermic reaction of magnetite ore provides homogeneous heat transfer which improves the strong sinter bond among the particles of iron ore and ultimately it improves the cold crushing strength of pellet [9].
Earlier studies on oxidation of magnetite iron ore pellets have described about the influence of various parameters like partial pressure of oxygen, pellet porosity, pellet size, Blaine number and additives, i.e., bentonite, limestone and low volatile anthracite coal/coke [9,10,11,12,13]. In the present study, the change of mineral phases with respect to temperature is highlighted and correlated with quality of pellet.
2 Materials and Methods
2.1 Materials
2.1.1 Magnetite Concentrate
The magnetite ore concentrate was collected from one of the mine in Bhilwara, Rajasthan. The magnetite ore was beneficiated by physical beneficiation process to generate high grade magnetite fine concentrate. The commercial grade bentonite and limestone were used as additives in the pelletisation process. The iron ore pellets were prepared by using the raw materials of magnetite iron ore concentrate (−100 μm size), bentonite (−75 μm size) and limestone (−100 μm size). The detail chemical analysis of the raw materials is given in the Table 1. The major elements of magnetite ore were analysed by wet chemical analysis. The minor and trace elements were determined by instrumental methods, namely Atomic Absorption Spectroscopy (PerkinElmer, USA) and ICP-OES (Thermo Scientific, USA). Blaine number of sample was determined using Blaine apparatus (Model: ToniPERM, Toni Technik GmbH, Germany). The size analysis and Blaine number of the magnetite fines are given in the Table 2.
2.2 Methods
2.2.1 Green Ball Formation
The green ball formation kinetics of the magnetite sample was studied using the laboratory scale disc pelletiser. The disc pelletiser has a diameter of 1 m with depth of 0.3 m. The rotation speed of the disc pelletizer was 18 rpm. The angle of inclination of the disc was 46° in all the experiments. Approximately 10 kg of moist magnetite concentrate fines and additives, bentonite and limestone were mixed to prepare the pellet feed material. The amounts of bentonite and limestone added were 1 wt. % of the total pellet feed for each.
Pelletisation process was started with production of seeds/nuclei formation. Nuclei were produced by scattering the feed material in small amounts on the rotating disc and then small amount of water was sprayed to initiate the nuclei formation. Once the nuclei were formed, the feed was fed at a constant rate ~100 g/min at the feeding point and water was sprayed at the water feeding point to attain the required moisture (~9%) and to ensure proper layering of the feed material on the nuclei. At this point, only growth of the pellets takes place along with breakage and attrition and no new nucleus was formed. This was continued for exact 10 min (180 disc revolutions) and the green pellets were discharged out of the pelletizer. These green pellets were then classified by the sieves at different sizes, i.e., 16, 12.5, 10, 8, 6.3, 5.6, 4.75 and 3 mm. Classified pellets in different size were then weighed and for each batch of pelletisation. The weight of the pellets falling in different sizes was used to calculate average pellet diameter from the equations given below:
where, n = number of pellets for a size class having mean diameter ‘d’, in mm; W t = total weight of pellets, in g; w = weight of pellet having mean diameter ‘d’, in g and; ρ = bulk density of the pellets, g cm−3.
Bulk density of the pellets was measured and it was found to be 2.2 g cm−3. Drop strength of the green pellets was measured just before discharging the pellets from the rotating disc. The green ball drop was measured by dropping the green ball on a steel plate from a height of 450 mm. Number of drops was recorded till the pellet breaks on the plate. Similar set of experiments for green balls formation were then carried out for different time intervals of 20, 30, 40, 50, 60, 70 and 80 min for the sample. Moisture content of the green pellets was measured by drying at ~110 °C in an air oven.
2.2.2 Pellet Induration
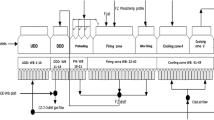
Heating/induration cycle is the particular stage of pelletisation process in which the green pellets are subjected to thermal treatment processes like upward and downward drying, preheating, firing and cooling zones. In this study, the dry pellets were indurated in a muffle furnace attached with a temperature control system. Silica crucibles of 1 l capacity were used for the induration experiments. Crucibles containing 500 g of pellets were thermally treated at different temperatures (800, 850, 900, 950, 1000, 1050, 1100, 1150, 1200 and 1250 °C) separately in a batch process. The rate of heating was at 20 °C/min followed by 10 min residence time in order to maintain the required temperature profiles for heating zones.
