Abstract
The increasing dependence on groundwater resources especially in developing countries requires an in-depth evaluation of the hydrogeochemical characteristics associated with any potential geological disposal site for radioactive waste. In particular, the borehole disposal system (BDS) for disused sealed radioactive sources (DSRS) interfaces with groundwater resources thereby necessitating detailed site characterization to generate requisite geostatistical information. In this study, the potential BDS site for the conditioned DSRS was characterized by assessing the hydrogeochemical nature and processes at varied depths of investigatory boreholes. Conventional hydrogeochemical, multivariate statistical, and multi-criteria decision-making (MCDM) analysis were used. Durov and Piper diagrams show that sodium chloride constituted the primary water type. Na versus Cl, and Gibbs diagrams demonstrated that weathering is a probable primary source of Na and Cl ions. Major ions ratios demonstrated the occurrence of both ion exchange and reverse ion exchange hydrogeochemical processes. Multivariate principal component analysis indicated three clusters of groundwater, whereby borehole BF cluster was influenced mainly by nitrate due to associated agricultural activities. Na+ and Cl− ions primarily influenced boreholes BH1 and BH2 clusters. MCDM preference ranking organization method for enrichment evaluation and geometrical analysis for interactive aid modeling demonstrated that the shallow depth BF borehole offered the most conducive reducing hydrogeochemical environment to host the borehole disposal system. The BF rock formation is associated with an alternating band of phyllite and quartzite. Schist mixed with a brownish quartzitic formation that is within 110–130 m depth in BH1 also provides favorable reducing conditions for the future disposal of conditioned DSRSs in Ghana.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radioactive wastes have been generated and stored in Ghana predominantly as disused sealed radioactive sources (DSRS) for future geological disposal using borehole disposal system (BDS) developed by the International Atomic Energy Agency (IAEA) and key stakeholders notably the South African Nuclear Energy Corporation (NECSA). The BDS is essentially an engineered borehole with multiple barrier system made up of stainless steel, concrete mixture and high density polyethylene (HDPE) casing within suitable host rocks located below thirty (30) m depth to isolate and contain the conditioned DSRS from the biosphere during the operational period of the disposal facility (Dawood et al. 2012; Glover and Essel 2020; IAEA 2011). Therefore, BDS interfaces with the natural hydrogeochemical environment. Significant global interest exists in the geological disposal sites for hazardous and radioactive waste due to the considerable public apprehension about the long-term safety and security of the disposal facility (Grambow and Bretesché 2014). The conduct of generic and site-specific characterization activities prior to the construction of the BDS is essential in ensuring the safety of both present and future generations from the hazardous effects of the waste (IAEA 2011; OECD 2003). The geoscientific information obtained from such investigations facilitate better comprehension of how the disposal site may evolve with time based on the natural temporal and spatial features, events and processes at the site (IAEA 2011). As a result, the information will lead to a well-informed decision making prior to the deployment of the BDS in a specific environment (Akortia et al. 2021; IAEA 2011).
Among the various site characterization activities, hydrogeochemical characterization describing the physicochemical characteristics and transformation of the chemical components within the geospatial environment of underground radioactive waste repositories is essential in determining the suitability of any potential disposal site (Lakshmanan et al. 2003). According to Grambow and Bretesché (2014) the detailed comprehension of the physicochemical mechanism of the disposal site is fundamental to the long-term efficiency and effectiveness of the disposal facility. The groundwater chemistry can affect the longevity of the engineered barrier systems of the BDS due to oxidation and reduction effects on the stainless-steel capsules and concrete matrix used in conditioning the radioactive sources. Groundwater characteristics can, therefore, affect the migration of radionuclides through the host rock and aquifer systems in the event that the integrity of the engineered barrier systems gets compromised (Grambow and Bretesché 2014). Moreover, the groundwater characteristics may also provide vital information on the origins of the water (Adewumi et al. 2018). Ghana is currently undertaking all the relevant site characterization activities particularly the hydrogeochemical investigations to meet both local and international requirements as part of the BDS implementation process.
