Abstract
In the process of CO2 geological sequestration, there is a risk that CO2 will leak into groundwater, resulting in a series of physical and chemical reactions, with influence on chemical compositions of groundwater. In this work, numerical simulation is conducted to study the influence of CO2 migration on the chemical composition of groundwater. The modeling results indicated that when CO2 leaks into groundwater, gaseous CO2 will migrate upward and diffuse laterally under the effect of buoyancy. The acidity of groundwater is enhanced and the pH is significantly reduced, leading to dissolution of calcite, illite, oligoclase, K-feldspar, chlorite and hematite, and precipitation of quartz, kaolinite, smectite-Na, siderite and smectite-Ca. The porosity of the formation increases. The concentrations of K+ and Fe2+ in groundwater gradually increase, the concentrations of Ca2+ and HCO3− basically remain unchanged after increasing to a certain value, the concentrations of Mg2+ and AlO2− first increase and then decrease, and the concentrations of Na+, Cl− and SO42− first decrease slightly and then gradually increase, but the change in concentration is small. Therefore, Fe2+, Mg2+, Ca2+ and pH can be used as important monitoring indicators of whether CO2 leakage into groundwater during geological storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the increasing concerns on global warming, CO2 geologic sequestration has been considered to be one of the most effective ways to reduce CO2 emissions and alleviate global warming (Cao et al. 2021). However, due to the uncertainty in geological conditions, the risk of CO2 leaking during geological storage has to be considered (Aines et al. 2009; Oldenburg et al. 2011). For example, sequestered CO2 may migrate into groundwater along faults, fractures and recovery wells (Du et al. 2012; Xie et al. 2017). Once CO2 migrate into groundwater, it will break the original hydrochemical equilibrium in groundwater and cause a series of problems on groundwater quality (Apps et al. 2010). Therefore, studying the impact of CO2 migration from geological storage on the quality of groundwater is an important part of the environmental risk assessment and development of early warning in CO2 geological storage.
Although the evaluation of the impact of CO2 geological storage on water quality has been studied long ago (Meer 1992), systematic study of the impact of CO2 on shallow groundwater quality began in 2004. Wang and Jaffe (2004) used numerical simulation for the first time to study the impact of CO2 migration on shallow groundwater. The results showed that if the monitored trace metal indicators were abnormal, it could be an indicator of CO2 leakage to shallow aquifers. Carroll et al. (2009) conducted reactive migration modeling on the impact of CO2 intrusion into American plateau aquifers, and showed that CO2 migration in aquifers can be detected by pH and carbonate chemical changes, and pointed out that the most appropriate monitoring location was the bottom of the waterproof roof. Fahrner et al. (2011) used PhreeqC to simulate the impact of CO2 migration to shallow groundwater on the electrical conductivity (EC) of groundwater, analyzed the impact of CO2 intrusion on the EC change of groundwater, and explored the use of monitoring groundwater EC changes to identify leakage. Kharaka et al. (2010) monitored the concentration changes of the main components, minor components, trace components and organic components in groundwater after CO2 injection, and the results showed that with CO2 injection, the concentrations of chemical components in groundwater changed significantly. Among them, pH dropped from 7.0 to 5.6, alkalinity increased from 400 to 1330 mg/L. The main components and trace components (including Pb, As, etc.) in groundwater increased significantly, but were lower than the Environmental Protection Agency (EPA) drinking water limit in the US. Keating et al. (2010, 2013) studied the impact of CO2 geological storage on the hydrochemistry of shallow groundwater in sandstone aquifers in New Mexico, USA. The study showed that high alkalinity and carbonate minerals mitigate the effects of CO2 intrusion on pH, and no migration of trace elements in the aquifer was observed, but the migration of As, U and Pb was observed in other saline aquifers. Therefore, they suggested that geochemical conditions determine the changes in the water quality of the aquifer and also affect the effectiveness of detecting CO2 migration. Chen (2013) studied the Ordos region of China and used the TOUGHREACT numerical simulation software to simulate the leakage of CO2 into shallow aquifers, and studied its impact on water quality. The results showed the migration of CO2 causes the migration of certain metals in the ore, which in turn affected the total amount of metal ions in the water.
