Abstract
In this work, the effects of the slag basicity (mass ratio CaO to SiO2) and the addition of FeSO4 or FeS2 into the slag on the stability of the mineralogical species in the slag containing chromium compounds were studied. The chemical stability was evaluated by leaching the slags with an acid solution. The main Cr-compounds in slags with low basicity (CaO/SiO2 = 1) and FeSO4 were FeCr2O4 and Ca3Cr2Si3O12, while Cr3S4 and Ca3Cr2Si3O12 were formed in slags with FeS2. The slags with high basicity (CaO/SiO2 = 2) and FeSO4 contained CaCr2O4, FeCr2O4 and Ca5(SiO4)2SO4. The slags with FeS2 and high basicity produced FeS·Cr2S3, FeCr2O4 and Ca3Cr2Si3O12. The results showed that the lowest chromium concentration levels in the leaching liquors corresponded to slags with CaO/SiO2 = 1 and high FeS2 contents, owing to the stable binding of chromium in the compounds FeCr2O4, Cr3S4 and Ca3Cr2Si3O12.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
There is an increasing interest in finding ecological solutions for the safe disposal of industrial by-products that can cause environmental risks due to the mobility of the toxic elements therein. In particular, environmental concerns are on the rise due to the heavy metal content in stainless steel and ferrochrome slags, especially Cr [1, 2].
The stone-like properties of metallurgical oxide slags make them attractive in the civil engineering field as construction material; therefore, to prevent the leaching of heavy metals such as chromium, it is desirable to treat them prior to its application or even landfilling. Chemical stabilization and solidification are very effective tools and are becoming standard processes in hazardous waste treatment and disposal. These processes aim to attain mineralogical control of the metals to be stabilized. It has been proposed to fix the chromium into stable mineral phases by adding some materials to the liquid slag in the transfer ladle [1]. For instance, blast furnace slags can be used to stabilize Cr(III) and Cr(VI) in contaminated soils to decrease their leachability to safe levels [3, 4]; blast furnace slags have also been used for both physical and chemical immobilization of chromium in Portland and blended cements [3].
The influence of several slag forming agents over the leaching behavior of Cr has been analyzed. For instance, adding MgO reduces the refractory wear down and modifies the slag composition, lowering the Cr-contents in the leachate [5] which can also be attained by adding spinel forming materials such as bauxite [6, 7]. In either case, the environment-friendly behavior of the slags is improved.
In a previous work [8] the authors studied the effect of slag basicity (CaO/SiO2) and MgO and Al2O3 contents on the stability of the mineralogical species and the leachability of slags. It was found that CaCr2O4 and CaCrO4 were present in slags prepared with neither MgO nor Al2O3. The Al2O3-based slags mainly produced Ca2Al2SiO7 and the Cr(VI)-containing oxide complex Ca4Al6CrO16, whilst MgO-based slags produced MgCr2O4 as main mineralogical species.
In this work, synthetic slags containing chromium have been prepared and the effects of slag basicity and FeSO4 and FeS2 contents on the stability of the mineralogical species formed have been evaluated. The morphology and composition of the slags have been analyzed by X-ray powder diffraction (XRD) and scanning electron microscopy coupled with energy dispersive spectroscopy (SEM–EDS). The chemical stability of Cr has been evaluated by analyzing the leaching levels of chromium according to the Mexican Waste Norms [9].
2 Materials and Experimental Procedure
The slags were prepared from laboratory reagent-grade compounds (CaO, FeSO4·7H2O, FeS2, CaF2, SiO2 and Cr2O3) which were previously ground into fine powder whose particle size was between 45 and 74 μm. Two synthetic slag systems were prepared:
The FeSO4 and FeS2 content in each type of slag ranged from 0 to 9 mass %, Cr2O3 and CaF2 were 10 % and the slag basicity, defined as the ratio of CaO content to the SiO2 content (mass %), was 1 or 2. The Cr2O3 content tested in these slags was higher than those actually found in ferrochrome or stainless steel production. Still this high Cr2O3 concentration was used in order to observe clearly the effect of chromium on the leaching behavior of the slags. Table 1 shows the mixture compositions for slags A. The experimental compositions for slags B were similar to those shown in Table 1, but FeS2 was used instead of FeSO4.
