Abstract
Malan loess is a wind-deposited sediment that is loose and porous and has high water permeability. It has experienced many dry–wet cycles during tens of thousands of years, resulting in unique engineering properties. The unique properties of loess are closely related to its texture and microstructure formed during dry–wet cycles. This paper investigates the influence of dry–wet cycles on the microstructure of Malan loess and its formation mechanism. The results show the following. (1) In an open environment, the ion content of the loess samples decreased after the dry–wet cycles, indicating that the cementation of the loess particles had weakened and the structural strength of the loess has decreased due to the loss of cementation materials as the cycle number increased. (2) The ion content of the soil samples did not change significantly after dry–wet cycles in a closed environment. However, after several cycles, the clay and colloid particles migrated and were adsorbed around the skeleton particles, and soluble salt ions were enriched in the form of cementation, with a slight increase in structural strength. The proposed dry–wet cycle model in this paper showed that the loess structure property was weaker in an open environment and stronger in a closed environment, revealing the formation and evolution of loess structure at the microscale. The results of this study provide very important theoretical and practical engineering significance for deep understanding of the mechanical and hydraulic properties of loess and the prediction and prevention of geological disasters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Loess soils occur worldwide but are most common in the dry mid-latitude zone. They are found in northwest and north China, and the Loess Plateau accounts for 72.4% of the loess area in China. The unique geographical location and climatic conditions result in differences in the composition, structural characteristics, and distribution of loess areas in different regions. Therefore, the Loess Plateau has the largest thickness, the most continuous distribution and the most typical characteristics of loess. During the formation of the Loess Plateau, the climate alternated between dry and cold conditions and warm and wet conditions many times. The climate is characterized by distinct seasonal changes, with humid and rainy summers and dry winters. The moisture content of the loess also changes with the change of climate, and the loess is often in the alternating state of dry and wet.
Loess is a wind-deposited sediment and is characterized by loose and porous soil with high water permeability. Accompanied by numerous dry–wet cycles in the process of deposition and soil-forming, the resulting loess is not only under-consolidated, loose and porous, which is conducive to water penetration, but also has strong water sensitivity. It is strongly affected by moisture and dry–wet cycles (Dijkstra et al. 2014; Ma et al. 2017; Zhang and Liu 2010). As a result, loess has unique engineering properties that make it prone to geological and engineering diseases. These characteristics of loess are attributed to its texture and microstructure.
Numerous studies have been conducted on the relationship between dry–wet cycles and the engineering properties of loess. Tang et al. (2016) believed that the dry–wet cycle caused an interaction between the soil and water. The mechanical properties of the soil samples degraded significantly after repeated cycles, and the permeability resistance at a given moisture content decreased with an increase in the number of cycles. The dry–wet cycle test of compacted loess showed that the porosity of the soil sample increased, and the density and cohesion decreased significantly with the cycle number. The volume deformation of the soil was irreversible, and the volume decreased with an increase in the compacting degree (Mao et al. 2013; Wan et al. 2015). A shear test of artificial sand after dry–wet cycles showed that the softening, deformation, and crushing of soil particles reduced the particle stiffness, and the shear strength decreased with the dry–wet cycle number (Wang et al. 2019; Xu et al. 2021). Liu et al. (2021), Xu et al. (2020), and Yuan et al. (2017) analyzed the effect of dry–wet cycles on the properties of loess. As the dry–wet cycle number increased, the number of cracks in the soil increased, and the shear strength increased and decreased. The cracks were irreversible. Hao et al. (2021) and Wang et al. (2020b) analyzed and verified the constitutive relationship of compacted loess under dry–wet cycles using triaxial shear tests. The settlement amount of the foundation soil increased and decreased with the cycle number. The mechanical and engineering properties of clay soil were investigated during dry–wet cycles (Khan et al. 2017; Tang et al. 2008). It was found that an increase in the number of cycles caused an increase in the number of irregular cracks on the soil surface and changes in the soil water content, density, and porosity. However, the micro-morphology of loess after dry–wet cycles remains unclear. Solanki and Zaman (2014) analyzed the engineering performance of subgrade soil after wetting and drying cycles and found that the strength of the undisturbed soil declined with the cycle number.
Li et al. (2016), Lu et al. (2015), and Wen and Yan (2014) stated that the migration of soluble salt in soil should be considered. Soluble salts in loess migrate during dry–wet cycles, affecting the soil strength and slope safety (Gao 1988; Higuchi et al. 2015; Ping et al. 2016; Xu et al. 2020; Yates et al. 2018). Computed tomography (CT) analysis showed that the wetting process in the dry–wet cycle accelerated the evolution of the loess structure, resulting in changes in the soil properties during the drying process (Li et al. 2020). Ye et al. (2020) conducted triaxial tests on samples with dry–wet cycles and found that the cycles had a negligible influence on the internal friction angle. CT analysis indicated that the dry–wet cycles increased the number of cracks in the samples. Mady and Shein (2020) used X-ray CT (X-CT) to quantify the changes in the pore space during drying and wetting. They found that the pore space and the total porosity of the samples increased (decreased), and the effective saturation decreased (increased) during drying (wetting). The wetting process caused the migration of particles in the loess, and the subsequent drying process resulted in the deformation of some small pores (Zhao et al. 2022). Zhang et al. (2022) found that shrinkage during wetting significantly affected the formation and evolution of the loess structure in the early stage of the dry–wet cycles, changing the current understanding of loess lithification process.