2.2.3 Indurated Pellet Properties
2.2.3.1 Pellet Surface Colour
The pellet samples were collected from each batch of induration process operated at temperatures of 800, 850, 900, 950, 1000, 1050, 1100, 1150, 1200 and 1250 °C. The appearance of the different batches of pellet was compared on the basis of their surface colour.
2.2.3.2 Chemical Composition
Representive pellet samples were collected from each batch of induration process to carry out the total Fe and FeO analysis.
2.2.3.3 Pellet Morphology
The pellet samples from each batch were cut at the cross section and mounted on araldite to study the morphology under optical microscope (LEICA-DM-2500P). The mineral phases developed with increasing temperature (110–1250 °C) were analysed using XRD (PANalytical, Netherland).
2.2.3.4 Compressive Strength of Pellet
Compressive strength measures the strength of product pellets to withstand the handling, transportation and reduction process. Pellets should have a mean product pellet compressive strength value of average 250 kg/pellet with the <150 kg/pellet fraction less than 5%. Compressive strength of pellets was determined using the universal tensile tester (Shanta Engineering, Kolkata) consisting of digital display of load. The maximum load that a pellet can withstand was recorded as compressive strength in units of kg/pellet (kg/p).
3 Results and Discussion
3.1 Green Ball Formation Kinetics
The kinetics of green pellet formation was explained with the average pellet diameter as a function of number of revolutions of disc pelletiser and shown in Fig. 1 [14,15,16,17]. This green ball growth curve shows an increasing order of pellet diameter formed with increase in time of batch balling process. The time required for 12.5 mm size of pellet was about 85 min or 1500 revolution. Figure 2 represents the variation of bulk density of the green pellet with time of pelletisation. The bulk density was varied from 2.17 to 2.4 g cm−3. Green pellet should have strength so that it can resist from the breakage during handling and feeding to induration machine. This green strength of the pellet was measured by the drops test. The drops strength of pellet as a function of time of pelletisation is shown in Fig. 3.
3.2 Physical Properties of Pellet at Different Temperatures
The photographs of the pellets collected at different temperatures of 110, 800, 850, 900, 950, 1000, 1050, 1100, 1150, 1200 and 1250 °C separately in a batch manner is shown in Fig. 4. Initially at 110 °C, colour of pellet was black similar to the colour of pellet feed material. At 800 °C magnetite phase starts oxidising to hematite phase and outer surface of the pellet looks brownish colour. Then gradually the colour changes to deep brown after 900 °C. After that at 1000 °C, the particles within the pellet start sintering and the color changes to grey and it becomes more greyish as temperature reaches 1250 °C.
3.2.1 Compressive Strength of Pellets
Compressive strength was recorded for 20 numbers of pellets collected at different induration temperatures [18]. Figure 5 represents the variation of average, maximum and minimum values of compressive strength of the pellets (out of 20 numbers) with rise in temperature of induration. The strengthening mechanism of pellets during temperature transition is most probably due to two reasons [19]:
-
(a)
The oxidation of magnetite to hematite, and
-
(b)
The crystal growth and recrystallization of magnetite grains
With an increase in temperature, the microstructure of pellets appears to be a duplex type with hematite in the shell and magnetite in the core causing severe lattice and bond strains [20]. Compressive strength of pellets gradually increase with an increase of temperature and reached its maximum value for pellets fired at 1250 °C, since at higher temperature the iron ore particles fuse together and make stronger bonding. The desired average value of strength (250 kg/pellet) for fired pellets was obtained for pellets fired at lower temperature i.e., 1150 °C. The figure also indicates that compressive strength of the indurated pellets decreases sharply after 1250 °C due to the formation of wustite (FeO).