The borehole disposal system interfaces with groundwater resources, and according to the UN (2022) World Water Development Report 2022, groundwater accounts for circa 99% of all freshwaters on Earth and approximately 25% of the water used in irrigation. The groundwater in the study area for the BDS in Ghana is used for both domestic and agricultural purposes. In this context, the necessity of managing and protecting groundwater sustainably cannot be overlooked (UN 2022). Therefore, there is the need to ensure that the BDS for conditioned DSRSs will contain and isolate the waste in the long term (IAEA 2005, 2011) thereby protecting the groundwater and promoting the continuous reliance on this vital natural resource in the area. In this context, the specific objective of the current study is to assess the hydrogeochemical characteristics of the proposed site for the BDS, and to unravel the physical and chemical features at different depths of the boreholes. The study will also evaluate the water–rock interaction, and sources of solutes in the groundwater. This study will generate vital geoscientific information that will ensure that adequate engineered multi-barrier systems are incorporated into the design of the BDS against any potential unfavorable oxidizing condition. However, it must be noted that although hydrogeological site characterization entailing porosity, permeability, transmissibility amongst others are equally important geoscientific parameters in terms of the BDS implementation, it is not considered in this current study. In line with the study objectives, geostatistical tools such as multivariate statistical and multi-criteria decision-making (MCDM) methods were deployed in evaluating the measured data so as to ascertain the actual hydrogeochemical outlook of the site.
Materials and methods
Study area
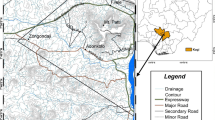
The study was conducted in the most urbanized and densely populated city, Greater Accra Region of Ghana, West Africa. More than 50% of Ghana’s population dwell in urban settings with its attendant challenge of increasing demand for natural resources such as water. The study area is located between 5° 6′ 7″ N and 5° 6′ 9″ N latitude and 0° 21′ W to 0° 26′ W longitude with an average elevation of 64 m. The area is in the tropical region of West Africa and is characterized by dry and rainy seasons. The area experiences two rainy seasons normally from April to July, and September to November with average annual relative humidity of approximately 80%. The sampling points are located within the Ga East District of Accra as shown in Fig. 1, and Fig. S1 in the Supplementary Information. The mean annual rainfall in the area ranges from 1000 to 1400 mm (Logah et al. 2013). The area currently hosts the Ghana Atomic Energy Commission (GAEC) where a nuclear research reactor and Ghana’s National Radioactive Waste Management facility are located. Three boreholes (BH1, BH2 and BF, see Fig. 1) meant for the site-specific characterization activities for the future geological disposal of conditioned DSRS are located within the GAEC site. The groundwater resource in the area serves as an important resource for irrigational activities at both research and commercial levels.
The area is made up of two main geological formations, namely the Togo structural units and the Dahomeyan supergroup as shown in Fig. 1. The Togo units are associated with Upper Precambrian age. The Togo units consist of granitoid, phyllite and phyllonite, biotite gneiss, sandstones, quartz schist, quartzite, and quartzite minor mica schist rocks. On the other hand, Dahomeyan supergroup is connected with Middle-Late Precambrian age basement rocks. The Dahomeyan supergroup is composed of Schistose marbles, orthogneiss, amphibolites and metamicrogabbro, and quartz schist (Doku 2013). As represented in Fig. 1, the Togo series occupy the north-western section and the highland areas of GAEC, whilst the Dahomeyan-outcrop occupies the low-lying areas (Nude et al. 2009). The area is characterized by unconfined and confined aquifers which occur mainly within massive crystalline rocks (Agbevanu 2015). The geologic formation associated with the groundwater is characterized by secondary porosity features such as joints, fractures, weathered zones and faults. The aquifers can also be classified into fractured and weathered zone aquifers. The unconsolidated layers near the land surface have high transmissivity active zone which serves as major conduits for groundwater movement. The underlying clay layer beneath the unconsolidated layer serves as aquitard, thereby separating the active zone from the deeper depth rocks. The Dahomeyan and Togo rocks in the area are normally associated with low permeability (Akiti 1987; Salifu et al. 2015). The borehole in the area generally yield between 0.41 to 29.8 m3/h of water (Agbevanu 2015). The groundwater resource in the area is essential for domestic and irrigation purposes, thus, there is a need to guard against any future anthropogenic source of contamination.