The literature review indicates that most researchers focused on the influence of the dry–wet cycle on the engineering properties of loess. Many scholars have realized the importance of the dry–wet cycle to the loess structure and the effect on the engineering properties of loess.
Loess is composed of particles, detrital minerals, clay minerals, and other components that affect the soil structure (Li et al. 2019; Meng and Zhang 2018). It is a typical structural soil, and its structural problem is the core problem of soil mechanics in the twenty-first century, and the fundamental internal factor affecting the mechanical properties of loess. The structural properties of loess affect and control its mechanical properties, and the physical properties presented in different geological environments are closely related to the structure of loess, and its unique structural characteristics are also the material basis and necessary conditions for the breeding, occurrence and evolution of geological disasters in loess areas. In short, the root cause of many loess geological disasters is structural problems. Due to the structural destruction of the loess, the influence of its mechanical properties provides a prerequisite for the occurrence of disasters.
In recent years, more and more geological disasters occur in loess area, which affect the development of all kinds of engineering construction in loess area, and pose a threat to the life and property safety of surrounding residents. At present, there is relatively little research on the structural mechanism of loess, but many scholars have gradually realized the importance of dry–wet cycle to the structural properties of loess. The dry–wet cycle is closely related to the formation of loess structure, and also affects its mechanical properties. It can be seen that the dry–wet cycle is very important to the structure and engineering properties of loess. Therefore, it is of great theoretical and practical significance to reveal the internal mechanism of the formation and evolution of the loess structure from the experimental point of view. The research results can provide an important theoretical basis for the design and construction of various projects and the prevention and control of geological disasters in the loess distribution area, and promote the rapid development of economic construction in the loess area.
Therefore, this article mainly investigates the effects of dry–wet cycles on the structure of Malan loess in different environments. By adding the metal salt NaCl to the soil samples, Na+ was selected as the marker ion, and microscopic test and shear tests were carried out to. The changes of ion migration under different cycles were observed in the microscopic test to verify whether there is significant ion migration under the dry–wet cycle and whether it has an impact on the structural properties of loess. Finally, shear tests were carried out to add evidence.
Test scheme and method
Physical properties of loess
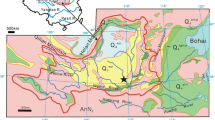
The sample collection region is located in Chanhe, Xi 'an, Shaanxi Province, as shown in Fig. 1. The terrain of the sampling area is high in the southeast and low in the northwest, forming multilevel terraces with open and flat terrain. Manual sampling was conducted on a fresh section of a secondary loess terrace at a depth of 4 m. The physical properties of the loess were determined by laboratory tests, including water content, specific gravity, compaction, and limit water content tests (Table 1).
Sample preparation
Na ion was used as a tracer ion to analyze the migration and redistribution of soluble salt ions in the loess under dry–wet cycles. The main reason is that Na+ has a relatively low affinity for soil (Wang and Yue 1998), and its limited adsorption to clay particles facilitates the analysis of the migration and redistribution. The NaCl solutions with different concentrations were added to the sample. After the water was evenly distributed, it is used to prepare the loess sample with a water content of 17% (optimal water content) and a dry density of 1.65 g/cm3 (the maximum dry density after compaction required by general engineering).
Test conditions and schemes
The Loess Plateau is a typical arid and semi-arid region. Soil profiles have shown alternating loess-paleosol depositions, representing dry and cold and relatively wet periods, respectively. Rainfall was relatively rare during the dry and cold climate; thus, most of the thick-layer loess is in the unsaturated state. However, most of the thick layer loess in the unsaturated state cannot produce seepage carrier. The differences in the moisture content of the loess were mainly caused by the comprehensive action of temperature gradient, humidity gradient and pore pressure gradient (Wu et al. 2019), and there was no exchange of clay particles, colloids, and ions with the external environment in this layer. This environment is referred to as the closed environment. In contrast, seepage can occur in the saturated loess stratum in some cases (such as the negative terrain that collects), which is called the open environment. Therefore, we divided the areas into open and closed environments to analyze the effect of the dry–wet cycles on the loess samples.
For the test of the loess samples from the open environment, cylindrical test blocks with h = 20 mm and d = 61.8 mm were prepared by a standard ring cutter. Ten hours of vacuum saturation was carried out in a vacuum saturator to wet the samples. The drying process was simulated by natural air drying in a cool and ventilated place for 20 days (Fig. 2a). For the test in a closed environment, we used a cylindrical test block with h = 10 mm and d = 39.8 mm. An atomizer was used in the humidifying device to wet a specific certain area of the loess sample until the surface of the soil sample no longer absorbs water (Fig. 2b). The drying process was simulated by natural air drying in a cool and ventilated place for 10 days to minimize the loss of clay particles, colloidal granules, and ions in the sample.