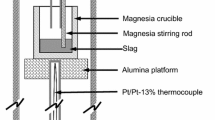
100 g of each slag system were prepared as follows. The powders were homogeneously mixed and placed in an Al2O3 crucible which was placed in a graphite protecting crucible inside an open induction furnace. Each mixture was heated for 30 min at a temperature 50 °C above its melting point, to ensure complete homogenization. It was worth to note that it was difficult to estimate theoretically the melting temperature of these slags; then, the slags were heated between 1450 and 1550 °C, depending on the composition, and the melting temperature was checked mechanically using a stainless steel bar which was introduced in the melt. The furnace temperature was controlled within ± 5 K with an R-type thermocouple (Pt–Pt, 13 %Rh).
The slags were left inside the furnace to cool down slowly until room temperature, to promote the formation of well defined crystalline structures. The cooling rate from the melting point to complete solidification was about 8 °C/min. The morphology, shape and compositions of the solid crystallized phases were investigated: the presence of crystals with homogeneous composition and well-developed facets, was taken as an indication of the attainment of equilibrium.
Samples of each slag were crushed into fine powders and characterized by X-ray diffraction (XRD Bruker D8 Focus) and scanning electron microscopy coupled with energy dispersive spectroscopy (SEM–EDS, Jeol 6300).
The chemical stability of the Cr-containing species present in these materials was evaluated by the following leaching technique, according to the Mexican environmental regulations [9]: 25 g of each slag was crushed below 74 μm and contacted with 500 cm3 of an aqueous acetic acid solution at pH = 2.88 ± 0.05 in a rotary system during 20 h at 30 ± 2 rpm and 23 ± 2 °C. The solid residues were filtered through ashless filter paper (Whatman 542) and the chromium present in the leachate was determined by atomic absorption spectrophotometry. It was worth noting that this chemical analysis gave the total chromium in the leachate, including Cr3+, Cr5+ and Cr6+.
3 Results and Discussion
3.1 Result of Slags A (FeSO4)
Figure 1 shows the XRD patterns of the slag A without FeSO4 with different basicity ratios. Cuspidine (Ca4Si2O7F2), calcium chromate (Ca3(CrO4)2), calcium chromite (CaCr2O4) and free Cr2O3 are present in both cases, despite the difference in basicity. The valence of chromium in the compounds CaCr2O4 and Ca3(CrO4)2 are 3+ and 5+, respectively. Figure 2 shows the SEM micrographs and the EDS semi-quantitative analysis for some chemical species found in this slag (CaSiO3, Cr2O3 and CaCr2O4).
The XRD patterns for slags A with 9 % FeSO4 are shown in Fig. 3. FeCr2O4, CaCr2O4 and the complex compound Ca5(SiO4)2SO4 are obtained for both the slag basicities. However, calcium silicates of the garnet group, andradite (Ca3Fe2Si3O12) and uvarovite (Ca3Cr2Si3O12), are present in slags with CaO/SiO2 = 1. The Ca5Cr2SiO12 with valence Cr5+, is observed in slags with CaO/SiO2 = 2. The garnet group comprise of several minerals with related chemical formulas. The generic formula for the common garnets is: X3 2+Y2 3+Si3O12, where X represents Ca, Fe2+, Mn or Mg, and Y represents Al, Cr or Fe3+. The species Ca5Cr2SiO12, a pentavalent Cr5+ compound, is formed in the slags with high basicity. Cr5+ and Cr6+ form stable anions in aqueous solution [10] and it is expected that the leachability of chromium by acid solutions may be high in these slags.