Sampling of groundwater
The boreholes were purged of stagnant water using submersible pump prior to groundwater sampling. Thirty-five (35) groundwater samples were collected at varied depths as indicated in Table S2 from the three boreholes using Solinst discrete interval sampler (Model 425) as shown in Fig. S2 during September 2020. This sampling approach minimizes the mixing of samples from varied depths due to the minor perturbation associated with the operation of the equipment, thereby resulting in highly representative samples at a particular depth. Further information on the standard operating procedure of the equipment is provided in the Supplementary Information. The samples for anions analysis were filtered through 0.45 µm acetate cellulose filter paper whilst those for cations were acidified after filtration to a concentration of 0.2% v/v using ultra-pure nitric acid. The air-tight samples in labelled pre-cleaned 330 mL HDPE bottles were preserved in a refrigerator at 4 °C for 3 days prior to analysis (Noble et al. 2011; Salifu et al. 2015).
Sample analysis
Physicochemical parameters such as temperature, electrical conductivity (EC), pH, and total dissolved solids (TDS) were determined in-situ using digital multi-parameter meter. De-ionized water was used to rinse the digital multi-parameter meter. Daily calibration of the meter was done using calibration solutions. Field blanks were analyzed intermittently to assess possible errors encountered during the sampling and analysis of samples. The major ions entailing potassium (K+) sodium (Na+), calcium (Ca2+), and magnesium (Mg2+), constitute the cations, whilst the anions consisting of chloride (Cl−), sulphate (SO42−), nitrate (NO3−), bicarbonate (HCO3−), and silicate (SiO44−) were measured using standard operating procedures as outlined in Table S1 of the Supplementary Information. All the analyses were conducted at the environmental chemistry laboratory of the Water Research Institute (WRI), Centre for Scientific and Industrial Research (CSIR), Accra, Ghana.
Data analysis
The generated physicochemical data as shown in Table S2 was subjected to conventional graphical hydrogeochemical analysis (such as Piper, Durov and Gibbs diagrams), multivariate statistical and multi-criteria decision-making (MCDM) techniques. These techniques facilitated comprehensive characterization of the hydrogeochemical processes. The simultaneous utilization of these geostatistical methods maximizes the latent information in the research data, thereby minimizing misinterpretation of the data to promote attainment of accurate inferences and conclusions (Gbeddy et al. 2020a; Kim et al. 2005). Principal component analysis (PCA) was the main multivariate method deployed since it provides both data reduction, pattern and cluster information about the processes influencing the hydrochemistry (Gbeddy et al. 2020b; Kim et al. 2005).
MCDM preference ranking organization method for enrichment evaluation (PROMETHEE) and geometrical analysis for interactive aid (GAIA) were used to assess and model the role of key physicochemical parameters such as chloride ion concentration due to its influence on the corrosive properties of groundwater. PROMETHEE determines the ranking of an object compared to others for a set of criteria based on relevant modelling scenarios. The scenarios entail whether maximized (high) or minimized (low) criteria are preferred, allocation of weighting factors, and the selection of suitable preference function. A subtraction based pairwise comparison of all the data matrix entries in all possible permutations is performed resulting in difference (d) for each comparison. The preference function then transforms the difference into a degree of preference varying from zero to one; the objects with results closer to one are preferred to those nearer to zero. GAIA on the other hand represents the pictorial display or pattern between objects and criteria for the PROMETHEE results (Ayoko et al. 2003, 2007; Doyi et al. 2018; Gbeddy et al. 2020a). The software namely StatistiXL Version 1.8 and Visual PROMETHEE Academic Edition Version 1.4.0.0 were used for the PCA, and PROMETHEE and GAIA, respectively. More information on these techniques can be found in Ayoko et al. (2003), Ayoko et al. (2007), Gbeddy (2020) and Gbeddy et al. (2020b).