Preparation of samples for microscopic analysis
After the dry–wet cycle test, the samples were cut into small circular blocks with a diameter of about 10 mm and a height of about 10 mm. The central part of the sample block was selected for quantitative analysis and observation to prevent the error caused by the disintegration of the sample edge. The flowchart in Fig. 3 shows the preparation method of the samples for microscopic analysis. Due to the loose structure of the loess particles, it is usually hardened first with hardener solution. The hardening solution consisted of epoxy resin, ethylenediamine, acetone, and dibutyl phthalate with a ratio of 100:7:200:2 (Li et al. 2019; Wang et al. 2010). The small soil sample was placed in a suitable container and vacuumized by a vacuum pump to better ensure that the hardening solution penetrated the sample. The solution was slowly dripped onto the sample. After it had permeated the sample, the solution was dripped onto the soil sample until it was completely immersed and the hardening solution level was 2–3 mm above the top of the soil sample. The samples were placed in a room for about 30 days to ensure hardening. The hardened sample was sliced and polished to smooth the surfaces for the X-ray fluorescence (XRF) and scanning electron microscopy (SEM) analyses. Prior to the SEM observation, gold was applied to the surface of samples using an SCD-005 sputter coater to obtain high-quality SEM images. The microscope images of the processed samples were acquired using an M4 TORNADO high-performance XRF spectrometer and a Quanta 400 FEG scanning electron microscope.
Analysis and results
The basic structural unit of loess
According to the morphological characteristics of different structural units and their functions at the microscale, the loess sample units were divided into single mineral particles and aggregates. The single mineral particle is the smallest structural unit of loess and affects the mechanical strength and stability of the loess. Its size is between a fine sand particle and a clay particle. Aggregates consist of many single mineral particles and clay minerals, carbonates, colloidal microcrystals, and other adhesive materials. The clay minerals, carbonates, and other adhesive materials bind the particles together. According to the cementation between particles and adhesive materials, they can be divided into the following four types: viscous aggregates, mononuclear aggregates, multinuclear aggregates and composite aggregates.
Viscous aggregates are generally composed of microclastic minerals, colloidal particles and clay particles; they are the smallest aggregates. Mononuclear aggregates consist of a single particle surrounded by clay minerals, carbonate minerals, or clastic minerals. Due to their simple structure, they are referred to as primary aggregates. If there are two or more single mineral particles as a core, and the outer layer consists of adhesive material, they are referred to as multinuclear aggregates. Composite aggregates are the largest aggregates and consist of several smaller aggregates. The aggregates range from single mineral particles to viscous, mononuclear, multinuclear aggregates, and composite aggregates. Figure 4 is the conceptual model of the basic structural unit of loess.
The adhesive material in loess binds the structural units together at different levels. It determines the pore size, quantity, and connectivity and affects the mechanical properties, morphological characteristics, and cementation strength of the loess basic structural units. In addition, due to the strong water sensitivity of adhesive particles, the mechanical properties and functional units of loess depend on the change of water content. Therefore, adhesive particles are the main reason for the strong water sensitivity and special water properties of loess.
Among high-grade aggregates, there are more pores in them and the distribution of pores is not uniform, most of the adjacent aggregate units are cemented by forming bridges between the aggregates with adhesive materials. The connection strength between aggregate units at all levels also depends on the strength and distribution of viscous bridges. The main components of bridges are adhesive particles, carbonates, and other adhesive substances. Adhesive particles are critical in the loess microstructure due to their strong adhesiveness. The more adhesive substances, the stronger the cementation between units and the more stable the bridges. Calcium carbonate is a crucial cementing material. The main components of calcium carbonate are dolomite and calcite, and secondary calcium carbonates are generated during leaching. They are encapsulated in skeleton particles or mixed with other clay particle and played a major cemented role (Guo et al. 2004; Meng and Li 2019).
Single mineral particles, such as clay, silt, and fine sand particles, exist independently in the loess and are connected to adhesive materials to form aggregate structures of different grades. In the aggregate unit, the fine sand and powder particles are commonly connected by adhesive particles. The structural strength of aggregates depends on the degree of cementation between materials such as fine sand, silt, and adhesive particles. In the cemented connection between single mineral particles and various aggregates, the adhesive particles and carbonates are critical in connecting single mineral particles and various aggregates, and are also important factors affecting the structural strength of loess.
Results of dry–wet cycle test in an open environment.
The loess samples with salt contents of 0, 0.3%, and 3% were subjected to a dry–wet cycle test with 0 and 12 times in an open environment. Subsequently, XRF and SEM–EDX (energy dispersive X-ray spectroscopy) analyses were conducted.
