Abstract
The Ethiopian Rift Valley (ERV) is characterized by arid and semi-arid climate with groundwater as the most important water resource used for drinking and irrigation purposes. However, in the region, people are suffering from severe water scarcity exacerbated by climate effect. Besides water availability, endemic water quality issues are critical and affect the suitability of the water and human health risks. The present study evaluates the suitability of groundwater for drinking and agricultural purposes in the Ziway Lake Basin (ZLB) of the ERV. Groundwater used for drinking contains multiple inorganic contaminants in levels that surpass the World Health Organization recommended limits. The most frequent of these violations were for Na+, K+, HCO3−, F− and few samples for Mn, As, U, Pb and Mo. The modeled Drinking Water Quality Index (DWQI) values of the groundwater show wide variation ranging from 12.7 (Excellent category) to 714 (Unsuitable category) with mean value of 94. Likewise, Irrigation Water Quality Index (IWQI) computed by considering EC, SAR, Na%, RSC and PI of the groundwater varies from 13.2 to 520 with a mean value of 106. Both DWQI and IWQI values suggest that groundwater is generally of Excellent quality for drinking and irrigation use in the headwater regions of the ZLB and progressively becomes extremely Unsuitable toward the rift floor. The exceptionally high DWQI values to the west of Lake Ziway is mainly associated with the co-occurrence of multiple toxic elements from a groundwater from the Quaternary sediments and rhyolitic volcanic aquifers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater represents more than 90% of the world’s readily available freshwater resources (Boswinkel 2000) and has a vital role in supporting the life of mankind (WRI, UNEP, UNDP, World Bank 1998). As groundwater is less vulnerable to contamination than surface water and provides a buffer against climate change, it has been increasingly used as water source for human consumption, irrigation, and industrial water supply (Wada et al. 2014). However, getting enough water with the required quality to satisfy the demand for various purposes is one of the major challenges in the twenty-first century (Amangabara and Ejenma 2012; Fienen and Arshad 2016).
The Ethiopian Rift Valley (ERV), a volcano-tectonically active part of the East African Rift system, provides an example of the challenges to water supply posed by groundwater quality. The ERV is about 1000 km long and 70–80 km wide, bordered by large and discontinuous normal fault systems (WoldeGabriel et al. 2000). As the rift margins are 2000–3000 m above the rift floor, the rainfall within the region is highly variable both in space and time. As a result, the available surface water resources are unreliable and not sufficient to fully support the sharply increasing population and socioeconomic development in the region. Consequently, groundwater becomes the only dependable water resource for community water supply, especially for the people residing in the semi-arid to arid section of the ERV. However, groundwater of the ERV has a significant water quality problem and does not comply with the established drinking water quality norms (Tekle-Haimanot et al. 1987; Tekle-Haimanot 1990; Kloos and Tekle-Haimanot 1999; Reimann et al. 2003). Several researchers (Chernet 1982; Darling et al. 1996; Ayenew 1998; Gizaw 1996; Yirgu et al. 1999; Chernet et al. 2001; Reimann et al. 2003; Tekle-Haimanot et al. 2006; Rango et al. 2010) noted that high salinity and fluoride levels appear to be the most widespread causes of water quality degradation in the region. According to Kassa (2007), the majority (80%) of groundwater well failures and abandonment in the central ERV are linked to these causes. The poor water quality in the region seems to be linked to the mobilization of contaminants naturally occurring in the volcanic rocks and lacustrine sediments and thus the enrichment of groundwater, e.g., with toxic elements (As, U, Mo and B), as it flows from rift escarpments to rift valley floor (UNDP 1973; Darling et al. 1996; Gizaw 1996; Rango et al. 2009, 2010, 2013).
Elevated geothermal gradients and emergence of mantle gases (e.g., CO2) escaping through fault zones are very common and enhance silicate weathering (Darling et al. 1996; Mechal et al. 2017), further facilitating the release of major and trace elements. As a result, the tolerance limit of these elements is often exceeded in drinking water, and over 13 million people living in the ERV are at risk from continuous exposure to high levels of multiple naturally occurring toxic chemical elements in the groundwater they consume (Reimann et al. 2003; Rango et al. 2012). Therefore, it is of great concern to assess the quality of groundwater for human consumption and health risks as well as for sustainable economic development, particularly its suitability to agricultural production (irrigation).
Several hydrochemical studies (e.g., Reimann et al. 2003; Kebede et al. 2010; Rango et al. 2009, 2010, 2013) have attempted to assess the groundwater water quality by comparing individual water quality parameters with standard values based on their uses. However, this type of assessment is not capable of providing a comprehensive picture of groundwater quality, especially when there is a co-occurrence of multiple water quality degraders for decision makers who require concise information. Moreover, the existing studies were mainly based on data points focused on the rift axis and very little information is available about the extension of water quality problems in the areas toward to the escarpment of the rift. As a result, the groundwater of ERV is crudely considered as unfit for any purposes. Thus, it is essential to evaluate the overall water quality status at basin wide scale to identify the most suitable areas for the development of groundwater resources for the desired purposes.
Analyses of surface and groundwater chemistry have been widely used to determine the water quality status. Water quality indexes (WQIs), based on the chemical composition of the water, are efficient techniques to determine the overall water quality by aggregating several water quality parameters into a single number that helps managers and decision makers (Gebrehiwot et al. 2011; Rajankar et al. 2010; Ravikumar et al. 2013; Ocampo-Duque et al. 2013; Sutadian et al. 2016). Nowadays, several types of WQIs have been formulated and, in particular, used to determine the appropriateness of groundwater for drinking purposes (Drinking Water Quality Index, DWQI) by many authors (Babiker et al. 2007; Singh and Khan 2011; Selvam et al. 2013; Sutadian et al. 2016; Boateng et al. 2016; RamyaPriya and Elango 2018).
The hydrochemical constituents of irrigation waters can have negative impacts on crop production and soil fertility (e.g., Singh et al. 2018). The projected results of IPCC (2013) suggest that the combined increase in evaporation and variability in rainfall over ERV due to global warming might lead to stress in surface water availability and quality. As a result, groundwater can potentially substitute for unreliable surface water sources for irrigation, especially under climate change, and thus evaluation of the groundwater suitability for agricultural purposes is essential for future use of the water resource. Many hydrochemical indices are used to study the quality of water for irrigation (e.g., Abbasnia et al. 2018; RamyaPriya and Elango 2018): electrical conductivity (EC), sodium percentage (Na %), sodium adsorption ratio (SAR), residual sodium carbonate (RSC), permeability index (PI), and irrigation water quality index (IWQI).
To enhance the understanding of the spatial variability of water quality and support the identification of groundwater resources suitable for drinking or irrigation purposes within the ERV, this work examines the example of Ziway Lake Basin (ZLB). The prime objective of this research is to: (1) assess the spatial distribution of major and trace element concentrations in the groundwater, (2) evaluate the appropriateness of groundwater for drinking and agricultural purposes, and (3) finally develop water quality maps of ZLB that will help for easy identification of areas for remediation and development of the groundwater resource.
Study area
Location, physiography, and climate
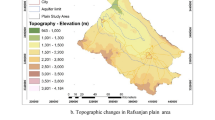
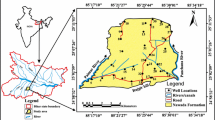
The study area, ZLB, lies in the northern sector of ERV with an aerial extent of 7291 km2, out of which 430 km2 is covered by Lake Ziway (Fig. 1). Geographically, it is bound between 7.36–8.47° N latitude and 38.22–38.4° E longitude.
In the ZLB, two prominent physiography regions exist, namely: the rift floor [< 2000 m above sea level (m a.s.l.)] and the adjoining escarpments (2000–4181 m a.s.l.). Although, the altitude shows wide ranges, about 60% of the basin area belongs to the rift floor while the remaining 40% belongs to the bordering escarpment on either side of the rift floor. Lake Ziway occupies the center of the rift floor and is fed by the two major rivers Ketar and Meki originating from the eastern and western flanks of the rift escarpments, respectively (Fig. 1).