Lu et al. (2010) conducted CO2 injection experiments on actual water-bearing media in the Gulf Coast area of the United States. The results showed the pH in the water decreased sharply (by about 3 units) at the beginning of CO2 injection, and then gradually recovered to a stable value. The concentration of some metal elements such as Ca, Mg, Zn, etc., increased rapidly when CO2 was just injected, and then tended to stabilize. The concentration of other metal elements such as Fe, Al, Cu, etc., increased at the beginning of the injection, and then began to decrease and became lower than before the test. Little and Jackson (2010, 2011) conducted more than 300 days of indoor injection experiments using aqueous media in 17 regions of the United States. The results showed that the H+ concentration in all aqueous media samples increased by 1 to 2 orders of magnitude. The concentration of alkali elements, alkaline earth elements, Mn, Co, Ni, and Fe in the water increased by more than 2 orders of magnitude. The concentration of U and Ba in individual media samples increased significantly. The migration of trace metal elements, the buffering effect of carbonate minerals, and the redox conditions of shallow aquifers control the effect of CO2. Because the concentrations of Mn, Fe, and Ca tend to increase at the beginning of the experiment, their concentrations can be used as the basis for monitoring CO2 leakage. Zhang et al. (2016) studied the impacts on pH value, DO, HCO3− and COD of surface water by changing the leakage time, leakage rate, and the temperature of carbon dioxide. They suggested that pH, DO and HCO3− could be used as the surface water monitering indicators for CO2 geological storage.
Natural isotope tracers are an important way to detect CO2 leakage. Sr isotopes were used to record the dissolution of carbonate rocks at CO2-enhanced oil recovery sites (Quattrocchi et al. 2005), and were coupled with carbon isotopes to track the movement of CO2 plumes during a controlled leak of CO2 into a shallow aquifer (Newell et al. 2014). Kim et al. (2020) evaluated the application of 222Rn in groundwater as a tracer for monitoring CO2 plume migration on shallow groundwater, and the results indicated that 222Rn could be used as a sensitive tracer to directly monitor CO2 leakage. Gardiner et al. (2021) demonstrated that multiple isotope system (δ13CDIC, 87Sr/86Sr, 234U/238U) could be used to identify and measure the impact of CO2 leakage at sequestration sites.
CO2 storage involves the flow of multi-phase and multi-component fluids in geological media, and processes such as mechanical and chemical reactions. To accurately simulate the effects of CO2 migration on the chemical composition of groundwater, it is necessary to couple the hydrodynamic and chemical reaction processes that may occur during the movement of multi-phase fluids in the formation. In this work, the Yanchang Oilfield in China was studied with numerical simulation to establish the reaction transport model of CO2 migration to groundwater during geological storage. By analyzing the influence of CO2 migration during geologic sequestration on the chemical components of groundwater, we can screen out monitoring indicators that are more sensitive to changes of CO2 leakage, and provide basis for similar CO2 geological storage monitoring programs, and reduce the possible risks in carbon capture and storage (CCS) projects.
Model setup
Modeling code and conceptualization
The non-isothermal reactive geochemical transport code TOUGHREACT V3.32-OMP (Xu 2001; Xu et al. 2006, 2014) was used in this work. TOUGHREACT is an extension of TOUGH2 (Pruess et al. 1999) and the fluid property module ECO2N was used for H2O–CO2–NaCl mixtures (Pruess et al. 2004).
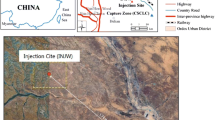
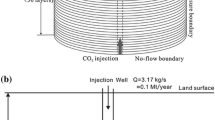
The depth of groundwater in the Yanchang Oilfield in China is 10 m, and the aquifer is sandstone. It is assumed that CO2 is leaking into the groundwater through cracks in the formation (Fig. 1). The vertical thickness of the groundwater is 60 m, which is divided into 6 grids, and the length in the horizontal direction is 500 m, which is divided into 50 grids (Fig. 2). The volume of the lateral boundary mesh is set to infinity. The annual average ground temperature in this area is 10.8 °C, the temperature gradient is about 3.1 °C /100 m, and the hydrostatic pressure gradient is 10 bar/100 m. Therefore, the bottom temperature of the model is about 13 °C and the pressure is 8 bar. The CO2 leakage point is set at X = 250 m, the CO2 leaking time and simulation time are set to 100 years. The constant leakage rate of CO2 is 0.0001 kg/s, which is estimated by the following formula.