X-ray fluorescence results
Figure 5 shows the element distribution (Al, Ca, Si, Na, K, and other elements) in the micro-regions of the loess samples. The higher the element content, the higher the brightness of the corresponding representative colors. It can be seen that in the distribution diagram of Ca, Si, Al, and other elements, the brightness of some areas is significantly higher than that of other areas, corresponding to the main element composition of quartz, calcite, feldspar, dolomite, montmorillonite, kaolinite and other clay minerals in loess.
In the distribution diagram of Na ion (tracer ion), the overall brightness is roughly uniform and there is no obvious place of high brightness, even in those finished 12 dry–wet cycles. This finding demonstrates that the XRF images do not clearly capture the migration of Na ions. There are two main reasons. First, most soil colloidal surfaces are negatively charged. Cationic adsorption occurs during the cycles, and the particles of clay minerals are very small. Although they are highly dispersed, they still have crystal structure. Almost all the crystal structure of clay minerals deviates from the ideal structure model, and all have certain structural distortion and crystal defects. Due to structural distortion, there are many incomplete crystal surfaces, and many cations, such as Al3+, Ca2+, K+, H+, and Na+, are attracted to these surfaces. Due to their adsorption capacity (Brady et al. 2000), it is not easy to ion migration. Second, the Quaternary loess experienced many wet and dry periods in geological history, and these conditions are difficult to simulate in an indoor dry–wet cycle test. In a very limited number of indoor dry–wet cycles, only a small number of cations will migrate. When the number of cycles is increased, cumulative effects can often be observed (Hua et al. 2020).
Although XRF provides information on the element composition and distribution, its low resolution will also have a large impact on ion observation. We failed to capture this phenomenon in the XRF results after 12 dry–wet cycles due to the low resolution, but we could deduce the ion migration based on the percentage of elements derived from the XRF results (Table 2).
Table 2 lists the percentage contents of each element in the samples with different numbers of cycles. The proportions of Na, Ca, Al, Fe, and other elements are lower in the samples finished to 12 cycles than in those finished to 0 cycle. This result indicates that during the humidification process in the open environment, the ions in the soil exchange with the external environment, and part of the soluble salt in the soil is taken out of the soil, which leads to the decrease of the soluble salt content in the soil. (Gao et al. 2019; Zhang and Luo 2014). The changes in the percentage content of elements during the dry–wet cycles, indicate that the ions in the open environment have obvious migration phenomenon, and the general trend of migration is to migrate from the saturated sample to the outside along with the direction of seepage.
SEM–EDX results
Figure 6 shows the SEM–EDX images of the different loess samples. The higher the element content, the brighter the color. Figure 6a, c, and e show the loess samples not exposed to the dry–wet cycles. Most of the Na+ is evenly distributed, but some areas show higher brightness than others. These indicate Na+-containing minerals, such as albite (Na[AlSiO3O8](Na2O·Al2O3·6SiO2)), montmorillonite ((Na,Ca)0.33(Al, Mg)2(OH)2•nH2O), and hornblende ((Ca,Na)2–3(Mg2+,Fe2+,Fe3+,Al3+)5[(Al, Si)8O22](OH)2) and some crystals of undissolved NaCl salt. In contrast, the images of the samples exposed to the dry–wet cycles (Fig. 6b, d, f) show that the areas with high brightness are no longer just complete particle shape or spot-like. Instead, they wrap around the particles in strips or irregularly. Compared with the loess samples that have not been cycled, Na+ in the loess samples that have been cycled through dry–wet cycles is more likely to accumulate around the particles, and the outline of particle shapes can be clearly observed in the figure. For loess samples in open environment, we only conducted 12 dry and wet cycles to capture the obvious phenomenon of ion migration. It can be imagined that in the history of thousands of dry and wet cycles of actual loess in open environment, the amount of ion migration caused by the cumulative effect of ion migration must be very considerable.
Structural effects of dry–wet cycles on loess in an open environment
Most of the migrating ions are the components in the cementation material, so most of the migrating ions are also cements, which is of great significance for the change of loess microstructure. However, the loss of loess under the action of saturated seepage is not limited to soluble salt ions in an open environment. Clay particles and colloids may also be lost, and the level of loss depends on the porosity, pore connectivity, seepage pressure, and flow rate. Ions and colloidal particles are the most prone to leaching due to their small particle size and ease of migration, affecting the degree of cementation between particles and the structural strength. Clay particles are larger and less prone to leaching. They cement skeleton particles due to their strong cementation capacity. The ion electrostatic force significantly affects the cementation strength and aggregate strength of loess. A low clay content corresponds to a low ion electrostatic force generated by the interaction between negatively charged clay minerals and various metal cations and weak cementation between particles. During the subsequent drying cycle, the remaining adhesive particles and soluble salt ions migrate and are redistributed, and the adhesive particles and soluble salts occur between the skeleton particles or around the particles. Since soluble salt ions and clay and colloidal particles act as cement, the cementation strength between the loess and aggregates decreases with the loss of soluble salt ions and clay and colloidal particles during the dry–wet cycles in an open environment. As a result, the structural strength of loess decreases. According to results, a structural weakening model of the loess exposed to dry–wet cycles in an open environment was established (Fig. 7).