The climate of ZLB varies greatly from semi-arid in the rift floor to humid in the bordering escarpment. The long-term (1981–2010) average annual rainfall of the study area is estimated to be 880 mm/year (Firdissa 2019). Nevertheless, in the escarpment bounding the rift floor, precipitation surpasses 1200 mm/year, whereas at the lowest altitude in the rift floor rainfall is frequently below 625 mm/year. Mean monthly temperature varies from 21.8 °C in the rift floor to less than 13.5 °C in the adjoining escarpments. The strong spatial variability of rainfall and temperature thus is explained by large differences in elevation.
Geological and hydrogeological setting
Being located in the heart of ERV, the geological setup of the study area, ZLB, is the result of different series of volcanic eruptions accompanied by tectonic events and denudation processes. The volcanic series and geological structures of the ERV have been extensively explored (Mohr 1962; Di Paola 1972; Merla et al. 1979; Kazmin et al. 1980; Woldegabriel et al. 1990; Di Paola et al. 1993; Boccaletti et al. 1998; Halcrow 2008; Dainelli et al. 2001; Benvenuti et al. 2002). Broadly, the outcropping lithology of the area can be subdivided into three major groups: Precambrian and Mesozoic sedimentary rocks, volcanites and volcano-clastic rocks, and Quaternary sedimentary deposits.
The Precambrian and Mesozoic sedimentary rocks cover a very limited area in the northwestern part of the study area. They comprise Precambrian biotite gneiss overlain by layers of Mesozoic sedimentary successions (sandstones, shales, marls, and limestones). A large part of the ZLB is occupied by a variety of volcanic rocks in association with volcano–clastic sedimentary deposits. The adjacent escarpments comprise basaltic lava flows with interlayered rhyolitic ignimbrite and tuff horizons. On the eastern side, these rock units are underlain by shield volcanoes comprising mainly trachytes with subordinate basalts and phonolites. Most parts of the rift floor are covered by silicic pyroclastic materials mainly consisting of peralkaline rhyolitic ignimbrites, poorly welded ignimbrites, and pumice fall with minor basaltic lava flows. Overlying the rift volcanic series, recent Quaternary sediments occupy the lake margins. The volcanic rocks and sediments of the ZLB have undergone extensive faulting resulting from the prevailing tectonic activity (Fig. 1).
As can be understood from the geological map (Fig. 1), the hydrogeology of the study area is highly heterogeneous as a result of variability in lithologic units and structures. Despite the complexity in lithology, the rock units can be grouped into three major hydrostratigraphic units: Quaternary sediments, fractured and weathered volcanics, and volcanics with low permeability (Ayenew 1998; OWWDSE 2016).
The Quaternary sediments are highly permeable and thick (> 30 m) in the rift floor; whereas in the steep scarps of the escarpments, the sediments are almost nonexistent and of low permeability. The fractured and weathered volcanic units form the major regional aquifer system. These units include basalt, ignimbrite and in some places rhyolites with varying permeability that generally increases toward the rift floor due to the increasing intensity of fracturing. Volcanics mainly comprising highly welded ignimbrites, tuff, rhyolite and trachyte with very low permeability occupy large parts of eastern and western mountainous regions.
In the study area, groundwater generally flows to the rift center from the adjacent escarpments indicating a topographic driven process at regional and sub-regional scale (Ayenew 1998; Ayenew et al. 2008, 2009). As a result, the rift floor is considered as regional discharge area where perennial rivers, lake and springs exist. However, locally in the rift floor, the groundwater flow is strongly influenced by geological structures manifested by wetlands, swamps, and thermal springs emerging from active faults.
Groundwater use
In ZLB, groundwater is used for different purposes, with the largest proportion for human consumption. Groundwater provides drinking water for almost the entire population in the region, as surface water resources such as rivers and lakes nearby the community are seasonal and usually carry higher pathogenic risks due to fecal contamination. Groundwater sources for drinking use ranges from springs to deep wells. However, many groundwater wells are contaminated with high levels of fluoride and other naturally occurring toxic trace elements (As, U, Mo, and B) (Reimann et al. 2003; Rango et al. 2009, 2010). Major towns in the region receive somewhat treated water from rivers and high discharge springs (fresh water). In contrast, the vast rural community largely relies on untreated groundwater wells and experiences dental fluorosis and skeletal fluorosis due to long-time exposure of fluoride-contaminated groundwater with clearly unknown health impacts of other toxic trace elements (Rango et al. 2012, 2014, 2017).
Agriculture activities are the main source of income for the majority of people living in the study areas and, hence, the overall economic growth of these areas is highly dependent on the success of the agriculture sector. Alongside surface water resource such as Meki and Katar rivers and Ziway Lake, groundwater is also used in the region by small-scale commercial irrigation, agro-industries, and floriculture farms. Irrigation is mainly sustained by surface water, but less than one-quarter of the irrigated areas depend on groundwater (Rodriguez de Francisco 2008; Pascual-Ferrer et al. 2014). Agricultural practice in the region is highly tied with the unreliable wet season of a year. During the last years, irrigation agriculture has increased rapidly in the study areas using shallow and deep groundwater wells especially in the rift floor. But the exploitation of groundwater for irrigation use is highly hampered by high salinity (e.g., Na, Cl, and B) (Rango et al. 2010, 2012).
Apart from the above-mentioned uses, groundwater has immense importance for the existence of rift lakes and wetlands. According to local water agencies working in the areas of water resource development, there is high pressure on the water resource utilization in the study areas, especially in case of drinking water supply and irrigation water utilization during dry seasons. This and related reasons have brought conflicts among community members in different parts of the study area (WWDSE 2016). To satisfy the demands of different groups and support economic development of water resources, further detailed water quality mapping specifically considering those parameters that are relevant for different purposes is needed.
Materials and methods
Water sampling and analysis
Groundwater samples were collected systematically along the NE–SW and E–W transects representing the major physiographic regions of ZLB. Accordingly, 55 representative groundwater samples from springs (10) and wells (45) were collected during the transition period between the wet and dry season (November to December) of 2018. The sampling depth of the groundwater in wells generally increase from a few meters (27 m) below the ground surface in the mountainous escarpments toward the center of the rift with a maximum depth of 300 m. The locations of the sampling sites are presented in Fig. 1.
During sample collection, inactive wells were pumped for about 10–20 min to remove the standing water column in the well to make sure fresh groundwater was sampled. The water samples were collected while pumping. Springs were sampled at their emerging place using a plastic syringe. All water samples were filtered with a 0.45-μm filter membrane and kept in clean and dry 100 ml standard double-caped HDPE plastic bottles. The water samples for cation and trace element analyses were acidified with ultra-pure 6 N HNO3 to prevent precipitation/adsorption during storage. Field-based water parameters such as temperature, pH, total dissolved solids (TDS), and electrical conductivity (EC) were measured onsite.
Both major and trace elements analyses were carried out in the Institute of Geology and Geophysics laboratory, Chinese Academy of Sciences, Beijing China. Major ions (Na, K, Ca, Mg, F, SO4, NO3, and Cl) were analyzed by ion chromatography using a Dionex DX-120 equipment. Samples with high Na+ (> 75 mg/l) concentrations were diluted to avoid oversaturation. Titration techniques were applied to determine total alkalinity (HCO3). Arsenic (As) was analyzed by LC-6500 atomic fluorescence photometer. Other trace elements (Li, Be, Sc, Ti, V, Cr, Mn, Co, Ni, Cu, Zn, Ga, Rb, Mo, Cd, Sb, Ba, Pb, Bi, U, and Sr) were measured by inductively coupled plasma mass spectrometry (ICP-MS) using NexION300D ICP/MS equipment. During the trace element analysis, a blank and two water standards were run at the start and end of the analyses. Measurement uncertainties for all parameters were evaluated and controlled using regular laboratory duplicates of samples and verifying the precision/calibration of the instruments through regular runs of various primary standard solutions.
Drinking water quality index (DWQI)
DWQI presents the overall picture of water quality status for drinking purposes (Amiri et al. 2016; Herojeet et al. 2016; Darvishi et al. 2016). It converts selected water quality parameters into a single dimensionless number so that the water quality of different sources and sites can be evaluated and compared. The calculation procedure was described in detail by many researchers (e.g., Brown et al. 1972; Saeedi et al. 2010; Abbasi and Abbasi 2012) and generally involves four steps (Sutadian et al. 2016). In the first step, the water quality parameters to be included in the DWQI calculation are selected. Here, 35 water quality parameters were analyzed. However, only 16 parameters (pH, EC, Ca, Mg, Na, K, HCO3, Cl, SO4, NO3, F, As, Mn, Mo, U, and Pb) that have great influence on water quality for drinking purpose were considered (Table 1).