where QG is the gas leakage rate, kg/s; Cd is the gas leakage coefficient, and Cd = 1 when the shape of the crack is round, Cd = 0.95 when the shape of the crack is triangular, Cd = 0.9 when the shape of the crack is rectangular; A is the area of the crack, m2; P is the pressure, MPa; Y is the outflow coefficient; M is the relative molecular mass, and the relative molecular mass of CO2 is 0.044 kg/mol; K is the adiabatic index; R is the gas constant, the value is 8.314, J/(mol·K); T is the temperature, K.
For the hydrogeologic parameters of groundwater, the porosity and permeability values are from geological surveys of the Yanchang oilfield in China, and the other hydrogeological parameters are from Xu et al. (2010). The hydrogeologic parameters are shown in Table 1.
The liquid relative permeability and capillary pressure models applied in this model are the Van Genuchten model (Van Genuchten 1980), and the gas relative permeability model is the Corey model (Corey 1954). The specific parameters are extracted from Xu et al. (2010). The models of relative permeability and capillary pressure are shown in Table 2.
The mineral composition of the formation
The primary mineral composition of the aquifer and the possible secondary minerals are shown in Table 3, extracted from Xu et al. (2014).
The volume fractions of primary mineral, possible secondary mineral, and their reaction kinetics parameters are shown in Table 4. Since the reaction rate of calcite is fast, it is assumed at equilibrium throughout the simulations. The dissolution and precipitation of other minerals are controlled by kinetics, and the kinetic parameters are extracted from Lasaga et al. (1994).
Kinetic rates could be functions of non-basis species as well. Usually the species appearing in rate laws happen to be basis species. TOUGHREACT (Xu et al. 2006) uses a general form of rate expression (Lasaga et al. 1994).
where positive values of rn indicate dissolution, and negative values precipitation, kn is the rate constant, An is the specific reactive surface area per kg H2O, Ωn is the kinetic mineral saturation ratio. θ and η are usually taken equal to one.
The temperature dependence of the reaction rate constant can be expressed reasonably well via an Arrhenius equation (Lasaga, 1984; Steefel and Lasaga, 1994). Because many rate constants are reported at 25 °C, it is convenient to approximate rate constant dependency as a function of temperature, thus
where k25 is the rate constant at 25 °C; R is gas constant; Ea is the activation energy; T is absolute temperature.
The reaction rate constant calculated using the above formula is usually considered only with pure water (neutral mechanism), while the dissolution and precipitation of minerals are often catalyzed by H+ (acid mechanism) and OH− (base mechanism). The kinetic rate constant k includes each of these three mechanisms (Lasaga et al. 1994; Palandri and Kharaka 2004).
where superscripts or subscripts nu, H, and OH indicate neutral, acid and base mechanisms, respectively; Ea is the activation energy, (KJ/mol); k25 is the rate constant at 25 °C, (mol/(m2 s)); R is gas constant, (KJ/(mol K)); T is absolute temperature, (K); a is the activity of the species; and n is power term (constant). Notice that parameters θ and η are assumed the same for each mechanism.
The reaction surface area A changes as the reaction progresses. In TOUGHREACT, the reaction surface area is approximated as a spherical model.
where \(\phi\) is porosity, r is the particle radius of a particular porous medium.
Chemical composition of shallow groundwater
The initial chemical composition is measured using groundwater samples collected from the Yanchang Oilfield in China. The salinity of groundwater is about 0.867 g/L and the density is 1 × 103 kg/m3. The groundwater is reacted with aquifer minerals listed in Table 3 to obtain equilibrium concentration. The initial and equilibrium concentrations are given in Table 5.