Results and discussions
Geospatial hydrogeochemical characterization of groundwater
The geology and geochemical processes occurring in groundwater system influence the chemical composition of groundwater. Therefore, it is essential to characterize the relationship between these processes and groundwater (Kazemi and Mohammadi 2012). This characterization is particularly important for hydrogeological environment meant for the disposal of conditioned disused sealed radioactive sources. According to Larson and Skold (1958), the presence of SO42− and Cl− anions in groundwater enhances the occurrence of corrosion whilst bicarbonates impede corrosion. Detailed hydrogeochemical information will therefore lead to well-informed decision making with respect to the suitability of a prospective DBS site.
The Durov and Piper diagrams in Figs. 2 and 3, respectively depict the synopsis of groundwater physicochemical parameters, and these diagrams are significant in characterizing the hydrogeochemical nature of an area (Kazemi and Mohammadi 2012). With respect to the ionic composition of the groundwater, Figs. 2 and 3 show that Na+ and Cl− constitute the predominant cation and anion, making up over 60% and 80%, respectively, in the study area. On the other hand, the concentration of another corrosion causing SO42− anion in the study area can be considered as negligible as shown in Figs. 2 and 3. Furthermore, there exist a high positive correlation of 0.983 between Na+ and Cl− in Table S3 of the Supplementary Information. This clearly indicates that sodium chloride (NaCl) is the primary water type in the area. This observation may pose some chemical challenges to the longevity of the engineered barrier system incorporated in the BDS due to the corrosive properties of Cl−. In this regard, the multi-barrier system for the future BDS must be designed and developed taking into consideration the corrosive properties of the hydrogeochemical environment.
Furthermore, the geospatial distribution of the chloride ions in the groundwater with reference to the water level is illustrated in Fig. 4. The borehole, BF measured the lowest concentration of Cl−, and the estimated concentration remained constant across various depths of the borehole. In this context, it can serve as a viable baseline borehole for future of the BDS. The two boreholes, BH1 and BH2, recorded high concentration of Cl− in excess of the World Health Organization (WHO) stipulated concentration threshold of 250 mg/L (Salifu et al. 2015). The concentrations of Cl− in BH1 was not characterized by any significant variations across varied borehole depths compared to BH2, especially with lower depths around 100 m downwards.
Origin of ions and associated hydrogeochemical processes
The Gibbs diagram in Fig. 5a indicates that the major ions in the groundwater reside within the rock dominance region thereby showing that weathering of rocks constitutes the fundamental factor in ions distribution in the groundwater. According to WHO (2003), weathering is responsible for the leaching of chlorides from rocks into water, thereby supporting the high chloride levels measured in this study. The Na versus Cl plot in Fig. 5b indicates that the groundwater samples from BH1 and BF plotted above the theoretical 1:1 equilibrium line, indicating the predominance of Na ions over Cl ions. However, in the case of BH2 whilst most of the samples plotted above the equiline, few samples can be found below and on the line. Weathering via dissolution of halite may be a probable source of chloride and sodium ions in BH2 groundwater samples that aligned with the equiline (Zaidi et al. 2015).
The hydrogeochemical processes taking place in the study area were evaluated using ion ratio assessment notably major ion/Cl− ratios versus Cl− concentration (McLean et al. 2011) as shown in Fig. 6. All the ratios for the major ions exhibited relative stability across varied depths of the boreholes whereby BH1 and BH2 form a cluster and BF also form another cluster. This may be an indication of relative stability in the hydrochemistry of the groundwater across various depths of the boreholes. However, BF measured lower concentrations of Cl− and higher ratios for Na/Cl (Fig. 6a), Mg/Cl (Fig. 6b), K/Cl (Fig. 6c) and HCO3/Cl (Fig. 6d) above the respective dashed Atlantic Ocean Seawater lines, thus depicting excess Na, Mg, K and HCO3 ions in the BF groundwater. Most of the groundwater samples from BH1 and BH2 borehole samples plotted below the Seawater Na/Cl line in Fig. 6a. As a result, reverse ion exchange process may be responsible for Na ions elimination from the groundwater (McLean et al. 2011).