Results of dry–wet cycle test in a closed environment
The number of dry–wet cycles in the test in the closed environment was 10, 30, and 50, and the salt content of the samples was 0, 0.3%, and 3%.
X-ray fluorescence results
Figure 8 shows the element distribution in the micro-regions of different samples in a closed environment. As can be seen from Fig. 8, most of the elements in the sample are evenly distributed. Due to the limitation of the adsorption of cations and the number of cycles, and mainly because of the low resolution, the ion migration could not be clearly seen, so a higher resolution scanning electron microscope detection was subsequently performed. Table 3 lists the element percentage content of the samples with different cycles in a closed environment. After different numbers of dry–wet cycles, the percentage of element content did not change significantly, and there was no material exchange with the external environment, indicating that there was no significant loss of soluble salt ions in the closed environment.
SEM–EDX results
Figure 9 shows the element distribution of the loess samples in a closed environment.
Na+, as a marker ion, moves with the increase and decrease of water under multiple dry and wet cycles. Mineral particles, such as albite, montmorillonite, and hornblende, contain more Na+ than other particles, so the particle shape is clearly outlined in the figure. Regional Na enrichment is observed in the loess samples after the dry–wet cycles (Fig. 9). After 10 dry and wet cycles, the bright parts of the loess samples were spots and a few stripes. In the Na element mapping map of loess samples after 50 cycles, few spots were found, and most of them were distributed in stripes.
Figure 10 shows the Na distribution of the control sample overlaid on the SEM image. Figure 10a shows the superimposed mapping of Na elements in the reshaped samples. The reshaped sample did not add Na+ and did not occur wet and dry cycles. In the figure, the brightness of some feldspar particles containing Na is relatively high, but there is no obvious Na+ accumulation around the particles. Figure 10b shows the Na distribution of the control samples exposed to 10 dry–wet cycles. The overall brightness is low, but some local areas show high brightness around the particle edges, indicating a high Na+ content. The Na+ has migrated around the skeleton particles after 10 dry–wet cycles. Figure 10c shows the Na distribution of the control sample and the sample after 50 dry–wet cycles. The areas with high brightness are larger than in Fig. 10b, and more Na+ is observed at the grain edges. As the number of cycle increases, the Na+ migration to the surrounding particles is more pronounced. Figure 10d shows the results of the sample with 3% Na+ content after 50 dry–wet cycles. Compared with other samples, the Na+ content is higher than in other samples, and the image brightness is also relatively high. After 50 wet and dry cycles, a large number of Na+ have migrated around the particle. Na+ surrounds most of the particles and is uniformly dispersed around the particle edges. These results demonstrate that the migration phenomenon of Na+ becomes clear with the increase of the number of cycles, and the higher the Na+ content, the more obvious the migration phenomenon.
Structural effects of dry–wet cycles on loess in a closed environment
During the wetting process in a closed environment, clay minerals and soluble salts are partially hydrolyzed. The clay particles and colloidal particles and the cation move freely due to gravity, and the metal cations migrate and are adsorbed. Subsequently, some of the water dissipates and the water content decreases during the drying process. The ions in the water move around the particles. A layer of water film will be formed outside the particles as a weakly bonded water film, but the binding force between the particles is relatively weak. After continuous drying, the water film becomes thinner, and there are still a small amount of pore water exists on the surface of the clay particles to form a stronger water film and resulting in a strong viscous effect. The ions and clay particles migrate and aggregate between particles, and the binding force between skeleton particles increases. The water content continues to decrease, causing the surrounding clay and colloidal particles and cation ions to adhere to the particle surfaces, gather at the junction of skeleton particles, or fill irregular particles. Under the action of polar water molecules, metal cations attract particles surrounded by anions and combine with them. The double electric layer repulsion in the near saturation state changes into the bonding attraction between particles, increasing the cementation strength between particles. When the loess is almost completely dry, the adhesive particles and soluble salt ions cement the particles, resulting in a solid connection.
When the number of cycles is sufficient, the ions migrate and move to the skeleton particles, generating ion electrostatic attraction and participating in the cementation between the skeleton particles to strengthen the structural connection between particles. The loess samples were in a closed environment, and there was no obvious ion loss. Therefore, after drying, the cementation between the skeleton particles of loess becomes dense and the strength increases obviously. According to results, a structural enhancement model of the loess exposed to dry–wet cycles in a closed environment was established (Fig. 11).
Direct shear test results
An unconsolidated undrained shear test was carried out in accordance with the latest geotechnical test specification GB/T50123-2019 using the ZJ strain-controlled direct shear instrument. The direct shear rate was 0.8 mm/min, and the vertical pressure was increased successively from 100 to 200, 300, and 400 kPa. We tested the direct shear samples in open and closed environments. We prepared the cyclic samples with 1, 3 and 6 cycles in open environment, and the loess samples with 10, 20 and 30 cycles in closed environment, original sample and reshaped sample. After the cycle is complete, the samples were air-dried. The test was performed after the sample was air-dried.