Results and discussion
Change of CO2 concentration in groundwater
As shown in Fig. 3, after CO2 migrates to groundwater, because the density of CO2 is smaller than the density of water, CO2 will migrate upward under buoyancy and gradually diffuse to the surroundings. As CO2 gradually diffuses, the CO2 gas saturation in the groundwater formation gradually increases. By 100 years, the CO2 gas saturation at the leaking point is about 0.36(Fig. 5a). CO2 basically diffuses into the entire area and gather at the top of groundwater (Fig. 3f). A fraction of CO2 will dissolve in groundwater and participate in water–rock interactions. As CO2 gradually migrates, the region with dissolved CO2 in groundwater gradually expands (Fig. 4). The dissolved CO2 concentration increases gradually at the point source of CO2 injection. By 10 years the concentration of dissolved CO2 changes little at the leaking point. By 100 years, the concentration of CO2 dissolved at the point source of CO2 is about 0.38 mol/L (Fig. 5b).
Chemical reaction in groundwater and change of mineral volume fraction
With the gradual migration of CO2, the amount of CO2 dissolved in groundwater continues to increase, and the spatial distribution of pH gradually expands (Fig. 6), resulting in a significant decrease in the pH value of groundwater and an increase in formation porosity (Fig. 7). By 100 years, the pH of groundwater has decreased from 6.67 to about 5.3 (Fig. 8a), and the chemical composition of groundwater is greatly affected. The porosity of the formation increases in 0–10 years, and when the mineral dissolution and precipitation reach a steady state, the porosity basically remains unchanged. By 100 years, formation porosity is approximately 0.20027 (Fig. 8b). The main reason for the decrease in pH value of groundwater is that CO2 dissolves in groundwater to form carbonic acid, which is unstable and decomposes into H+ and HCO3−. The reaction equations are as follows.
The change in volume fraction (abundance) of mineral is defined by the following formula.
where V0 is the initial volume fraction of mineral, V1 is the volume fraction of mineral at a given time t. Negative values indicate dissolution, positive values indicate precipitation.
CO2 migration to groundwater changes the acidity of the groundwater and the original water chemistry, leading to dissolution of calcite, oligoclase, K-feldspar, chlorite and hematite (Fig. 9a). The volume fraction of calcite remains almost unchanged after 10 years. The volume fractions of oligoclase, K-feldspar and chlorite gradually decrease, while illite begins to precipitate after 10 years (Fig. 9b). The dissolution reactions are as follows:
The dissolution of minerals consumes H+ in the groundwater, alleviating the pH value of the groundwater, and leads to precipitation of secondary minerals. When the dissolved CO2 in groundwater gradually increases, K-feldspar is gradually transformed into kaolinite. In addition, due to the massive dissolution of oligoclase and chlorite, the concentration of Na+ and Mg2+ in the groundwater increases significantly, resulting in the precipitation of smectite-Na. Figure 9c shows that the mineral volume fraction of quartz, kaolinite and smectite-Na increases gradually, and the amount of mineral precipitation increases accordingly, among which the volume fraction of smectite-Na changes the most. By 100 years, the volume fraction of smectite-Na changes by about 1.65 × 10–5, while the volume fraction of hematite remains almost unchanged. The precipitation reactions occur as follows.