From Fig. 7a, all but two of the groundwater samples have Ca + Mg versus SO4 + HCO3 plotted below the 1:1 theoretical line. In this regard, the two BH1 and BH2 groundwater samples that lie above the 1:1 line indicates the potential occurrence of reverse ion exchange for these two high salinity samples (McLean et al. 2011). These high salinity samples also displayed Na/Cl ratios that plotted below the Seawater line in Fig. 6a. In this context, the study area is characterized potentially by both ion exchange and reverse ion exchange processes, as earlier evident in Fig. 5b by the high Na ions and enriched Cl ions, respectively in some of the groundwater (Lufuno 2017). Ca + Mg versus Cl + SO4 plot (see Fig. 7b) shows that all BH1 and BH2 groundwater samples plotted below the 1:1 line thereby indicating the potential occurrence of equilibrium between Cl + SO4 with alkalis (Kazemi & Mohammadi 2012). Furthermore, Fig. 7c indicates the predominance of alkalis over alkali earth metals in the hydrogeochemical environment of the study area since most of the samples plotted below the 1:1 line.
Hydrogeochemical evaluation using multivariate and multi-criteria decision-making analysis
Multivariate analysis
A data matrix consisting of 35 objects by 13 variables was subjected to principal component analysis (PCA) to facilitate pattern recognition in the data. The data were standardized due to the differences in units and variance of the variables. Using an Eigenvalue > 1, only two principal components (PCs) were established to be significant and accounting for 85% of variance in the data.
The biplot of the analysis in Fig. 8 shows that the objects segregated into three clusters. These clusters dovetail the various locations of the groundwater. The cluster on the negative axis of PC1 represents the borehole located at the research site of the Biotechnology and Nuclear Agriculture Research Institute (BNARI). The site is associated with crop and animal research and thus, the attendant nitrate (NO3−) concentration as evident in Fig. 8. The nitrate in the groundwater may be emanating from organic and inorganic fertilizers laden in the upper soil layer. The shallow depth of the BF borehole facilitates the hydrogeochemical interaction or migration of the nitrate into the groundwater. The second and third clusters on the positive axis of PC1 are made-up of the two boreholes, BH1 and BH2 as shown in Fig. 8 for the disposal project of conditioned DSRSs. BH1 cluster is influenced mainly by calcium (Ca), sodium (Na) and potassium (K) ions whilst BH2 cluster is affected predominantly by magnesium ions. However, both clusters are influenced by chloride and sodium ions. Considering the very high correlation of 0.983 between Na and Cl as shown in Fig. 8, and Table S3 of the Supplementary Information, it can be estimated that the main water type is sodium chloride (NaCl) as stipulated earlier.
The distance between the BF cluster, and the BH1 and BH2 clusters as shown in Fig. 8, clearly demonstrates that varied underlying natural conditions and anthropogenic activities influence the hydrogeochemistry of the GAEC site. The BF borehole is of shallow depth whilst BH1 and BH2 are relatively deeper. The hydrogeochemical properties of BF does not correlate with the hydrogeochemical characteristics of the shallow depth sections of either BH1 or BH2. These are clear indications of varied geological features and anthropogenic activities at different depths in the study area.
Multi-criteria decision-making analysis
The data matrix comprising of 35 actions by 13 criteria was further evaluated using PROMETHEE-GAIA to rank the various geological depths in terms of favorable hydrochemical condition for hosting the BDS. In this context, a favorable hydrochemical condition refers a reducing groundwater quality that will mitigate the rapid corrosion of the multiple engineered barrier system in the BDS. Two different modeling scenarios were used to evaluate the data. In the first model, pH was maximized (that is reducing condition) whilst all other criteria were minimized in order to model a reducing environment. Equal weighting was assigned to all the criteria, indicating that all the criteria were given equal consideration during the modelling. The generated complete ranking of the objects/samples (PROMETHEE-II) is shown in Table 1. As already specified under the data analysis section, the objects with Phi results closer to one are preferred to those nearer to zero.