In the second step, each of the chemical parameters was assigned a weight (AWi). Unequal weight (Brown et al. 1972) was assigned based on their relative importance in the overall quality of water for drinking uses (Table 1). The assigned weight ranges from 1 to 5. The highest weight of 5 was assigned to parameters which have the major effects on drinking water quality (F, As, and NO3) and a minimum of 1 was assigned to parameters (K) that are considered as not harmful (Srinivasamoorthy et al. 2008; Vasanthavigar et al. 2010). Other parameters were assigned a weight between 1 and 5 based on their significance in the overall quality of water for drinking uses (Table 1; Ketata-Rokbani et al. 2011). Finally, the relative weight (RW) of each parameter was calculated:
where AWi is the assigned weight of each parameter, n is the number of parameters and RW is the relative weight. the assigned weight (AW) and the calculated relative weight (RW) values are presented in Table 1.
In the third step, a quality rating scale (Qi) is calculated for each parameter:
where Qi is the quality ranking, Ci is the concentration of each parameter in individual water sample in mg/l and Si is the WHO standard for each parameter in mg/l (Table 1).
Finally, in the fourth step, after the water quality sub-index SIi was obtained by multiplying the quality grade by the RW of each parameter (Eq. 3), the integrated DWQI was obtained by totaling all the sub-indices (Eq. 4).
Irrigation water quality
The quality of irrigation water has a substantial impact on the soil salinity, growth, and yield of agricultural products. In general, water applied for irrigation always contains different concentrations of dissolved constituents originating through natural (rainfall, weathering, and dissolution of rocks and soils) and anthropogenic (domestic and industrial) processes (Jarvie et. al. 1998). Therefore, the quality of the irrigation water needs to be assessed to avoid or reduce adverse impacts on agriculture (Mohammed 2011). The irrigation suitability of the groundwater was tested based on SAR in association with EC values, Na %, RSC, and PI as calculated by the following formulas (Table 2).
In addition to the above indices, IWQI was computed to determine the overall appropriateness of the groundwater for irrigation based on several water quality parameters (Table 3). The IWQI concept was primarily developed and introduced by Meireles et al. (2010) and later used by several authors (e.g., Abbasnia et al. 2018). However, in this study, the IWQI model used by RamyaPriya and Elango (2018) which is similar to the assessment procedure applied in DWQI. Five parameters including EC, SAR, Na (%), RSC, and PI, which dominantly influence the water quality for irrigation purpose, were considered for computing IWQI. The IWQI was calculated using Eq. (5).
where RWn is the relative weight of the nth parameter, Qn is the sub-index of the corresponding parameter. The assigned weights and sub-index of all the variables considered in this study are presented in Table 3.
Result and discussions
Hydrochemistry and drinking water quality assessment
Field-based parameters and major ions
The analytical results of the physicochemical parameters were statistically examined to provide the general chemical characteristics of the groundwater. Table 4 presents the minimum, maximum, mean, and the standard deviation for each variable. The permitted limits for drinking water set by WHO (2011) was included. For some elements that are not addressed by the guideline WHO (2011), US EPA (2003) was used for comparison purpose.
Field-based variables such as temperature, pH, TDS, and EC of the groundwater show wide variability across the basin; however, a clear spatial pattern is observed from the escarpment toward the rift floor (Table 4; Fig. 2a–d). The temperature of the groundwater varied from 14.3 to 60.7 °C. The pH of the groundwater varied from slightly acidic to slightly alkaline, ranging from 6.17 to 8.8. TDS of the groundwater varied between 25 and 4725 mg/l; whereas EC ranged from 38.75 to 6300 μS/cm. In general, temperature, pH, TDS, and EC of the groundwater show a steady increase from the escarpment to the rift floor (Fig. 2a–d) with local anomalies associated with thermal waters. This is attributed to strong water–rock interaction along the groundwater flow direction assisted by high geothermal gradient prevailing in the area (Darling et al. 1996; Gizaw 1996; Mechal et al. 2017).
a–d Box plot showing the spatial variation in temperature, pH, TDS, and EC from the escarpment to the rift floor. The broken line represents the "permissible" value (WHO 2011)
Water samples collected from high altitudes (western and eastern escarpments) are characterized by low TDS and EC fully complying with the WHO (2011) drinking water quality guideline (Fig. 2c, d). The high rainfall rate in the escarpment plays a main role in keeping the TDS and EC signature of the groundwater below the permissible value. However, in the rift floor more than 50% of the groundwater samples depicts high TDS and EC surpassing the permissible limit and are not suitable for drinking purposes. Almost all groundwater samples exhibited a pH value within the tolerable limit of 6.5–8.5 (WHO 2011), except a few acidic and alkaline groundwater samples situated in the upper escarpments and rift floor, respectively.
Unlike the field measured parameters, the main dissolved ions show a wide variability across the basin (Table 4; Fig. 3a–h). The variation in cation concentrations is more evident than that of anions. Mg2+ ranged from below detection limit (bdl) to 33.4 mg/l with a mean value of 7.3 mg/l. On the other hand, Ca2+ concentration varied from 0.5 to 113.8 mg/l with an average value of 28.9 mg/l. Due to the weathering of olivine and pyroxene minerals in the source rocks (basalt), Ca2+ and Mg2+ are relatively predominant in the aquifers of the upper escarpment, but gradually drop toward the rift floor. Both Ca2+ and Mg2+ concentration in the groundwater are within the permissible limit in the majority of the study area (Fig. 3a, b). On the contrary to Ca2+ and Mg2+, Na+ and K+ exhibit positive trends from the escarpment to the rift floor (Fig. 3c, d). The Na+ content ranged between 2.3 and 858 mg/l with an average value of 133.6 mg/l. Na+ is the dominant cation, but highly variable as characterized by the standard deviation being larger than the mean value (Table 4). Twenty-five percent of the groundwater samples had high level of Na+ crossing the tolerable limit in drinking water (> 200 mg/l). Similarly, the K+ content varied between 0.66 and 152 mg/l with an average value of 16.7 mg/l. According to WHO (2011), 42% of the groundwater samples exceeded the allowable limit (12 mg/l). These groundwater samples exceeding the allowable limit for Na+ and K+ are located in the rift floor (Fig. 3c, d), suggesting that the groundwater in elevated (> 2000 m a.s.l.) regions satisfies the WHO guideline and is suitable for drinking purposes.
a–h Box plot showing the spatial variation of major ions (Ca2+, Mg2+, Na+, K+, HCO3−, Cl−, SO42−, and NO3−) from the mountains to the rift floor. The broken line represents the "permissible" guideline (WHO 2011)
Anions varied with the following trend: HCO3− > Cl− > SO42− > NO3− (Table 1; Fig. 3e–h). The HCO3− content varied from 43.6 to 2547 mg/l with an average value of 433 mg/l. Bicarbonate is the dominant anion characterized by an increasing trend from the escarpment toward the rift floor, which corresponds to the increase in Na+ and K+. In the majority of the study area (85.5% of the samples), HCO3− exceeded the desired limit of 120 mg/l set by WHO (2011). The Cl− and SO42− content is quite low and below the WHO (2011) guideline value of 250 mg/l (Table 1; Fig. 3f, g). In the study area, the content of NO3− varied from bdl to 41.1 mg/l with an average value 25.3 mg/l. The relatively higher content of NO3− in the rift floor is associated with agricultural and other anthropogenic activities that contribute to the contamination of groundwater. However, the concentration of NO3− in all water samples is below the permissible limit (WHO 2011) of 50 mg/l throughout the basin (Fig. 3h).