The dissolution and precipitation of the initial minerals lead to significant changes in the concentration of chemical components in the groundwater, which then recombine to form secondary minerals. In 100 years, the secondary minerals are mainly smectite-Ca and siderite. As shown in Fig. 9d, the volume fraction of smectite-Ca and siderite gradually increases, and the volume fraction of siderite changes the most. By 100 years, the volume fraction of siderite changes about 3.29 × 10–8. The reactions that occur are as follows:
Changes in the concentration of chemical components in groundwater
Figure 10a shows that the concentration of K+ gradually increases at the point source of CO2 leaking. The increase in K+ concentration is mainly due to the dissolution of K-feldspar and illite. Illite begins to precipitate after 10 years, which consumes K+, making the concentration of K+ increase slowly after 10 years. By 100 years, the concentration of K+ at the leaking point is 1.67 × 10–4 mol/L. The dissolution of calcite and oligoclase for the generation of secondary mineral smectite-Ca offers a large amount of Ca2+. In the initial time of CO2 leakage, the concentration of Ca2+ increases significantly. By 10 years, the concentration of Ca2+ is 2.09 × 10–3 mol/L at the leaking point, after 10 years, the concentration of Ca2+ increases more slowly. By 100 years, the concentration of Ca2+ is 2.18 × 10–3 mol/L (Fig. 10b) at the leaking point. The change trend of the concentration of Ca2+ is basically the same as that of calcite. The dissolution of oligoclase significantly increases the concentration of Na+ in groundwater, while the formation of smectite-Na consumes part of Na+. Figure 9a, c shows that the volume fraction change of oligoclase is about 2.07 × 10–5, and the volume fraction of smectite-Na is about 1.65 × 10−5at 100 years. Since the initial volume fraction of oligoclase is much larger than that of smectite-Na, the concentration of Na+ gradually increases (Fig. 10c).
Figure 10d shows that the concentration of Mg2+ increases first and then decreases. The dissolution of illite and chlorite significantly increase the concentration of Mg2+ in groundwater, and the precipitation of smectite-Na and smectite-Ca consume part of Mg2+. It can be seen from Fig. 9c, d the volume fraction change of smectite-Na and smectite-Ca increase significantly and consume a large amount of Mg2+. Therefore, the concentration of Mg2+ decreases significantly after 10 years, but it is still higher than the initial value, indicating that the content of Mg2+ produced by the dissolution of illite and chlorite is still greater than the Mg2+ consumed by smectite-Na and smectite-Ca precipitation. It can be seen from Fig. 10e the concentration of Fe2+ increases significantly. The dissolution of chlorite provides a large amount of Fe2+ for the precipitation of siderite. Comparing Fig. 9a, b, it can be seen that the change in the volume fraction of chlorite is 2–3 orders of magnitude higher than the change in the volume fraction of siderite. In 10 years, the volume fraction of siderite changes significantly. Therefore, the concentration of Fe2+ increases significantly in 0–10 years. Due to the increase in the amount of siderite precipitation and the consumption of part of Fe2+, the increasing trend of Fe2+ began to slow down.
The change of HCO3− concentration is partly caused by the decomposition of carbonic acid, and partly by the dissolution of calcite and the precipitation of siderite. According to Fig. 10f, by 10 years, the concentration of HCO3− is 0.86 mol/L at the leaking point, and from the 10 years, the concentration of HCO3− changes little. Comparing Fig. 9a, d, it can be seen that the variation of the volume fraction of calcite is significantly greater than that of siderite, and the variation trend of the concentration of HCO3− is basically similar to that of the dissolved CO2 and Ca2+ in groundwater. As can be seen from Fig. 10g, the concentration of AlO2− increases first and then decreases. The increase of AlO2− concentration is mainly due to the precipitation of illite. In addition, the dissolved CO2 in the water also reacts with part of AlO2− (Formula (22)), leading to the decrease of AlO2− concentration, but the concentration of AlO2− is still higher than the initial value. Due to SO42− and Cl− in the groundwater is not involved in the mineral dissolution and precipitation reaction, therefore, the concentration of SO42− and Cl− change very little, and basically maintain at the initial value (Fig. 10h, i).
Monitoring indicators
As the pH of groundwater decreases significantly with CO2 leakage, the acidity of groundwater increases significantly, and the monitoring of pH value is more convenient and intuitive, therefore, we should focus on monitoring the change characteristics of the pH value of groundwater. It is recommended to lay an in-situ online monitor in the target aquifer to monitor the change characteristics of the pH of groundwater. The frequency of in-situ online monitoring is recommended to be once every 10 min, and sampling monitoring is recommended to be once a month. By comprehensively improving the monitoring frequency of groundwater, the changes of groundwater monitoring indicators can be timely and accurately grasped. The data obtained from monitoring should be compared with the previous environmental background value to eliminate fluctuations in monitoring indicators caused by non-CO2 leakage such as environmental factors and human activities, so as to avoid the wrong judgment of CO2 leakage. If the pH of groundwater is abnormal, it is highly likely to be caused by CO2 leakage, and remedial measures should be taken immediately.