The result indicates that the shallow depth borehole BF offers the most conducive reducing hydrogeochemical environment for the disposal of conditioned DSRSs followed by borehole 1 (BH1) and then 2 (BH2). Considering the conducive reducing hydrogeochemical environment of the BF borehole which is characterized by phyllite and quartzite rocks as specified in Table S4, it can be inferred that these host rocks may offer suitable redox condition for the safe disposal of conditioned DSRSs. However, since phyllites are characterized by marked fissility, orientation of foliation surface, and anisotropy (Andrade and Saraiva 2010; GeologyScience 2022) there is the need to explore greater depth groundwater and rock formations in BF. Conditioned DSRSs ought to be disposed at depths that will minimize human intrusion, thus further exploration on the BF may lead to more geoscientific information to aid the decision-making process. Groundwater from geological depths of 110–130 m for BH1 indicates favorable reducing environment for the disposal of conditioned DSRSs by virtue of the high rank in Table 1 next to BF. The main underlying lithology around the 110–130 m depth in BH1 influencing the conducive condition is schist mixed with brownish quartzitic materials as indicated in Table 1.
To further verify the accuracy in the interpretation of the generated data, a second modelling scenario entailing maximized pH, all other criteria been minimized, and chloride and sulphate allotted weighting of five (5) to help select the most reducing hydrogeochemical environment. The PROMETHEE-II output for the analysis is presented in Table 2, and the result indicates similar pattern for the top ranking and most reducing groundwater condition as observed using the first model.
Conclusions and recommendations
The present study characterizes the hydrogeochemical processes within the potential geological disposal site for disused sealed radioactive sources (DSRS) in Ghana. With the aid of Piper and Durov diagrams, sodium and chloride ions were found to be the major cation and anions, respectively in the groundwater thereby resulting in sodium chloride water type. Gibbs diagram shows that the major ions plotted within the rock dominance region thereby implying that weathering of rock minerals is the main source of ions in the area. The major ion/Cl− ratio versus Cl− concentration mostly indicated the prevalence of alkalis over alkali earth metals in the hydrogeochemical environment. Principal component analysis shows that the groundwater in borehole, BF is influenced mainly by nitrates that may be emanating from the upper soil layer via leaching. Preference ranking organization method for enrichment evaluation (PROMETHEE) and geometrical analysis for interactive aid (GAIA) multi-criteria decision-making analysis indicates that the hydrogeochemical environment around BF offers the most conducive reducing condition for any future implementation of the borehole disposal system for conditioned DSRSs in Ghana. Considering the location of the study area relative to the Atlantic Ocean, the potential movement of saline water (seawater intrusion) and its influence on the water type of the area may be investigated using relevant isotopic and chemical techniques in order to produce a holistic knowledge of the hydrogeochemical characteristics of the area.
Data availability
The underlying data in this study is not associated with human and animal subjects. The generated data is readily available in the Supplementary Information attached to this manuscript.
References
Adewumi AJ, Anifowose AYB, Olabode FO, Laniyan TA (2018) Hydrogeochemical characterization and vulnerability assessment of shallow groundwater in basement complex area, Southwest Nigeria. Contemp Trends Geosci 7(1):72–103. https://doi.org/10.2478/ctg-2018-0005
Agbevanu KT (2015) Modelling and simulation of groundwater flow and radionuclide transport in aquifers of dahomeyan system of the Accra Plains in Ghana University of Ghana, Legon-Accra
Akiti TT (1987) Environmental isotope study of ground water in crystalline rocks of the Accra plains (Ghana). Fourth Working Meeting Isotopes in Nature, German Democratic Republic