The SEM–EDS semi-quantitative analysis confirm the XRD results, as can be observed in the micrographs shown in Fig. 4, corresponding to the slag with 9 % FeSO4 and CaO/SiO2 = 1. The trapezohedral crystal (labeled A) contains calcium, chromium, silicon and oxygen. So it is believed that it corresponds to uvarovite (Ca3Cr2Si3O12). High contents of chromium, iron and oxygen are found in the octahedron crystals (labeled B). It has been reported [11] that chromite (FeCr2O4) crystallizes in cubes or octahedrons, such as those observed in this sample; thus, it is believed that chromite (FeCr2O4) is formed in this slag. The crystal labeled C, contains mainly calcium, silicon, sulfur and oxygen, and they probably correspond to Ca5(SiO4)2SO4, identified by XRD in Fig. 3.
Spinel-type compounds, such as FeCr2O4, are very resistant to oxidation and dissolution in acid environment; therefore low chromium levels of leaching are expected. It can be seen that the presence of FeSO4 leads to the formation of chromite FeCr2O4 instead of Ca3(CrO4)2 in these slags, diminishing the formation of leachable chromium compounds.
3.2 Result of Slags B (FeS2)
Figure 5 shows the X-ray diffraction patterns for slags B with 3 % FeS2 and CaO/SiO2 = 1 and 2. As has been observed in slags A with FeSO4, the slags with FeS2 contain cuspidine (Ca4Si2O7F2) and calcium silicates. The Cr-based compounds for the slags with CaO/SiO2 = 1 are Ca3Cr2Si3O12 and Cr3S4. The last compound (Cr3S4), named brezinaite, is a complex compound formed by CrS and Cr2S3, which has been reported to form at very reducing conditions. Figure 5b shows that increasing the slag basicity, the Cr-based compounds are Ca3Cr2Si3O12, CaCrSi4O10 and CaCr2O4. Brezinaite (Cr3S4) is also observed in the FeS2-based slags with high basicity.
Figure 6 shows the SEM micrographs and the EDS results for the crystalline phases of the slag with 3 % FeS2 and CaO/SiO2 = 1. Crystal labeled A shows a trapezohedral structure with calcium, chromium, silicon and oxygen, probably corresponding to uvarovite (Ca3Cr2Si3O12). Crystal labeled B contains mainly chromium and sulfur and it is believed that it corresponds to brezinaite (Cr3S4), identified by XRD in Fig. 5. The triclinic crystal observed in this slag corresponds to wollastonite (CaSiO3), according to the EDS results.
Figure 7 shows the X-ray diffraction patterns for slag B with 9 % FeS2 and CaO/SiO2 = 1 and 2. The X-ray results show that the mineralogical species obtained in this slag are basically the same as those obtained with 3 % FeS2. It is worth noting that the main difference regarding the Cr-based compounds is that, with low basicity, brezinaite is obtained, whilst at high slag basicity, calcium chromite (CaCr2O4) and FeS·Cr2S3 are formed. Magnetite (Fe3O4) is also detected in the slags with FeS2.
Figure 8 shows micrographs of the crystalline structures obtained in slags B with 9 % FeS2 and CaO/SiO2 = 2. The octahedron crystals (labeled A), which contain mainly iron, chromium and oxygen, probably correspond to chromite (FeCr2O4), as identified by XRD (see Fig. 7). The crystals labeled B contain mainly chromium, iron and sulfur, and it is possible that they correspond to the complex compound FeS·Cr2S3, observed in the XRD results. Elongated crystals (labeled C) correspond to calcium chromite (CaCr2O4). Hashimoto et al. [12] also obtained needle-like CaCr2O4 crystals by heating a powdered mixture of CaCO3 and Cr2O3, which are very similar to the crystals shown in Fig. 8.
3.3 Leaching Trials Results
Figure 9 shows the effect of FeSO4 and the CaO/SiO2 ratio in the slags on the leaching behavior of chromium. As can be seen, the maximum chromium extractions of 8.8 and 34.3 mg/l Cr are reached when FeSO4 is not added to the slag and the basicities are 1 and 2, respectively; whilst 4.8 and 27.1 mg/l Cr are leached for slags with 9 % FeSO4 and CaO/SiO2 equals 1 and 2, respectively. These results show that the lowest chromium concentration levels in the leaching liquors correspond to FeSO4-based slags and low slag basicity (CaO/SiO2 = 1) owing to the stable binding of chromium in the spinel (FeCr2O4) and the garnet compound uvarovite (Ca3Cr2Si3O12).