Due to the small changes in formation porosity, it is not suitable as a monitoring indicators. As AlO2− has a strong ability to bind H+, it is very easy to form Al(OH)3 precipitation, making it is not suitable as a monitoring indicator. The relative change of ion concentration in groundwater is calculated by the following formula.
where C1 is the ion concentration at a given moment, (mol/L); C0 is the initial ion concentration, (mol/L).
Figure 11 shows that the concentrations of Fe2+, Mg2+ and Ca2+ in groundwater change significantly after 10 and 100 years of CO2 leaking. The concentrations of K+ and HCO3− change slightly, while the concentrations of Na+, Cl− and SO42− remain almost unchanged. Therefore, it is recommended to adopt Fe2+, Mg2+ and Ca2+ as important monitoring indicators of CO2 leakage. Ion concentration is mainly monitored by sampling. It is recommended to take samples once a month before CO2 injection and storage, twice a month during CO2 injection and storage, and once a month in the later stage of CO2 injection and storage. In the monitoring process, the sampling frequency and analysis intensity should be adjusted according to the actual situation, and the CO2 leakage situation should be judged jointly with the monitoring instrument.
These indicators are recommended to be monitored to detect CO2 in time and provide early warnings. Once data abnormalities are found, corresponding measures should be taken. In addition, some monitoring indicators (such as conductivity, temperature and pressure, etc.) can be appropriately added based on the actual situation on site, and the monitoring indicator system can be further improved on the basis of accurate data.
Conclusions
In this work, the multi-phase reaction transport code TOUGHREACT-OMP/ECO2N is used to model the influence of CO2 migration on shallow groundwater. The following conclusions are made from our simulations.
-
1.
After CO2 migrates to groundwater, it migrates upward due to buoyancy in gaseous form and gradually diffuses to the surroundings, and the CO2 gas saturation in groundwater gradually increases. A fraction of CO2 will be dissolved in the groundwater. As the dissolved CO2 gradually increases, the acidity of the groundwater is significantly increased and the pH is significantly reduced, causing dissolution/precipitation of the initial minerals, resulting in a slight increase in the porosity of the formation.
-
2.
The dissolved minerals are calcite, illite, oligoclase, K-feldspar, chlorite and hematite; the precipitated minerals are quartz, kaolinite and smectite-Na. The secondary minerals are siderite and smectite-Ca.
-
3.
The dissolution and precipitation of minerals change the concentration of chemical components in the groundwater. The concentrations of K+ and Fe2+ in the groundwater gradually increase. The concentrations of Ca2+ and HCO3− remain basically unchanged after increasing to a certain value. The concentration of Mg2+ and AlO2− increase first and then decrease, but is still higher than the initial value. The concentration of Na+, SO42− and Cl− do not change much.
-
4.
It is suggested to choose pH as an important indicator to judge whether CO2 leakage into groundwater during geological storage. At the same time, the changes of Fe2+, Mg2+, and Ca2+ should be monitored. These indicators could provide early warning of CO2 leakage.