Akortia E, Glover ET, Nyarku M, Dawood AMA, Essel P, Sarfo EO, Ameho EM, Aberikae EA, Gbeddy G (2021) Geological interactions and radio-chemical risks of primordial radionuclides 40K, 226Ra, and 232Th in soil and groundwater from potential radioactive waste disposal site in Ghana. J Radioanal Nucl Chem 328(2):577–589. https://doi.org/10.1007/s10967-021-07675-2
Andrade PS, Saraiva AA (2010) Physical and mechanical characterization of phyllites and metagreywackes in central Portugal. Bull Eng Geol Env 69(2):207–214. https://doi.org/10.1007/s10064-009-0251-9
Ayoko GA, Bonire JJ, Abdulkadir SS, Olurinola PF, Ehinmidu JO, Kokot S, Yiasel S (2003) A multicriteria ranking of organotin(IV) compounds with fungicidal properties. Appl Organomet Chem 17(10):749–758. https://doi.org/10.1002/aoc.520
Ayoko GA, Singh K, Balerea S, Kokot S (2007) Exploratory multivariate modeling and prediction of the physico-chemical properties of surface water and groundwater. J Hydrol 336(1–2):115–124. https://doi.org/10.1016/j.jhydrol.2006.12.013
Dawood AMA, Glover ET, Essel P, Adjei-Kyereme Y, Asumadu-Sakyi GS, Akortia E, Nyarku M (2012) Borehole disposal concept for radioactive waste disposal-the GAEC project. Elixir Pollut 47:8752–8756
Doku MS (2013) Seismological and geological investigation for earthquake hazard in the Greater Accra Metropolitan Area, University of Ghana, Accra, Ghana
Doyi I, Essumang D, Gbeddy G, Dampare S, Kumassah E, Saka D (2018) Spatial distribution, accumulation and human health risk assessment of heavy metals in soil and groundwater of the Tano Basin, Ghana. Ecotoxicol Environ Saf 165:540–546. https://doi.org/10.1016/j.ecoenv.2018.09.015
Gbeddy G (2020) Transformation and degradation of organic pollutants on urban road surfaces [by publication, Queensland University of Technology]. Australia
Gbeddy G, Egodawatta P, Goonetilleke A, Ayoko G, Jayarathne A, Chen L, Russell S (2020a) Optimized simultaneous pressurized fluid extraction and in-cell clean-up, and analysis of polycyclic aromatic hydrocarbons (PAHs), and nitro-, carbonyl-, hydroxy-PAHs in solid particles. Anal Chim Acta 1125:19–28. https://doi.org/10.1016/j.aca.2020.05.021
Gbeddy G, Goonetilleke A, Ayoko GA, Egodawatta P (2020b) Application of multivariate data techniques in photochemical study of polycyclic aromatic hydrocarbons (PAHs) and transformed PAH products in road dust. Ecotoxicol Environ Saf 196:110478. https://doi.org/10.1016/j.ecoenv.2020.110478
GeologyScience (2022) Phyllite: Composition, Properties, Formation, Uses. GeologyScience. https://geologyscience.com/rocks/phyllite/. Accessed 7 Nov 2022
Glover ET, Essel P (2020) Implementation of the borehole disposal system for safe and secure management of disused radioactive sources in Ghana. IAEA International Conference on Nuclear Security Vienna, Austria
Grambow B, Bretesché S (2014) Geological disposal of nuclear waste: II. From laboratory data to the safety analysis—addressing societal concerns. Appl Geochem 49:247–258. https://doi.org/10.1016/j.apgeochem.2014.05.015
IAEA (2005) Safety of radioactive waste disposal. International Conference on the Safety of Radioactive Waste Disposal, Tokyo, Japan
IAEA (2011) Geological disposal facilities for radioactive waste : specific safety guide (IAEA Safety Standards for protecting people and the environment, Issue. I. A. E. Agency
Kazemi GA, Mohammadi A (2012) Significance of hydrogeochemical analysis in the management of groundwater resources: a case study in Northeastern Iran, hydrogeology - a global perspective. In: Kazemi GA (ed). InTech, Rijeka, Croatia. Available from: http://www.intechopen.com/books/hydrogeology-a-global-perspective/significance-ofhydrogeochemicalanalysis-in-the-management-of-groundwater-resources-a-case-study-in
Kim J-H, Kim R-H, Lee J, Cheong T-J, Yum B-W, Chang H-W (2005) Multivariate statistical analysis to identify the major factors governing groundwater quality in the coastal area of Kimje, South Korea. Hydrol Process 19(6):1261–1276. https://doi.org/10.1002/hyp.5565
Lakshmanan E, Kannan R, Kumar MS (2003) Major ion chemistry and identification of hydrogeochemical processes of ground water in a part of Kancheepuram district, Tamil Nadu, India. Environ Geosci 10(4):157–166