Shear test results
Figure 12 shows the results of direct shear tests with different cycles. In an open environment, at the same positive pressure, the shear strength of the samples after the dry–wet cycles decreases with an increase in the number of cycles. The shear strength of the samples exposed to the dry–wet cycles is significantly lower than that of the original sample. In the closed environment, the shear strength of the soil samples increases slightly with the cycle number increased at the same positive pressure, and the rate of change is the largest a positive pressure of 400 kPa.
The soluble salt ions exchange materials with the external environment due to the close contact between the sample and the water in the open environment, reducing the amount of cementation materials between the soil particles. Adhesive particles and soluble salts migrate to the surrounding particles during the cycles, increasing the cementation between particles. However, due to the continuous reduction in the content of adhesive particles and ions, the overall structural strength of the particles decreases. Therefore, at the same positive pressure, the shear strength decreases with an increase in the cycle number in an open environment. The soluble salts and other substances in the soil sample migrate along with the water molecules during the wetting cycle in the closed environment. However, there is no material exchange with the external environment and a negligible loss of ions. As the water molecules dissipate, the soluble salt ions and adhesive substances adhere to the surrounding particles, increasing the cementation level between particles and enhancing the structural strength. At the same positive pressure, the shear strength of samples in a closed environment is significantly different from that of original specimens. However, as the cycle number increases, the shear strength of the samples in the closed environment increases slightly.
However, it is difficult to achieve ideal conditions. The number of cycles in this test was far lower than that under actual conditions. When the number of cycles in the closed environment is sufficient, we believe that the trend of the increasing shear strength of the soil samples will be clearer due to the cumulative effect, increasing the structural strength of the loess.
Discussion
Most dry–wet cycle studies are carried out in an open environment. The structural and mechanical strength of the loess decreases with the cycle number. The loess stratum which can form seepage due to temporary saturation is considered as an open environment. On the contrary, most of the other thick loess in the unsaturated state, we refer to the environment where this loess is located as a closed environment. We conducted dry–wet cycle tests in open and closed environments. It was concluded that the structure strength of loess is weakened in an open environment and slightly strengthened in a closed environment. The vast majority of Malan Loess has existed in the unsaturated zone of the huge thick layer for a long time. Under the premise of no water retention, the moisture increases and decreases repeatedly, and no material exchange occurs with the external environment. The closed environment test simulated the environment of Malan loess in the stratum. After several dry and wet cycles, the shear strength increased slightly, and the loess structure improved slightly. In the original stratum, under the action of rainfall and drying cycle year after year, and the cementation materials among the loess skeleton particles dissolve, migrate, and are redistributed around the particles many times. The connection between the skeleton particles was strengthened, and the cementation strength between the particles was higher. The structural strength of the loess particles stabilized as the number of dry–wet cycles increased. In addition, long-term stratigraphic deposition and various geological processes increase the stability of the loess structure, and finally formed the unique natural structure of original loess. The enhanced loess structure model proposed in a closed environment in this paper also better explains the genetic mechanism of Malan loess structure. At the same time, it provides a theoretical basis for the structural strength of undisturbed loess is higher than that of disturbed loess, and the differences in mechanical properties and water properties caused by loess structure.
Conclusion
Through the above test results, the following conclusions can be drawn:
-
(1)
The basic structural unit of loess are divided into single mineral particles, viscous aggregates, mononuclear aggregates, multinuclear aggregates and composite aggregates. The adhesive particles and carbonates bind the structural units together at different levels, and are also important factors affecting the structural strength of loess.
-
(2)
The XRF microscopic observation of soil samples failed to clearly see the migration changes of particles due to its limited resolution. According to the statistics, the element contents of the samples in the open environment were decreased after the dry–wet cycles, whereas those in the close environment exhibited no change significantly.
-
(3)
According to SEM–EDX results, some ions migrated and distributed around the particles in the open environment, and the ion content decreased to a certain extent. The cementation strength between particles and the structural strength of the loess were lower after the dry–wet cycles. Therefore, structural weakening model of loess exposed to dry–wet cycles in an open environment is established. In a closed environment, there was no significant loss of ions, and soluble salt ions and adhesive particles migrated. With the increase of the number of dry and wet cycles, the cemented connection between the loess particles was strengthened, and the structural strength of the loess particles was also slightly enhanced. Therefore, structural enhancement model of loess exposed to dry–wet cycles in a closed environment is established.
-
(4)
The shear test results of the samples exposed to dry–wet cycles showed that the shear strength of the loess samples in the open environment decreased with the cycle number at the same positive pressure. At the same positive pressure, the shear strength of the loess samples in the closed environment increased slightly with the cycle number.
Availability of data and materials
All data used in the study are confidential in nature, so the research data are not shared.