References
Aines RD, Leach MJ, Weisgraber TH, Simpson MD, Friedmann SJ, Bruton CJ (2009) Quantifying the potential exposure hazard due to energetic releases of CO2 from a failed sequestration well. Energy Proc 1(1):2421–2429. https://doi.org/10.1016/j.egypro.2009.02.003
Apps JA, Zheng L, ZhangY XuTF, Birkholzer JT (2010) Evaluation of potential changes in groundwater quality in response to CO2 leakage from deep geologic storage. Transport Porous Med 82(1):215–246. https://doi.org/10.1007/s11242-009-9509-8
Cao F, Eskin D, Leonenko Y (2021) Modeling of carbon dioxide dissolution in an injection well for geologic sequestration in aquifers. Energy 221(7):119780. https://doi.org/10.1016/j.energy.2021.119780
Carroll S, Hao Y, Aines R (2009) Geochemical detection of carbon dioxide in dilute aquifers. Geochem T 10(1):1–18. https://doi.org/10.1186/1467-4866-10-4
Chen K (2013) The impact of CO2 leakage from reservoir on the water quality of the shallow aquifer. Dissertation, Jilin University, CN
Corey AT (1954) The interrelation between gas and oil relative permeabilities. Prod Mon 01:38–41
Du SH, Su XS, Zheng LG (2012) Advances in deep CO2 leakage impact on shallow groundwater quality. Adv Water Sci 023(006):875–880. https://doi.org/10.14042/j.cnki.32.1309.2012.06.013
Fahrner S, Schaefer D, Dahmke A (2011) Reactive transport modeling to assess geochemical monitoring for detection of CO2 intrusion into shallow aquifers. Energy Proc 4:3155–3162. https://doi.org/10.1016/j.egypro.2011.02.230
Gardiner JB, Capo RC, Newell DL, Stewart BW, Hakala JA (2021) Tracking natural CO2 migration through a sandstone aquifer using Sr, U and C isotopes: Chimayó, New Mexico, USA. Int J Greenh Gas Con 104:103209. https://doi.org/10.1016/j.ijggc.2020.103209
Keating EH, Fessenden J, Kanjorski N, Koning DJ, Pawar R (2010) The impact of CO2 on shallow groundwater chemistry: observations at a natural analog site and implications for carbon sequestration. Environ Earth Sci 60(3):521–536. https://doi.org/10.1007/s12665-009-0192-4
Keating EH, Hakala JA, Viswanathan H, Carey JW, Pawar R, Guthrie GD, Fessenden-Rahn J (2013) CO2 leakage impacts on shallow groundwater: Field-scale reactive-transport simulations informed by observations at a natural analog site. Appl Geochem 30(Complete):136–147. https://doi.org/10.1016/j.apgeochem.2012.08.007
Kharaka YK, Thordsen JJ, Kakouros E, Ambats G, Herkelrath WN, Beers SR, Birkholzer JT, Apps JA, Spycher NF, Zheng L (2010) Changes in the chemistry of shallow groundwater related to the 2008 injection of CO2 at the ZERT field site, Bozeman. Montana Environ Earth Sci 60(2):273–284. https://doi.org/10.1007/s12665-009-0401-1
Kim J, Lee S, Ha S, Joun W, Ju YJ, Lee K (2020) Natural 222Rn as a tracer of mixing and volatilization in a shallow aquifer during a CO2 injection experiment. Hydrol Process 34(26):5417–5428. https://doi.org/10.1002/hyp.13953
Lasaga AC (1984) Chemical kinetics of water-rock interactions. J Geophys Res 89:4009–4025. https://doi.org/10.1029/JB089iB06p04009
Lasaga AC, Soler JM, Ganor J, Burch TE, Nagy KL (1994) Chemical weathering rate laws and global geochemical cycles. Geochim Cosmochim Acta 58:2361–2368. https://doi.org/10.1016/0016-7037(94)90016-7
Little MG, Jackson RB (2010) Potential impacts of leakage from deep CO2 geo-sequestration on overling freshwater aquifers. Environ Sci Technolog 44:9225–9232. https://doi.org/10.1021/es102235w
Little MG, Jackson RB (2011) Response to comment on “Potential impacts of leakage from deep CO2 geo-sequestration on overlying freshwater aquifers.” Environ Technol 45(7):3175–3176. https://doi.org/10.1021/es200421f
Lu J, Partin JW, Hovorka SD, Wong C (2010) Potential risks to freshwater resources as a result of leakage from CO2 geological storage: a batch-reaction experiment. Environ Earth Sci 60(2):335–348. https://doi.org/10.1007/s12665-009-0382-0
Meer L (1992) Investigations regarding the storage of carbon dioxide in aquifers in the Netherlands. Energ Convers Manage 33(5–8):611–618. https://doi.org/10.1016/0196-8904(92)90063-3