Larson TE, Skold RV (1958) Laboratory studies relating mineral quality of water to corrosion of steel and cast iron. Corrosion 14(6):43–46. https://doi.org/10.5006/0010-9312-14.6.43
Logah FY, Obuobie E, Ofori D, Kankam-Yeboah K (2013) Analysis of rainfall variability in Ghana. Int J Latest Res Eng Comput (IJLREC) 1(1):1–8
Lufuno L-M (2017) Investigation of the groundwater hydrogeochemistry characteristics in Beaufort West, South Africa. University of the Free State, South Africa
McLean W, Brown S, Scarff S, Rochford L (2011) NSW Office of Water 2011 characterisation of hydrogeochemistry and risks to groundwater quality. Parsons Brinckerhoff Australia Pty Limited
Noble RRP, Gray DJ, Gill AJ (2011) Field guide for mineral exploration using hydrogeochemical analysis. Commonwealth Scientific and Industrial Research Organisation (CSIRO), Canberra
Nude PM, Shervais JW, Attoh K, Vetter SK, Barton C (2009) Petrology and geochemistry of nepheline syenite and related carbonate-rich rocks in the Pan-African Dahomeyide orogen, southeastern Ghana, West Africa. J Afr Earth Sci 55(3–4):147–157. https://doi.org/10.1016/j.jafrearsci.2009.03.010
OECD (2003) Geological disposal: building confidence using multiple lines of evidence. First AMIGO Workshop, Yverdon-les-Bains, Switzerland
Salifu M, Yidana SM, Anim-Gyampo M, Appenteng M, Saka D, Aidoo F, Gampson E, Sarfo M (2015) Hydrogeochemical and isotopic studies of groundwater in the middle Voltaian aquifers of the Gushegu district of the Northern region. Appl Water Sci 7(3):1117–1129. https://doi.org/10.1007/s13201-015-0348-1
UN (2022) The United Nations World Water Development Report 2022. Groundwater: Making the invinsible visible, Issue
WHO (2003) Chloride in drinking-water. Background document for development WHO Guidelines for drinking-water quality, issue. WH Organization, Geneva
Zaidi FK, Nazzal Y, Jafri MK, Naeem M, Ahmed I (2015) Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: a case study from northwestern Saudi Arabia. Environ Monit Assess 187(10):607. https://doi.org/10.1007/s10661-015-4828-4
Acknowledgements
The authors express their deepest gratitude to the Radiation Protection Institute (RPI) of the Ghana Atomic Energy Commission (GAEC) for creating the enabling research environment and provision of logistical support for this study. The authors also acknowledge the expert advice and logistical support from the International Atomic Energy Agency (IAEA). Finally, our sincere thanks go to the technical staff of the Water Research Institute (WRI) of the Centre for Scientific and Industrial Research (CSIR), Ghana for the efficient analysis of groundwater samples.
Funding
No funding or grant was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Study conceptualization, investigation, data collection and analysis, writing of first draft of the manuscript were performed by GG. Study supervision, review and editing of first draft of the manuscript were done by EG. Investigation, review and editing of first draft of the manuscript were done by EA. Review and editing of the manuscript were done by AD and CE. Investigation, review and editing of first draft of the manuscript were done by PE. Investigation and data collection were done by EOS, EA and EAA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Animal research
This study does not involve any human or animal subject. Moreover, it does not entail any human and animal data or biological material. In this context, the study did not require any ethical approval.
Consent to participate
This study excludes human subjects, thus it does not warrant any consent to participate.
Consent to publish
This study excludes human participants, hence it does not require any informed consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gbeddy, G., Glover, E., Akortia, E. et al. Geostatistical and hydrogeochemical characterization of a probable borehole disposal site for radioactive waste in Accra, Ghana. Environ Earth Sci 82, 199 (2023). https://doi.org/10.1007/s12665-023-10900-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-023-10900-8