Figure 10 shows the leaching results for the slags with FeS2. It is clear that FeS2 is better than FeSO4 in controlling the chromium leachability, which can be due to the stable binding of chromium in brezinaite (Cr3S4) and uvarovite (Ca3Cr2Si3O12).
3.4 Potential-pH Diagrams
Potential-pH diagrams can be used to identify the conditions required to dissolve a metallic compound in an aqueous solution at specific temperature and pressure. The FACTSage computational thermodynamic package [13] and its database have been used to determine the potential-pH diagrams at 298.15 K (25 °C) for the Ca–Cr–H2O, Ca–Cr–Si–H2O and Fe–Cr–H2O systems. The E-pH diagrams are shown in Figs. 11 and 12. Water is only stable in the region bounded by the dashed lines. The equilibrium conditions that can be utilized for the leaching of minerals or any solid compound in aqueous solutions at ambient temperature are constrained to those defined by the region of water stability.
Figure 11 shows the pH values calculated specifically to compare the domains of Ca3Cr2Si3O12 and CaCr2O4 and in this way to establish which Ca–Cr compound makes the slag less vulnerable to leaching. The molality of all the aqueous species in these systems has been fixed to m = 1. Figure 11a shows that CaCr2O4 becomes unstable when the pH of the leaching solution is slightly acidic, about pH < 6, where Ca2+ and Cr(OH)2+ ions are produced. The domain of Ca3Cr2Si3O12 is larger than that corresponding to CaCr2O4 and it is stable in solutions with pH > 3. This is in agreement with the results of the work by Tae and Morita [14] who reported that the mineralogical species in the immobilization process of hexavalent chromium in wastewater using a granulated BF slag and hydrothermal treatment is the uvarovite (Ca3Cr2Si3O12). It is worth noting that the thermodynamic information of Ca3(CrO4)2, Ca5Cr2SiO12 and CaCrSi4O10 are not available and these species have not been included in the E-pH diagrams.
Figure 12 shows the potential-pH diagrams at 298.15 K (25 °C) for the Fe–Cr–H2O systems with two ranges of the Fe/(Fe + Cr) molar ratio. The main Cr–Fe compound in this system is the solid iron chromite (FeCr2O4). This figure shows that with low Fe/(Fe + Cr) ratio (between 0 and 0.333) FeCr2O4 is stable in relatively high acid solution (about pH > 2.2); however, Cr3+ and Cr(OH)2+ are dissolved in the liquid solution. The stable species for the systems with Fe/(Fe + Cr) > 0.333 are FeCr2O4 and Fe2O3, avoiding in this way the leaching of any Cr-ion species. In the slags of the present work, the value Fe/(Fe + Cr) > 0.333 is obtained for the systems with 9 mass % of FeSO4 or FeS2.
3.5 Effect of the Slag Basicity
Different chromium oxides exist in silicate melts. Turkdogan [15] reported that chromium is present in silicate melts, depending on the temperature and composition, in bi- tri- or hexavalent states. The ratio between the concentrations of Cr2+, Cr3+ and Cr6+ is regulated by the reactions
In oxidizing conditions, the anions of chromates are stable in basic melts, but decompose to form an ion of trivalent chromium in acid melts. In silicate slags silicon is present in the orthosilicate anion which is formed by the reaction:
Consequently, the solution of silica reduces the concentration of free anions of oxygen (O2−). The addition of SiO2 diminishes the slag basicity and the equilibrium of reaction (2) is shifted to the left and the hexavalent chromium is reduced to the trivalent state.
It is worth to note that the experiments in this work have been carried out in an open furnace with the oxygen partial pressure close to PO2 = 0.21 atm. Even though the alumina crucible is placed in a graphite protecting crucible, the experimental setup is not exposed to a reducing atmosphere above the slag system. If air is used as an atmosphere, hexavalent chromium compounds appear in the high-lime region, whereas at high silica slag content, all the chromium is virtually in the +3 oxidation state as has been reported by Glasser and Osborn [16]. So, if air is used as atmosphere, the stable chromium compounds depend only on the CaO/SiO2 ratio.