3.2.2 Pellet Morphology
In order to validate the reason for change of pellet surface colour and pellet strengthening with increasing temperature, the morphology and microstructure of pellets at different temperatures were examined under optical microscope [21]. The microscopic images are shown in Fig. 6a–j. In the microscopic images, different images were taken from cross sectional surface as well as periphery of two cross sections of the same pellet. These findings reveal that the pellets with size (12.5–16 mm in diameter) are indurated uniformly during thermal treatments in batch manner. The mineral phases of magnetite and hematite are identified on the microscopic images. With suitable alignment of polarization, hematite appears bright and magnetite appears to be darker. Magnetite phase is denoted by ‘M’ and hematite phase is denoted by ‘H’. In Fig. 6a, availability of magnetite is dominant. Few hematite crystals are formed in the magnetite region at the temperature of 850 and 900 °C, as shown in Fig. 6b, c, respectively. The periphery of two pellet cross-sections is shown in Fig. 6d–j. The thickness of the periphery of indurated pellets starting from temperature 950 °C is observed to be broadening towards core with the increase of temperature of pellet induration. It indicates the conversion of magnetite to hematite starts at around 950 °C. Figure 6i, j show the pellet characteristics at the temperature of 1200 and 1250 °C, respectively. Almost all the magnetite particles oxidize to hematite. At 1200 °C, the hematite particle formation increases and the particles are sintered together which results in high compressive strength of the pellet at temperature 1200–1250 °C. While in pellet at 1250 °C, very fine particles of magnetite which was associated with the quartz also starts oxidizing to hematite as shown in the Fig. 6j.
3.3 Chemical Analysis of Pellets at Different Temperatures
The total Fe and FeO content of the pellets with increasing temperature were analysed by chemical analysis. Total Fe content with respect to increasing temperature of pellets is shown in Fig. 7. It is observed that the total Fe content is in the range of 64.0 to 64.6 which is not so diversified. FeO content with respect to increasing temperature of pellets is shown in Fig. 8. It is observed that the FeO content decreases with increasing temperature. It indicates that with increase in temperature magnetite converts to hematite as per the microscopic study of cross section of pellets.
3.4 Mineralogy of Pellets at Different Temperatures
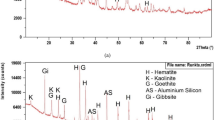
The quantitative mineral phase analysis of the pellets at different temperatures was carried out using XRD patterns. High score plus software of PANalytical instrument was used for the purpose. The pellets are named according to their indurating temperature, i.e., Pellet-110 means pellet was indurated at temperature of 110 °C. The quantitative phase analysis report of selected samples such as Pellet-110, 850, 1050 and 1250 °C are shown in Fig. 9a–d, respectively. The mineral phases like, magnetite, hematite, quartz, cummingtonite, muscovite, calcite and goethite are present in the Pellet-110 as shown in Fig. 9a. It mostly contains magnetite which is similar to the pellet feed. The pellet also contains goethite which disappears at temperature of 850 °C as shown in Fig. 9b. Rest of the mineral phases are present other than goethite. An increase in the amount of hematite at the expense of magnetite indicates the oxidation of magnetite to hematite. In Pellet-1050, mineral phases hematite, magnetite, quartz and pseudobrookite are observed as shown in Fig. 9c. It indicates that with increase in induration temperature, the mineral phases like cummingtonite, muscovite, calcite and goethite are destabilized whereas the available small amount of TiO2 is converted to titanite and pseudobrookite. At high temperature of 1250 °C, silica is converted to cristobalite alpha as shown in Fig. 9d.
The change of the mineral phases at different temperatures of pellets is derived from the XRD quantitative analysis and is summarised in Table 3. It indicates that magnetite mineral phase decreases whereas hematite phase increases with increasing temperature. This corroborates indications from microscopic image analysis and chemical analysis of pellets at different thermal conditions. The amount of quartz remains in the range of 5–6%. The fired pellet finally at 1250 °C contains hematite, magnetite, quartz and cristobalite (a polymorph of quartz).
4 Conclusions
This study was carried out to evaluate the effect of temperature profile in the induration of the magnetite green pellets with respect to their chemical, mineralogical and physical characteristics. The conclusions are as follows;
-
1.