Newell DL, Larson TE, Perkins G, Pugh JD, Stewart BW, Capo RC, Trautz RC (2014) Tracing CO2 leakage into groundwater using carbon and strontium isotopes during a controlled CO2 release field test. Int J Greenh Gas Con 29:200–208. https://doi.org/10.1016/j.ijggc.2014.08.015
Oldenburg CM, Jordan PD, Nicot JP, Nicot JP, Mazzoldi A, Gupta AK, Bryant SL (2011) Leakage risk assessment of the In Salah CO2 storage project: applying the certification framework in a dynamic context. Energy Proc 4:4154–4161. https://doi.org/10.1016/j.egypro.2011.02.360
Palandri J, Kharaka YK (2004) A compilation of rate parameters of water-mineral interaction kinetics for application to geochemical modeling. US Geology Surv Open File Report 2004-1068
Pruess K, Oldenburg C, Moridis G (1999) TOUGH2 User's Guide, Version 2.0. LBL-43134, Lawrence Berkeley National Laboratory, Berkeley, CA. https://doi.org/10.2172/751729
Pruess K, Garcia J, Kovscek T, Oldenburg C, Rutqvist J, Steefel C, Xu T (2004) Code intercomparison builds confidence in numerical simulation models for geologic disposal of CO2. Energy 29(9/10):1431–1444. https://doi.org/10.1016/j.energy.2004.03.077
Quattrocchi F, Barbieri M, Bencini R, Cinti D, Durocher K, Galli G (2005) Strontium isotope (87Sr/86Sr) chemistry in produced oilfield waters: the iea weyburn CO2 monitoring and storage project. Greenh Gas Control Technol 65:2111–2114. https://doi.org/10.1007/1-4020-4471-2_20
Steefel CI, Lasaga AC (1994) A coupled model for transport of multiple chemical species and kinetic precipitation/dissolution reactions with applications to reactive flow in single phase hydrothermal system. Am J Sci 294:529–592. https://doi.org/10.2475/ajs.294.5.529
Van Genuchten MT (1980) A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci Soc Am J 44(5):892–898. https://doi.org/10.2136/sssaj1980.03615995004400050002x
Wang S, Jaffe PR (2004) Dissolution of a mineral phase in potable aquifers due to CO2 releases from deep formations; effect of dissolution kinetics. Energ Convers Manage 45(18–19):2833–2848. https://doi.org/10.1016/j.enconman.2004.01.002
Xie J, Wei N, Wu LZ, Zhang KN, Xu M (2017) Progress in leakage study of geological CO2 storage. Rock Soil Mech S1:187–194. https://doi.org/10.16285/j.rsm.2017.S1.021
Xu TF (2001) Modeling multiphase non-isothermal fluid flow and reactive geochemical transport in variably saturated fractured rocks: 1. Methodology. AM J Sci 301(1):16–33. https://doi.org/10.2475/ajs.301.1.16
Xu TF, Sonnenthal E, Spycher N, Pruess K (2006) TOUGHREACT-A simulation program for non-isothermal multiphase reactive geochemical transport in variably saturated geologic media: applications to geothermal injectivity and CO2 geological sequestration. Comput Geosci-UK 32(2):145–165. https://doi.org/10.1016/j.cageo.2005.06.014
Xu TF, Kharaka YK, Doughty C, Freifeld BM, Daley TM (2010) Reactive transport modeling to study changes in water chemistry induced by CO2 injection at the Frio-I Brine Pilot. Chem Geol 271(3–4):0–164. https://doi.org/10.1016/j.chemgeo.2010.01.006
Xu TF, Sonnenthal E, Spycher N, Zheng LG (2014) TOUGHREACT V3.0-OMP sample problems. Earth Sciences Division, Lawrence Berkeley National Laboratory University of California, Berkeley, CA, 94720
Zhang L, Parthasarathy H, Karamalidis A (2016) Investigation on arsenopyrite dissolution and As(III) migration under geologic carbon storage conditions: a numerical simulation approach. Greenh Gases. https://doi.org/10.1002/ghg.1651
Acknowledgements
This work was supported by National Key Research and Development Program of China (Grant no. 2018YFB0605504).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, L., Wu, Y., Chong, S. et al. Influence of CO2 migration from geological storage on the chemical composition of groundwater and monitoring indicators. Environ Earth Sci 81, 73 (2022). https://doi.org/10.1007/s12665-022-10194-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-022-10194-2