In the studied region, the concentrations of F− ranged from bdl to 40 mg/l, with a mean of 3.4 mg/l (Table 1). It has been found that 24 (44%) out of 55 water samples had F− above the allowable limit (1.5 mg/l) (Table 1). Fluoride at optimum concentration is beneficial for the human body (Brunt et al. 2004; Mjengera, and Mkongo 2003; Firempong et al. 2013); however, drinking water with F− levels greater than 1.5 mg/l causes adverse human health effects (Feenstra et al. 2007; Peter 2009). High F−concentration is one of the major water quality problems affecting people residing in ERV including the study area (Tekle-Haimanot et al. 1987; Gizaw 1996; Kloos and Tekle-Haimanot 1999; Reimann et al. 2003; Rango et al. 2009; Mesele and Mechal 2020).
Figure 4 portrays the spatial distribution of the F− concentration in groundwater across the basin showing a general increase from both the western and eastern escarpment toward the rift floor. The escarpment shows low F− concentration satisfying the WHO water quality standard (< 1.5 mg/l). This suggests that groundwater from the escarpments is a safe source of water for drinking use. On the contrary, all the fluoride-rich groundwater in the study area crossing the threshold value is found in the rift floor. In the rift floor, F− is a major water quality degrader, especially for those communities living west and south of Lake Ziway, where F− reaches 40 mg/l (Fig. 4). Millions of people have been forced to use these water sources for prolonged period and as a result both dental and skeletal fluorosis are very common in the area (Rango et al. 2020).
Hydrochemical facies and controlling processes
The hydrochemical facies of groundwater in ZLB were studied by plotting the concentrations of major ions in the Piper plot (Piper 1944; Fig. 5). Three distinct hydrochemical facies (Ca/Mg–HCO3, Na/Ca–HCO3, and Na–HCO3) were recognized across the study area. The headwater of ZLB, western and eastern escarpments, show a weakly mineralized Ca/Mg–HCO3 hydrochemical facies. On the other hand, rift floor groundwater including thermal groundwater exhibits a highly mineralized Na–HCO3 hydrochemical facies with Na+ and HCO3− strong dominance over the other ionic species. Na/Ca–HCO3 hydrochemical facies were the mixture of the two extreme hydrochemical facies occupying the transition zone and shallow groundwater in the rift floor. In summary, groundwater systematically evolves from a Ca/Mg–HCO3 to Ca/Na–HCO3 and lastly to Na–HCO3 hydrochemical facies along the groundwater flow direction from the escarpments to the center of the rift floor.
Different hydrochemical processes can be involved during the hydrochemical evolution of groundwater along the groundwater flow path across the study area. To identify the major hydrochemical processes responsible for the evolution, the most widely used Gibbs diagram (Gibbs 1970) was constructed. As shown in Fig. 6, almost all groundwater samples cluster in the rock–water interaction field suggesting weathering of rock-forming minerals as the origin of the dissolved ions.
Among different water–rock interactions in silicate-dominated lithological classes, silicate weathering is one of the major hydrochemical processes governing groundwater chemistry (Garrels and Mackenzie 1967; Freeze and Cherry 1979). According to Rajmohan and Elango (2004) and Kumar et al. (2006), the importance of silicate weathering can be recognized by studying the scatter plots of total cations (TZ+) versus (Ca2+ + Mg2+) and (Na+ + K+). As illustrated in Fig. 7a, all escarpments and some rift floor groundwater samples linearly spread between 1:2 and 1:1 lines suggesting Ca2+ and Mg2+ have originated from the weathering of basaltic aquifers. Likewise, the plot of TZ+ and (Na+ + K+) shows the majority of the groundwater samples are plotted above the 1:1 line, indicating acidic rock weathering is the key hydrochemical process which adds Na+ and K+ to the groundwater. From these results, it can be inferred that silicate weathering is the prime sources of Na+, Ca2+, Mg2+, K+, and the balancing HCO3− into the groundwater in the study area. The exceptionally high Na+ and HCO3− content in some of the rift floor and thermal groundwater samples is probably related to silicate weathering aided by the uptake of mantle CO2 leaking through deep-seated faults in addition to the ordinary soil CO2 (Darling et al. 1996; Gizaw 1996; Mechal et al. 2017).
The dominance of Na–HCO3 hydrochemical facies in the rift floor aquifers cannot only be explained by silicate weathering. Besides silicate weathering, cation exchange might have an effect on groundwater chemistry as observed in a similar geological setting (Li et al. 2013; Wu and Sun 2016). To assess the occurrence of cation exchange reactions in ZLB, the two chloro‐alkaline indices (CAIs), CAI-I and CAI-II were applied as recommended by Schoeller (1965). These two indices are expressed as Eqs. (6) and (7) (the concentration of the ions are expressed in meq/l).
When there is an exchange between Na+ in groundwater with Ca2+/Mg2+ of the aquifer material, both indices CAI-I and II will be positive. Conversely, the negative CIAs values show a reverse ion exchange. Accordingly, the calculated CAI-I and CAI-II values are negative for all groundwater samples with an average value of − 13.54 and − 0.57, respectively. Thus, Ca2+/Mg2+ in groundwater exchange with Na+ in aquifer, forming rocks and sediments leading to the further increment of Na+ with parallel depletion of Ca2+/Mg2+ in the groundwater along the flow path. The hydrochemical evidence suggests that silicate weathering and cation exchange played a role for the evolution of the groundwater from the low mineralized Ca/Mg–HCO3 to high mineralized Na–HCO3 hydrochemical facies along groundwater flow.
As suggested by several authors (Gizaw 1996; Yirgu et al. 1999; Rango et al. 2009; Haji et al. 2018; Bianchini et al. 2020), the above-mentioned processes, silicate weathering and cation exchange, are also responsible for the elevated content of F− exceeding the permissible limit in the rift sector of the study area (Fig. 4). Fluoride is positively correlated with Na+ (R2 = 0.83), HCO3− (R2 = 0.65), TDS (R2 = 0.65) and negatively correlated with Mg2+ (R2 = − 0.18) and Ca2+ (R2 = − 0.3). These relationships suggest that silicate weathering gradually releases F− into the groundwater reservoir as it flows from the bordering escarpments to the rift axis. Simultaneously, cation exchange is also believed to remove Ca2+ from the groundwater to replace Na+ in aquifer material. This results in freely circulating F− in the rift center, as there is not sufficient Ca2+ to precipitate as CaF2 (Rango et al. 2010; Haji et al. 2018; Bianchini et al. 2020).
As stated by Keshavarzi et al. (2010), in alkaline water, F− is desorbed from solid phases, while in acidic water, F− is adsorbed on a clay surface; therefore, alkaline pH is more conducive for F− dissolution. In the present study, the gradual change in pH from the slightly acidic nature in the upstream to sub-alkaline water in the rift floor highly favored the dissolution of F−, which is reflected by a positive correlation with pH (Fig. 8; R2 = 0.42). The influence of alkalinity on F− dissolution is also recognized by several other authors (e.g., Saxena and Ahmed 2003; Jacks et al. 2005; Rafique et al. 2009; Haji et al. 2018).
Trace element distribution in the groundwater
According to Gaillardet et al. (2003), trace elements are known by their concentrations below 1 mg/l in groundwater. Despite their low concentrations, the level of some trace elements in water plays either a negative or positive role in human health (WHO 2011). To identify potentially toxic trace elements, the concentrations of trace elements in the groundwater samples were compared with existing drinking water quality guidelines (WHO 2011; US EPA 2003).
Apart from Be and Bi, the analyzed trace elements (Mn, As, U, Mo, Pb, Cr, Sr, Li, Sc, Ti, V, Co, Ni, Cu, Zn, Ga, Rb, Cd, Sb, and Ba) were detected in the majority (75%) of the collected samples in the ZLB. The low content of Be and Bi below the detection limit might be linked to the lower concentrations in aquifer materials (volcanic rocks). For most of the trace elements (Sr, Li, Sc, Ti, V, Co, Ni, Cu, Zn, Ga, Rb, Cd, Sb, and Ba) reported in Table 4, the measured concentrations were far below the guideline values and are not expected to cause harm to people. However, a few potentially toxic trace elements such as Mn, As, U, Pb, and Mo were above the permissible limit (WHO 2011).