During the green pellet formation of magnetite ore the green ball growth increases with increase of residence time in batch balling process. The bulk densities of the green pellets remain more or less constant whereas their drop number increases with time of pelletisation.
-
2.
The surface colour of the magnetite pellets changes from brown to grey with increasing temperature from 110 to 1250 °C.
-
3.
Compressive strength of pellets increases with an increase of temperature and it is maximum at 1250 °C.
-
4.
The microscopic image analysis indicates the abundance of magnetite grains in the pellet at 110 °C, whereas the population of the hematite grains increases with an increase in the induration temperature. The conversion from magnetite to hematite starts from surface to core of the pellet after 900 °C. This phenomenon also facilitates the improvement of pellet strength with increasing temperature.
-
5.
The FeO content of the pellets decreases with an increase of temperature which indicates conversion of magnetite to hematite.
-
6.
Mineralogical studies suggest that, in sharp contrast to the dried (110 °C) pellets which contain magnetite as the dominant phase, hematite is the dominant phase in the pellets heated at 1250 °C.
References
Mayer K, Pelletizing of Iron Ores, Springer, Berlin (1980).
Kawatra S K, and Ripke S J, Int J Miner Process 65 (2002) 165.
Fu J, Cheong Y, Reynolds G, Salman A, and Hounslow M, Powder Technol 140 (2004) 209.
Mohindra M, Prusti P, Sahu S N, Beuria P C, Sahu A K, and Biswal S K, in International Symposium on MRMMPI, Bhubaneswar (2013), p 143.
Sastry K V S, Dontula P, and Hosten C, Powder Technol 130 (2003) 231.
Liang R Q, Yang S, Yan F S, and He J C, J Iron Steel Res Int 20 (2013) 16.
Kumar T K S, Investigation of Sintering Kinetics of Magnetite pellets during Induration, Ph D Thesis, Luleå University of Technology, (2015).
Kumar T K S, Viswanathan N N, Ahmed H M, Andersson C, and Björkman B, Metall Mater Trans B 47 (2016) 309.
Forsmo S P E, Forsmo S E, Samskog P O, and Björkman B M T, Powder Technol 183 (2008) 247.
Forsmo S, Influence of Green Pellet Properties on Pelletizing of Magnetite Iron Ore, Doctoral Thesis, Luleå University of Technology Department of Chemical Engineering and Geosciences, Division of Process Metallurgy (2007).
Guo H W, Bai J L, Zhang J L, and Li H G, J Iron Steel Res Int 21 (2014) 9.
Cho H J, Tang M, and Pistorius P C, Metall Mater Trans B 45B (2014) 1213.
Tang M, Cho H J, and Pistorius P C, Metall Mater Trans B 45B (2014) 1304.
Kapur P C, and Fuerstenau D W, Trans AIME 229 (1964) 348.
Sastry K V S, and Fuerstenau D W, Powder Technol 7 (1973) 97.
Kapur P C, Adv Chem Eng 10 (1978) 55.
Abouzeid A Z M, Seddik A A, and El-Sinbawy H A, Powder Technol 24 (1979) 229.
Gupta R C, Theory and Laboratory Experiments in Ferrous Metallurgy, PHI Learning Private Limited (2010).
Sivrikaya O, and Arol A I, Int J Miner Process 110–111 (2012) 90.
Dwarapudi S, Devi T U, Rao S M, and Ranjan M, ISIJ Int 48 (2008) 768.
Umadevi T, Kumar P, Lobo N F, Mahapatra P C, Prabhu M, and Ranjan M, Steel Res Int 80 (2009) 709.
Acknowledgements
The authors are thankful to Prof. B.K. Mishra, Director of CSIR-Institute of Minerals and Materials Technology, Bhubaneswar for giving permission to publish this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prusti, P., Nayak, B.K. & Biswal, S.K. Study of Temperature Profile in the Induration of Magnetite Iron Ore Pellets. Trans Indian Inst Met 70, 453–462 (2017). https://doi.org/10.1007/s12666-016-1011-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-016-1011-8