4 Conclusions
Synthetic slag samples of the system CaO–CaF2–SiO2–Cr2O3–(FeSO4 and FeS2) were prepared in order to understand the effect of FeSO4 and FeS2 content and the slag basicity on the stability of the mineralogical species and the leachability of slags. The following results were obtained:
-
(a)
Slags without FeSO4 and FeS2 contained Ca3(CrO4)2, a pentavalent chromium species (Cr5+), and CaCr2O4. The FeSO4–based slags produced the complex compound Ca5(SiO4)2SO4, together with garnet-type compounds (Ca3Cr2Si3O12 and Ca3Fe2Si3O12). The slags with FeS2 formed Ca3Cr2Si3O12 and the sulfur-based compounds (Cr3S4 and FeS·Cr2S3).
-
(b)
The results showed that the slags without FeSO4 or FeS2 produced the highest chromium extraction in the leachates. The presence of FeSO4 in the slag led to the formation of chromite (FeCr2O4) and uvarovite (Ca3Cr2Si3O12), diminishing the formation of leachable chromium compounds; however, the CaO/SiO2 ratio was to be maintained at a low level in order to avoid the formation of Ca5Cr2SiO12, where Cr had a valence of 5+.
-
(c)
The leaching results showed that FeS2 was better than FeSO4 in controlling the chromium leachability, which could be due to the stable binding of chromium in chromite (FeCr2O4), brezinaite (Cr3S4) and uvarovite (Ca3Cr2Si3O12).
-
(d)
The potential-pH diagrams showed that the stability domain of Ca3Cr2Si3O12 and FeCr2O4 were larger than that corresponding to CaCr2O4; therefore, the formation of these compounds minimized the leachability of the slag.
References
Lee Y and Nassaralla C L, Metall and Mater Trans B 29B (1970) 405.
Lind B B, Fallman A-M and Larsson L B, Waste Management 21 (2001) 255.
Duchesne J and Laforest G, Cement and Concrete Research 34 (2004) 1173.
Allan M and Kukacka L E, Waste Management 15 (1995) 193.
Kindness A, Macias A and Glasser F P, Waste Management 14 (1994) 3.
Shen H, Forssberg E and Nordstrom U, Resources, Conservation and Recycling 40 (2004) 245.
Fallman A M and Aurell B, Science of the Total Environment 178 (1996) 71.
García-Ramos E, Romero-Serrano A, Zeifert B, Flores-Sánchez P, Hallen-López M and Palacios E G. Steel Research Int 79 (2008) 332.
Mexican Official Norms. NOM-053-ECOL (1993). http://www.Semarnat.gob.mx. Accessed 11 April 2014.
Brown T L, LeMay H E and Burste B E, Chemistry, The Central Science, Prentice Hall, New Jersey (1997) p 892.
Chesterman C W and Lowe K E (eds) The Audubon Society. Field Guide to North American Rocks and Minerals. Alfred A. Knopf, Inc. New York (1988), p 419.
Hashimoto S, Yamaguchi A and Takahashi Y, Mat Res Bull 32 (1997) 1593.
Thompson W T, Bale C W and Pelton A D. FACTSage-Facility for the Analysis of Chemical Thermodynamics (FACTSage), Ecole Polytechnique, Montreal. (2014). http://www.crct.polymtl.ca.
Tae S J and Morita K. ISIJ International 48 (2008) 1311.
Turkdogan E T, in Physical Chemistry of High Temperature Technology, (ed) Academic Press (1980).
Glasser F P and Osborn E F. J Am Ceram Soc 41 (1958) 358.
Acknowledgments
The authors wish to thank the Institutions CONACyT, SNI, COFAA, CNMN and IPN for their assistance to the Process Metallurgy Group at ESIQIE-Dep. Metallurgical Eng.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martinez-Morales, C., Romero-Serrano, A., Zeifert, B. et al. Stabilization of Chromium in Synthetic Slags with FeSO4 and FeS2 . Trans Indian Inst Met 70, 1399–1407 (2017). https://doi.org/10.1007/s12666-016-0938-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-016-0938-0