The concentration of these toxic trace elements in the groundwater were non-uniform across the watershed ranging from bdl to high values exceeding the health-based value for drinking water (Table 4). Manganese is an important element for humans, but can cause adverse health effects due to over- or insufficient consumption. Concentrations below 40 µg/l are usually acceptable to consumers (WHO 2011). According to Erikson and Aschner (2003) and Vartanian et al. (1999), overconsumption of Mn beyond the acceptable limit may result in infertility, malfunction of the immune system and Parkinson-like symptoms. Manganese concentration in the ZLB ranges from bdl to 466 µg/l with mean value of 37.6 µg/l. The manganese level is quite low in the western and eastern escarpments, but the central rift floor is highly enriched rising to ten times higher than the permissible limit (Fig. 9a). The concentration of Mn in this study is higher than those reported in Tertiary sedimentary sandstone aquifer in Nigeria (180 µg/l; Oyem et al. 2015) and Mississippi River Valley alluvial, sandstone and glacial aquifers in the USA (300 µg/l; McMahon et al. 2019), but significantly lower than the Nubian sandstone aquifer in Egypt (36, 340 µg/l; Khozyem et al. 2019) and Carboniferous and Devonian sedimentary aquifer in Scotland (1900 µg/l; Homoncik et al. 2010).
Arsenic is tasteless, odorless, and colorless, but has been proven to be toxic at very low concentrations, even below the recommended level of 10 μg/l (WHO 2011). Numerous epidemiological studies (e.g., Smith et al. 1998; Parvez et al. 2008; Tchounwou et al. 2019) have documented that drinking As-contaminated water can cause skin, liver, lung, kidney and bladder cancer. Arsenic in the groundwater samples ranged from bdl to 278 µg/l, with a mean of 30.2 µg/l. Arsenic shows strong correlation (R2 > 0.8) with EC, Na+, K+, HCO3−, and F−. It is negatively associated with Mg (R2 = − 0.27) and Ca2+ (R2 = − 0.31) and has almost no distinctive association with NO3 (R2 = − 0.07). These relationships suggest that As contamination in the study area is geo-genic and tied with Quaternary sediments and rhyolitic volcanic rocks instead of basic rocks (e.g., basalts) (Barringer and Reilly 2013). This finding is in agreement with the studies of Rango et al. (2010, 2013) and Bianchini et al. (2020). The spatial distribution of As concentrations in the groundwater (Fig. 9b) shows that areas to the west and south of Lake Ziway represent zones of high As concentrations (As > 10 µg/l). Arsenic levels were much lower and far below the permissible limit in the western and eastern escarpments of the study area. Some studies (e.g., Reimann et al. 2003; Rango et al. 2010, 2013) reported the occurrence of As contamination in the MER accompanied by high F content. The As content is more pronounced mainly in hot springs (up to 1019 µg/l) in the southern MER (Bianchin et al. 2020). It should also be noted that the content of As in the Quaternary sediments and rhyolitic volcanic aquifers of the study area is much higher than those of limestone aquifer in Nigeria (160 μg/l; Gbadebo 2005) and alluvial aquifer in India (68 μg/l; Yadav et al. 2020) and lower than crystalline basement aquifers in Burkina Faso (1630 μg/l; Smedley et al. 2007), fluvio-lacustrine aquifers in Mongolia (1800 μg/l; Smedley et al. 2003) and marine and fluvial origin alluvial sediment aquifer in Vietnam and Cambodia (1340 μg/l; Buschmannet al. 2008).
According to Milvy and Cothern (1990) and Brugge and Buchner (2011), uranium is considered to be a non-essential element for human health, and the exposure to extremely low concentration in groundwater results in potential problem in the kidney and bones. Uranium concentrations ranged between bdl and 43.9 µg/l with a mean of 4.73 µg/l. Uranium level was generally very low in the study area, but anomalous concentration exceeding the WHO (2011) threshold value (15 µg/l) was observed to the west of Lake Ziway (Fig. 1). These zones also overlap with high F > 1.5 mg/l, As > 10 µg/l and Mn > 40 µg/l. The range of U concentrations observed in this study area falls within the range found in Ethiopian Rift (Reimann et al. 2003; Rango et al. 2013). However, U concentrations observed in this study were found to be lower than that observed in granites and pegmatites in Sweden (470 μg/l; Selden et al. 2009) and Quaternary sediments in China (288 μg/l; Wu et al. 2014), but higher than that of alluvial sediments in Bangladesh (10 μg/l; Frisbie et al. 2009) and Vietnam (5.1 μg/l; Buschmann et al. 2008).
Lead content in groundwater obtained from this study ranged from bdl to 71.7 μg/l with a mean value of 4.1 μg/l. Only 5% of the water samples collected throughout the study area exceeded the WHO (2011)-recommended limit of 10 μg/l. Several authors (e.g., Cunningham and Saigo 1997; Baykov et al. 1996; Jaishankar et al. 2014) have shown that frequent overintake of Pb can initiate tiredness, hypertension, anemia behavioral changes, irritability and impairment of intellectual functions. Molybdenum is an indispensable trace element for human health; nonetheless, overdoses can be harmful (Smedley and Kinniburgh 2017). Similarly, molybdenum concentrations ranged from bdl to 104 µg/l with a mean value of 6.2 µg/l. Molybdenum concentration was generally very low in ZLB and only 5% of the groundwater samples, located in the rift floor to the west of Lake Ziway, had high concentrations exceeding the WHO (2011)-recommended value for drinking water (70 μg/l). Molybdenum has good association with As (R2 = 0.53), Pb (R2 = 0.21) and U (R2 = 0.71) suggesting that the reactive Quaternary sediments and rhyolitic volcanic rocks are also the potential source in the groundwater triggered by high geothermal activity in the area. (Fig. 1).
Multiple violations of trace elements (Mn, As, U, Pb, and Mo) are very common concurrently in the groundwater samples in the rift floor section of ZLB. This simultaneous occurrence of toxic elements could pose a further threat to human health through their mixture products (Bopp et al. 2018; Kortenkamp and Faust 2018). A mixture risk assessment is not considered in this work; however, the result suggests that groundwater of the study area possibly causes health concerns for the people consuming directly without any treatment from the groundwater sources.
Assessment of groundwater for irrigation
Water quality, soil types, and agricultural practices are essential factors for successful performance of irrigation. However, water quality can have a profound role in crop productivity as well as soil fertility. Therefore, evaluating the suitability of groundwater for irrigation purpose is vital for proper growth of crops and soil fertility. The most widely applied irrigation water quality indicator that can potentially show the quality of groundwater for irrigation purpose are EC, SAR, Na%, RSC, and PI. These indices were calculated by the relation provided in Table 3 and groundwater samples were classified according to its irrigation usage (Table 5).
Sodium adsorption ratio (SAR) and USSL diagram
Sodium adsorption ratio is used to measure the relative amount of Na+ concentration to those of Ca2+ and Mg2+ in a water sample. The excessive Na+ concentration relative to Ca2+ and Mg2+ reduces soil hydraulic conductivity and consequently hinders the supply of water required for plant growth. The excess Na+ or limited Ca2+ and Mg2+ content is determined by SAR value and can be evaluated using standard equation provided in Table 3 (Richards 1954).
The SAR values of the groundwater in the ZLB ranged from 0.13 to 237.4 meq/l with a mean value of 3.4 meq/l (Table 5). The SAR value of the groundwater displays a positive trend toward the rift floor. The increase in SAR value from the escarpment toward the rift center is linked to strong silicate hydrolysis aided by high geothermal gradient. Based on SAR values, more than 80% of the groundwater samples cluster in Excellent category and would be suitable for irrigation. The remaining 20% water samples classified as Doubtful to Unstable range and are mainly situated in the rift floor.
In addition to absolute SAR, the suitability of the groundwater for irrigation was also appraised based on US Salinity Laboratory (USSL) plot (Richards 1954), in which the EC is considered as salinity hazard while SAR as alkalinity hazard (Fig. 11a). The plot shows that 14 of the groundwater samples mainly belonging to the escarpments fall in the classes C1–S1 with low salinity and alkalinity/Na+ which are suitable for irrigation usage in almost all soil types. Twenty-six groundwater samples lie in the class C2–S1 and C3–S1 (medium to high salinity and low Na+). These waters can be utilized for irrigation application in almost all soil types with slight danger of exchangeable Na+. Most of the thermal and rift floor groundwater were classified as high to very high salinity water (C3 and C4) and high to very high Na+ (S3 and S4) field and thus cannot be applied in soils with limited drainage. Even with sufficient drainage, special management for salinity control is needed and crops with good salt tolerance should be preferred. In general, groundwater from the escarpments of the basin were suitable for irrigation usage with minor impact to the soil and crops. On the other hand, the water in the western lake margin areas is categorized as poor quality and requires treatment before irrigation application.