References
Brady NC, Weil RR, Brady NC (2000) Elements of nature and properties of soils. Prentice Hall, New York
Dijkstra TA, Wasowski J, Winter MG, Meng XM (2014) Introduction to geohazards of central China. Quart J Eng Geol Hydrogeol 47:195–199. https://doi.org/10.1144/qjegh2014-054
Gao G (1988) Formation and development of the structure of collapsing loess in China. Eng Geol. 25:235–245. https://doi.org/10.1016/0013-7952(88)90029-4
Gao Y, Yu YT, Zheng JG, Liang Y (2019) Strength characteristics of compacted loess during leaching. Rock Soil Mech. 40:3833–3843. https://doi.org/10.16285/j.rsm.2019.0072
Guo Y et al (2004) Composition of loess aggregate and its relationship with CaCO_3 on the loess plateau. Acta Pedologica Sinica. 41:362–368. https://doi.org/10.11766/trxb200303310306
Hao YZ, Wang TH, Cheng L, Jin X (2021) Structural constitutive relation of compacted loess considering the effect of drying and wetting cycles. Rock Soil Mech. 42:2977–2986. https://doi.org/10.16285/j.rsm.2021.0551
Higuchi K, Chigira M, Lee DH, Wu JH (2015) Rapid weathering and erosion of mudstone induced by saltwater migration near a slope surface. J Hydrol Eng. https://doi.org/10.1061/(ASCE)HE.1943-5584.0001105
Hua K, Xiao J, Li SJ, Li Z (2020) Analysis of hydrochemical characteristics and their controlling factors in the Fen River of China. Sustain Cities Soc. https://doi.org/10.1016/j.scs.2019.101827
Khan MA, Hossain MS, Khan MS, Samir S, Aramoon A (2017) Impact of Wet-Dry Cycles on the Shear Strength of High Plastic Clay Based on Direct Shear Testing, 3rd Conference on Geotechnical Frontiers, Orlando, FL. pp. 615–622.
Li L, Zhang K, Zhang Q, Mao Y, Li G (2016) Experimental study on the loess strength degradation characteristics under the action of dry-wet and freeze-thaw cycles. J Glaciol Geocryol. 38:1142–1149. https://doi.org/10.7522/j.issn.1000-0240.2016.0133
Li XA et al (2019) Characterization of the mechanisms underlying loess collapsibility for land-creation project in Shaanxi Province, China-a study from a micro perspective. Eng Geo 249:77–88. https://doi.org/10.1016/j.enggeo.2018.12.024
Li YR, Zhang WW, He SD, Aydin A (2020) Wetting-driven formation of present-day loess structure. Geoderma. https://doi.org/10.1016/j.geoderma.2020.114564
Liu S et al (2021) Experimental study on the effects of wet-dry cycles and suction on the mechanical properties of unsaturated Xiashu loess. Journal of Southeast University. Nat Sci Ed 51:473–479
Lu HJ, Li JX, Wang WW, Wang CH (2015) Cracking and water seepage of Xiashu loess used as landfill cover undern wetting-drying cycles. Environ Earth Sci 74:7441–7450. https://doi.org/10.1007/s12665-015-4729-4
Ma F, Yang J, Bai X (2017) Water sensitivity and microstructure of compacted loess. Transp Geotech 11:41–56. https://doi.org/10.1016/j.trgeo.2017.03.003
Mady AY, Shein EV (2020) Assessment of pore space changes during drying and wetting cycles in hysteresis of soil water retention curve in Russia using X-ray computed tomography. Geoderma Reg. https://doi.org/10.1016/j.geodrs.2020.e00259
Mao YC, Li GY, Lei JX, Zhang LR, Chen ZY (2013) Experimental Study on the Effects of Wetting-drying Cycles of Compacted Loess, 3rd International Conference on Civil Engineering and Building Materials (CEBM 2013), Hong Kong, PEOPLES R CHINA, pp. 326. https://doi.org/10.4028/www.scientific.net/AMR.831.326.