Percent sodium (% Na) and Wilcox diagram
Percent sodium is also utilized in the assessment of groundwater suitability for irrigation, as the concentration of Na+ reacts with soil to lower its permeability (Todd 1980). When the content of Na+ is elevated in irrigation water, Na+ tends to be absorbed by clay particles discharging Ca2+ and Mg2+ ions. This exchange process of Na+ in water for Ca2+ and Mg2+ in soil lowers the permeability and ultimately results in soil with low infiltration rate. As a result, air and water circulation is inhibited during wet situations, and such soils become typically hard when dry (Saleh et al. 1999).
The computed %Na for the ZLB ranged from 11.7 to 99.9% with mean value of 56.2% (Table 5). The Wilcox (1955) plot (Fig. 10b) relating %Na and EC illustrates that 38 (70%) groundwater samples belong to Excellent to Permissible, 4 (7%) to Permissible to Doubtful, 6 (11%) to Doubtful to Unsuitable, and 7 (13%) to Unsuitable categories.
Residual sodium carbonate (RSC)
Another limiting factor for irrigation water is the excess of CO3− and HCO3− concentrations with regard to Ca2+ and Mg2+. Excess CO3− and HCO3− in water can deteriorate the irrigation water quality as it results in the precipitation of the alkali metals, mainly Ca2+ and Mg2+. The precipitation of Ca2+ and Mg2+ as carbonate minerals may amplify the Na+ concentration and consequently increase SAR values (Eaton 1950). Based on theRSC value, groundwater can be categorized into Suitable (RSC < 1.5 meq/l), Unsuitable (RSC > 2.5 meq/l), and Marginally suitable (1.25–2.5 meq/l) for irrigation (Table 5) (Deshpande and Aher 2012).
According to RSC values, 38% of the water samples are Suitable for irrigation application, and about 18% of the water fall under Marginally suitable category and the remaining 44% of water samples are in the Unsuitable category. More than 50% of the water samples were found to be fairly Suitable for irrigation and identified in the escarpments part of the basin. On the contrary, the Unsuitable water samples were mainly located in the southeastern and central part of the basin. High RSC potentially can have a damaging effect to the physical properties of soil, as it induces dissolution of organic matter and finally generates a black stain on the soil surface when it dries (Kumar et al. 2006; Srinivasamoorthy et al. 2008).
Permeability index (PI)
The permeability index is one of the important irrigation water quality indicators, as the soil permeability is affected by continuous application of irrigation water, which is controlled by the Na+, Ca2+, Mg2+, and HCO3− contents of the soil. Doneen (1964) developed a benchmark for evaluating the appropriateness of water for irrigation usage based on PI, and waters can be categorized as classes Excellent (> 75%), Good (25–75%), and Doubtful (< 25%) (Table 5).
In the ZLB, the computed PI values of the groundwater varied between 39.9 and 136.4 meq/l, with mean value of 100.5 meq/l. Based on PI value, the greater proportion of the water samples (98%) clustered under Excellent to Good classes and are generally suitable for irrigation.
Water quality index
Drinking water quality index (DWQI)
DWQI was applied to get an overall picture of groundwater quality for drinking purpose in the LZB. The computed DWQI values are then used to categorize the groundwater into five classes as Excellent, Good, Poor, Very Poor, and Unsuitable (Table 6) for drinking use (Sahu and Sikdar 2008).
The calculated DWQI values of the groundwater in the basin range between 2.7 and 714 with an average of 94. Of the 55 groundwater samples, 56% of the samples belonged to Excellent and 20% to Good and were considered to be suitable for drinking purpose. However, the remaining 24% categorized as Poor to Unsuitable ranges (Table 6) and cannot be directly utilized for human consumption without proper treatment.
DWQI spatial distribution map (Fig. 11a) was created based on the calculated DWQI values. It can easily be observed that DWQI values varied from 12.7 Excellent class to 714 Unsuitable class for drinking purposes and systematically increases from the escarpments toward the rift axis. Lower DWQI values below 100 are observed in the upper escarpments, indicating the suitability for drinking purposes. The low value in the escarpments is associated with the fast groundwater replenishment and low rock–water interaction rate due to short travel time. As a result, the concentration of the highest influencing parameters (see Table 2) of DWQI is usually found to be below that of the drinking water guideline.
On the other hand, high DWQI values above 100 designated as Poor to Unsuitable water quality status were observed in the rift floor. As discussed in Sect. 4.1.2, silicate hydrolysis and cation exchange processes along the groundwater flow direction from the escarpments toward the rift floor through acidic volcanic rocks enrich the groundwater in F−, Na+, K+, and HCO3− with a corresponding increment of pH and EC. As a result, almost all influencing parameters of DWQI were found to be in excess and went above the permissible limit, which led to high DWQI value. The exceptional very high DWQI values to the west of Lake Ziway is mainly related to co-existence of the elevated concentration of toxic elements in Quaternary sediment and rhyolitic volcanic rock aquifer such as F, Mn, As, U, Pb, and Mo. From Figs. 4 and 9, it can be inferred that the areas that have high F, Mn, and As concentration also have high DWQI values. This substantiates the validity of the DWQI map. In general, the poor drinking water quality in ZLB is associated with the combined action of silicate hydrolysis and cation exchange as well the occurrence of very reactive lithotype.
Irrigation water quality index (IWQI)
Suitability of groundwater for irrigation purpose can be assessed either taking into consideration the chemical parameters (indices) (e.g., Li et al. 2013; Kawo and Karuppannan 2018) or a combination of many indices (e.g., Meireles et al. 2010; RamyaPriya and Elango 2018). Rating of water for irrigation purpose using individual chemical parameters can be helpful; however, the overall suitability based on many influencing parameters provides concise information for decision by managers and administrative organizations. The calculated IWQI values of the groundwater in the basin ranged from 13.2 to 520 with an average of 106. In groundwater, 28% of the samples belonged to Excellent, 46% to Good, 15% to Poor, 7% to Very poor, and 4% to Unsuitable category (Table 6).
The spatial variation of IWQI values in the ZLB is shown in Fig. 11b. Like the DWQI map, groundwater quality is generally characterized by an Excellent water quality for irrigation in the headwater regions of the ZLB and progressively become Unsuitable for irrigation water uses in the rift floor. In the rift floor, the parameters considered for the IWQI are characterized by high EC, SAR, Na%, RSC, and PI values leading to high IWQI values. This is directly associated with the gradual evolution of groundwater from low mineralized Ca/Mg–HCO3 type in the escarpment to strongly mineralized Na–HCO3− type in the rift floor.
Conclusion
Water quality is one of the most important factors for sustainable development in the ERV, which is characterized by arid and semi-arid climate and serious water shortage often associated with water quality problems. In this study, the suitability of groundwater for drinking and irrigation use was evaluated in LZB of the ERV. Water quality of the 55 groundwater samples was assessed for major and trace element concentrations. Results showed that groundwater samples from the escarpments are typically characterized by low TDS and Ca/Mg–HCO3 hydrochemical facies. The subsequent interaction of these waters with the acidic volcanic rocks along the flow path toward the rift axis prompts a general increase of TDS and evolution of hydrochemical facies toward Na–HCO3. Comparison of major and trace element concentrations of the water samples with drinking guidelines (WHO 2011) indicated that large percentages (for Na, K, F, and HCO3), and small percentages (for Mn, As, U, Pb, and Mo) of samples are found exceeding the permissible limits. Water rock interactions such as silicate weathering and ion exchange are the prime processes controlling groundwater quality with limited anthropogenic stress.
The overall groundwater quality for drinking purpose, DWQI values, show strong spatial variation in the ZLB ranging from 12.7 (Excellent class) to 714 (Unsuitable class) with an average of 94. DWQI suggests that groundwater is generally characterized by an Excellent quality for drinking water use in the head water regions of the ZLB and progressively becomes Unsuitable in the rift floor. The poor groundwater quality in the downstream section of the basin is associated with an increase in TDS coupled with the co-occurrence and accumulation of multiple toxic trace elements (F, Mn, As, U, Pb, and Mo) along groundwater flow. For the community residing in areas with very high DWQI values, especially to the west of Lake Ziway where major cities are located, it is necessary to look for alternative sources of water or device new methodologies that can remove multi-toxic elements from the groundwater.