Meng L, Zhang F (2018) The new concept of soil skeleton and the microstructure of loess. Constr Sci Technol. https://doi.org/10.16116/j.cnki.jskj.2018.13.002
Meng J, Li XA (2019) Effects of carbonate on the structure and properties of loess and the corresponding mechanism: an experimental study of the Malan loess, Xi’an area China. Bull Eng Geol Environ. 78:4965–4976. https://doi.org/10.1007/s10064-018-01457-z
Ping L, Vanapalli S, Tonglu L (2016) Review of collapse triggering mechanism of collapsible soils due to wetting. J Rock Mech Geotech Eng 8:256–274. https://doi.org/10.1016/j.jrmge.2015.12.002
Solanki P, Zaman M (2014) Effect of wet-dry cycling on the mechanical properties of stabilized subgrade soils. Geo-congress. https://doi.org/10.1061/9780784413272.351
Tang C, Shi B, Liu C, Zhao L, Wang B (2008) Influencing factors of geometrical structure of surface shrinkage cracks in clayey soils. Eng Geol 101:204–217. https://doi.org/10.1016/j.enggeo.2008.05.005
Tang CS, Wang DY, Shi B, Li J (2016) Effect of wetting-drying cycles on profile mechanical behavior of soils with different initial conditions. Catena 139:105–116. https://doi.org/10.1016/j.catena.2015.12.015
Wan Y, Xue Q, Wu Y, Zhao L (2015) Mechanical properties and micromechanisms of compacted clay during drying-wetting cycles. Rock Soil Mech. 36:2815–2824. https://doi.org/10.16285/j.rsm.2015.10.010
Wang Z, Yue B (1998) Diffussion characteristics of water soluable cations in engineering loess. Bull Soil Water Conserv 18:9–13. https://doi.org/10.3969/j.issn.1000-288X.1998.02.002
Wang M, Bai X, Yang J (2010) Method of specimen preparation for collapsible loess microstructure research. J Taiyuan Univ Technol. 41:283–286
Wang JJ, Zhou YF, Wu X, Zhang HP (2019) Effects of soaking and cyclic wet-dry actions on shear strength of an artificially mixed sand. Ksce J Civ Eng. 23:1617–1625. https://doi.org/10.1007/s12205-019-0896-2
Wang L et al (2020a) Characterization of the collapsible mechanisms of Malan loess on the Chinese Loess Plateau and their effects on eroded loess landforms. Human Ecol Risk Assess. 26:2541–2566. https://doi.org/10.1080/10807039.2020.1721265
Wang T, Hao Y, Wang Z, Cheng L, Li J (2020b) Experimental study on dynamic strength properties of compacted loess under wetting-drying cycles. Chin J Rock Mechan Eng. 39:1242–1251. https://doi.org/10.13722/j.cnki.jrme.2019.0945
Wen BP, Yan YJ (2014) Influence of structure on shear characteristics of the unsaturated loess in Lanzhou. China. Eng Geol. 168:46–58. https://doi.org/10.1016/j.enggeo.2013.10.023
Wu B et al (2019) In-situ monitoring of moisture field and estimation of unsaturated permeability coefficient of loess foundation. J Disaster Prev Mitig Eng. 39:691–699. https://doi.org/10.13409/j.cnki.jdpme.2019.05.001
Xu J, Li YF, Wang SH, Wang QZ, Ding JL (2020) Shear strength and mesoscopic character of undisturbed loess with sodium sulfate after dry-wet cycling. Bull Eng Geol Environ. 79:1523–1541. https://doi.org/10.1007/s10064-019-01646-4
Xu XT et al (2021) Effect of wet-dry cycles on shear strength of residual soil. Soils Found. 61:782–797. https://doi.org/10.1016/j.sandf.2021.03.001
Yates K, Fenton CH, Bell DH (2018) A review of the geotechnical characteristics of loess and loess-derived soils from Canterbury, South Island, New Zealand. Eng Geol 236:11–21. https://doi.org/10.1016/j.enggeo.2017.08.001
Ye WJ, Bai Y, Cui CY, Duan X (2020) Deterioration of the internal structure of loess under dry-wet cycles. Adv Civ Eng. https://doi.org/10.1155/2020/8881423
Yuan Z, Ni W, Tang C, Hu S, Gan J (2017) Experimental study of structure strength and strength attenuation of loess under wetting-drying cycle. Rock and Soil Mechanics. 38:1894. https://doi.org/10.16285/j.rsm.2017.07.007
Zhang M, Liu J (2010) Controlling factors of loess landslides in western China. Environ Earth Sci. 59:1671–1680. https://doi.org/10.1007/s12665-009-0149-7
Zhang N, Luo Y (2014) Influence of soluble salt on the strength characteristics of loess. Yellow River. 36:103–105. https://doi.org/10.3969/j.issn.1000-1379.2014.08.031
Zhang WW, Li YR, Wang R, Beroya-Eitner MA (2022) A model for the formation and evolution of structure of initial loess deposits. Catena. https://doi.org/10.1016/j.catena.2022.106273
Zhao L, Wu T, Yu S, Rong B (2022) Exploration on mechanical test method of improved loess under dry-wet cycles. MATEC Web of Conf 358:01031. https://doi.org/10.1051/matecconf/202235801031
Funding
This research was financially supported by the National Natural Science Foundation of China (Grant No. 42230712, 41877225).
Author information
Authors and Affiliations
Contributions
Rongrong Gao: Conceptualization, Methodology, Investigation, Writing-original draft, Writing-review & editing. Xi-An Li: Conceptualization, Funding acquisition, Proofread & review. Mingxiao An: Investigation, Writing-original draft. Zhitao Hao: Investigation, Writing-review & editing. Biao Qin: Writing-review & editing. Feng Wen: Proofread & review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, R., Li, XA., An, M. et al. Experimental study on structural effects of particle migration in Malan loess under different dry and wet conditions. Environ Earth Sci 83, 353 (2024). https://doi.org/10.1007/s12665-024-11675-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-024-11675-2