The spatial assessment of irrigation water quality inferred from water quality indicators such as IWQI, EC, SAR, Na%, RSC, and PI values showed the existence of different water quality classes across the study area. The significant proportion of rift floor groundwater samples fall in moderately Suitable to Unsuitable classes for irrigation use. These classes of groundwater might destroy the physical properties of soils (e.g., soil drainage) and lower crop productivity in the area. The occurrence of such irrigation water quality in this region is mainly attributed to the dominance of Na and HCO3 arising from the late stage evolution of the groundwater. On the contrary, the escarpment groundwaters fall in the Suitable category in any of the above irrigation water quality indicators.
The spatial assessment of groundwater quality has led to a better understanding of water quality degraders and overall water quality status of ZLB. DWQI and IWQI maps have provided integrated information about the suitability of the groundwater for different purposes, which helps policy makers and managers to identify water schemes falling in unsuitable areas and thus to take appropriate measures and identify new areas for future groundwater resource development.
References
Abbasi T, Abbasi SA (2012) Water quality indices. Elsevier Science, Burlington
Abbasnia A, Yousefi N, Hossein AM, Nabizadeh R, Radfard M, Yousefi M, Alimohammadi M (2018) Evaluation of groundwater quality using water quality index and its suitability for assessing water for drinking and irrigation purposes: case study of Sistan and Baluchistan province (Iran). Hum Ecol Risk Assess. https://doi.org/10.1080/10807039.2018.1458596
Amangabara GT, Ejenma E (2012) Groundwater quality assessment of Yenagoa and environs Bayelsa State, Nigeria between 2010 and 2011. Resour Environ 2(2):20–29
Amiri V, Nakhaei M, Lak R, Kholghi M (2016) Assessment of seasonal groundwater quality and potential saltwater intrusion: a study case in Urmia coastal aquifer (NW Iran) using the groundwater quality index (GQI) and hydrochemical facies evolution diagram (HFE-D). Stoch Environ Res Risk Assess 30(5):1473–1484
Ayenew T, Demlie M, Wohnlich S (2008) Hydrogeological framework and occurrence of groundwater in the Ethiopian aquifers. J Afr Earth Sci 52:97–113. https://doi.org/10.1016/j.jafrearsci.2008.06.006
Ayenew T, Fikre S, Wisotzky F, Demlie M, Wohnlich S (2009) Hierarchical cluster analysis of hydrochemical data as a tool for assessing the evolution and dynamics of groundwater across the Ethiopian Rift. Int J Phys Sci 4(2):76–90
Ayenew T (1998) The Hydrogeological system of the Lake District basin, central main Ethiopian Rift. Publ. no. 64, PhD Thesis, ITC, Enschede, The Netherlands
Babiker IS, Mohamed MAA, Hiyama T (2007) Assessing groundwater quality using GIS. Water Resour Manag 214:699–715. https://doi.org/10.1007/s11269-006-9059-6
Barringer JL, Reilly PA (2013) Arsenic in groundwater: a summary of sources and the biogeochemical and hydrogeologic factors affecting arsenic occurrence and mobility. In: Paul B (ed) Current perspectives in contaminant hydrology and water resources sustainability. InTechOpen, London, pp 82–116
Bauder TA, Waskom RM, Sutherland PL, Davis JG (2011) Irrigation water quality criteria. Colorado State University Extension Publication, Crop series/irrigation. Fact sheet no. 0.506
Baykov BD, Stoyanov MP, Gugova ML (1996) Cadmium and lead bioaccumulation in male chickens for high food concentrations. Toxicol Environ Chem 54:155–159
Benvenuti M, Carnicelli S, Belluomini G, Dainelli N, Di Grazia S, Ferrari GA, Iasio C, Sagri M, Ventra D, Atnafu B, Kebede S (2002) The Ziway-Shala basin (Main Ethiopian Rift, Ethiopia): a revision of basin evolution with special reference to the late quaternary. J Afr Earth Sci 35:247–269
Bianchini G, Brombin V, Marchina C, Natali C, Godebo TR, Rasini A, Salani GM (2020) Origin of fluoride and arsenic in the main Ethiopian rift waters. Minerals 10(5):453. https://doi.org/10.3390/min10050453
Boateng TK, Opoku F, Acquaah SO, Akoto O (2016) Groundwater quality assessment using statistical approach and water quality index in Ejisu–Juaben municipality. Ghana Environ Earth Sci 75:489
Boccaletti M, Bonini M, Mazzuoli R, Abebe B, Piccardi L, Tortorici L (1998) Quaternary oblique extensional tectonics in the Ethiopian Rift (Horn of Africa). Tectonophysics 287:97–116. https://doi.org/10.1016/S00401951(98)80063-2
Bopp SK, Barouki R, Brack W, Dalla Costa S, Dorne JLCM, Drakvik PE, Faust M, Karjalainen TK, Kephalopoulos S, van Klaveren J, Kolossa-Gehring M, Kortenkamp A, Lebret E, Lettieri T, Nørager S, Rüegg J, Tarazona JV, Trier X, van de Water B, van Gils J, Bergman Å (2018) Current EU research activities on combined exposure to multiple chemicals. Environ Int 120: 544–562. https://doi.org/10.1016/j.envint.2018.07.037.
Boswinkel JA (2000) Information Note, International Groundwater Resources Assessment Centre (IGRAC), Netherlands Institute of Applied Geoscience, Netherlands. In: UNEP (2002), Vital water graphics—an overview of the state of the world's fresh and marine waters, UNEP, Nairobi, Kenya
Brown RM, McCleiland NJ, Deininger RA, O'Connor MF (1972) A water quality index-crossing the psychological barrier. In: Proceedings of the International conference on water pollution research, Jerusalem, 18–24 June 1972, pp 787–797
Brugge D, Buchner V (2011) Health effects of uranium: new research findings Rev. Environ Health 26(2011):231–249
Brunt R, Vasak L, Griffioen J (2004) Fluoride in groundwater: probability of occurrence of excessive concentration on global scale, IGRAC
Buschmann J, Berg M, Stengel C, Winkel L, Sampson ML, Trang PTK, Viet PH (2008) Contamination of drinking water resources in the Mekong delta flood plains: arsenic and other trace metals pose serious health risks to population. Environ Int 34:756–764
Chernet T, Travi Y, Valles V (2001) Mechanism of degradation of the quality of natural water in the lakes region of the Ethiopian Rift Valley. Water Res 35:2819–2832
Chernet T (1982) Hydrogeology of the lakes region, Ethiopia (Lakes Ziway, Langano, Abitata, Shalla and Awassa). In: The provisional military government of socialist Ethiopia-ministry of mines and energy. Ethiopian Institute of Geological Surveys, Addis Ababa, Ethiopia
Cunningham WP, Saigo BW (1997) Environmental science: a global concern. Wm. C. Brown Publisher, New York, pp 389
Dainelli N, Benvenuti M, Sagri M (2001) Geological map of the Ziway–Shala lakes basin (Ethiopia). 1:250,000, Italian Ministry for University and Scientific and Technological Research (MURST)
Darling G, Gizaw B, Arusei M (1996) Lake–groundwater relationships and fluid–rock interaction in the East African Rift Valley: isotopic evidence. J Afr Earth Sci 22:423–430
Darvishi G, Kootenaei FG, Ramezani M, Lotfi E, Asgharnia H (2016) Comparative investigation of river water quality by OWQI, NSFWQI and Wilcox indexes (case study: The Talar River–IRAN). Arch Environ Prot 42(1):41–48
Deshpande SM, Aher KR (2012) Evaluation of groundwater quality and its suitability for drinking and agriculture use in parts of Vaijapur, District Aurangabad, MS, India. J Chem Sci 2(1):25–31
Di Paola GM (1972) The Ethiopian Rift Valley (between 7°00′ and 8°40′ lat. north). Bull Vulcanol 36:517–560
Di Paola GM, Seife MB, Arno V (1993) The Kella horst: its origin and significance in crustal attenuation and magmatic processes in the Ethiopian Rift Valley. In: Geology and mineral resources of somalia and surrounding regions, Ist. Agronom. Oltremare, Firenze, Relaz. Monograf, vol 113, pp 323–338
Doneen LD (1964) Water quality for agriculture. Department of Irrigation, University of California, Davis
Eaton EM (1950) Significance of carbonate in irrigation water. Soil Sci 69:12–133
Erikson KM, Aschner M (2003) Manganese neurotoxicity and glutamate—GABA interaction. Neurochem Int 43:475
Feenstra L, Vasak L, Griffioen J (2007) Fluoride in groundwater: Overview and evaluation of removal methods. International Groundwater Resources Assessment Centre Report nr. SP, (1)
Fienen MN, Arshad M (2016) The international scale of the groundwater issue. In: Integrated groundwater management: concepts, approaches and challenges, pp 21–48. https://doi.org/10.1007/978-3-319-23576-9_2
Firdissa M (2019) Estimation of groundwater recharge by using hydrological modelling: application to Lake Ziway watershed, Ethiopian Rift. MSc. Thesis Addis Ababa Science and Technology University, Addis Ababa, Ethiopia
Firempong C, Nsiah K, Awunyo-Vitor D, Dongsogo J (2013) Soluble fluoride levels in drinking water-a major risk factor of dental fluorosis among children in Bongo community of Ghana". Ghana Med J 47:16–23
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, Englewood Cliffs
Frisbie SH, Mitchell EJ, Mastera LJ, Maynard DM, Yusuf AZ, Siddiq MY, Ortega R, Dunn RK, Westerman DS, Bacquart T, Sarkar B (2009) Public health strategies for western Bangladesh that address arsenic, manganese, uranium, and other toxic elements in drinking water. Environ Health Perspect 117(3):410–416
Gaillardet J, Viers J, Dupré B (2003) Trace elements in river waters. Treatise Geochem 5:225–272
Garrels RM, Mackenzie FT (1967) Origin of the chemical compositions of some springs and lakes. Equilib Concepts Nat Water Syst 67:222–242
Gbadebo AM (2005) Arsenic pollution in aquifers located within limestone areas of Ogun State, Nigeria. In Natural arsenic in groundwater: proceedings of the pre-congress workshop ''Natural Arsenic in Groundwater'', 32nd international geological congress, Florence, Italy, 18–19 August 2004. CRC Press, pp 85–92
Gebrehiwot AB, Tadesse N, Jigar E (2011) Application of water quality index to assess suitability of groundwater quality for drinking purposes in Hantebet watershed, Tigray, Northern Ethiopia. ISABB J Food Agric Sci 1:22–30
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 17:1088–1090. https://doi.org/10.1126/science.170.3962.1088
Gizaw B (1996) The origin of high bicarbonate and fluoride concentrations in waters of the Main Ethiopian Rift Valley, East African Rift system. J Afr Earth Sci 22(4):391–402
Haji M, Wang D, Li L, Qin D, Guo Y (2018) Geochemical evolution of fluoride and implication for F-enrichment in groundwater: example from the Bilate River Basin of Southern Main Ethiopian Rift. Water 10:1799. https://doi.org/10.3390/w10121799
Halcrow (2008) Rift Valley Lakes integrated natural resources development master plan. Ethiopian Valleys Development Studies Authorities, Ministry of Water Resources, Addis Ababa, Ethiopia
Herojeet R, Rishi MS, Lata R, Sharma R (2016) Application of environ metrics statistical models and water quality index for groundwater quality characterization of alluvial aquifer of Nalagarh Valley, Himachal Pradesh, India. Sustain Water Resour Manag 2(1):39–53
Homoncik SC, MacDonald AM, Heal KVÓ, Dochartaigh BÉ, Ngwenya BT (2010) Manganese concentrations in Scottish groundwater. Sci Total Environ 408(12):2467–2473. https://doi.org/10.1016/j.scitotenv.2010.02.017
IPCC (2013) Climate change 2013: the physical science basis. In: Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 1535
Jacks G, Bhattacharya P, Chaudhary V, Singh KP (2005) Controls on the genesis of some high-fluoride groundwaters in India. Appl Geochem 20:221–228
Jaishankar M, Mathew BB, Shah MS, Gowda KRS (2014) Bio-sorption of few heavy metal ions using agricultural wastes. J Environ Pollut Hum Health 2(1):16
Jarvie HP, Whitton BA, Neal C (1998) Nitrogen and phosphorus in east coast British rivers: speciation, sources and biological significance. Sci Total Environ 210(211):79–109
Kassa N (2007) Groundwater resources and management issues in Central Ethiopian Rift. Unpublished MSc thesis, Addis Ababa University, Ethiopia, pp 128
Kawo NS, Karuppannan S (2018) Groundwater quality assessment using water quality index and GIS technique in Modjo River Basin, central Ethiopia. J Afr Earth Sci 147:300–311. https://doi.org/10.1016/j.jafrearsci.2018.06.034
Kazmin V, Seife MB, Nicoletti N, Petruccini C (1980) Evolution of the Northern Part of the Ethiopian Rift. In: Geodynamic evolution of the Afro-Arabic Rift system, Accad. Naz. Lincei, Roma, Atti Convegni, vol 47, pp 275–292
Kebede S, Travi Y, Stadler S (2010) Groundwaters of the Central Ethiopian Rift: diagnostic trends in trace elements, 18O and major elements. J Environ Earth Sci 61:1641–1655
Keshavarzi B, Moore F, Esmaeili A, Rastmanesh F (2010) The source of fluoride toxicity in Muteh area, Isfahan, Iran. Environ Earth Sci 61(4):777786. https://doi.org/10.1007/s12665-009-0390-0
Ketata-Rokbani M, Gueddari M, Bouhlila R (2011) Use of geographical information system and Water Quality Index to assess groundwater quality in El Khairat Deep Aquifer (Enfidha, Tunisian Sahel). Iran J Energy Environ 2(2):133–144
Khozyem H, Hamdan A, Tantawy AA, Emam A, Elbadry E (2019) Distribution and origin of iron and manganese in groundwater: case study, Balat-Teneida area, El-Dakhla Basin, Egypt. Arab J Geosci 12:523. https://doi.org/10.1007/s12517-019-4689-1
Kloos H, Tekle-Haimanot R (1999) Distribution of fluoride and fluorosis in Ethiopia and prospects for control. Trop Med Int Health 4:355e364
Kortenkamp A, Faust M (2018) Regulate to reduce chemical mixture risk. Science 361:224–226. https://doi.org/10.1126/science.aat9219
Kumar M, Ramanathan AL, Rao MS, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. J Environ Geol 50:1025–1039
Li P, Wu J, Qian H (2013) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang county, China. Environ Earth Sci 69(7):2211–2225. https://doi.org/10.1007/s12665-012-2049-5
McMahon PB, Belitz K, Reddy JE, Johnson TD (2019) Elevated manganese concentrations in United States groundwater, role of land surface-soil-aquifer connections. Environ Sci Technol 53:29–38. https://doi.org/10.1021/acs.est.8b04055
Mechal A, Birk S, Dietzel M, Leis A, Winkler G, Mogessie A, Kebede S (2017) Groundwater flow dynamics in the complex aquifer system of Gidabo River Basin (Ethiopian Rift): a multi-proxy approach. Hydrogeol J 25:519–538. https://doi.org/10.1007/s10040-016-1489-5
Meireles ACM, Andrade EMD, Chaves LCG, Frischkorn H, Crisostomo LA (2010) A new proposal of the classification of irrigation water. Revista Ciencia Agronomica 41:349–357. https://doi.org/10.1590/S1806-66902010000300005
Merla, G, Abbate E, Azzaroli A, Bruni P, Canuti P, Fazzuoli M, Sagri M, Tacconi P (1979) A geological map of Ethiopia and Somalia, and comment. CNR, Firenze
Mesele Y, Mechal A (2020) Hydrochemical characterization and quality assessment of groundwater in Meki River Basin, Ethiopian Rift. Sustain Water Resour Manag 6:117. https://doi.org/10.1007/s40899-020-00471-y
Milvy P, Cothern R (1990) Scientific background for the development of regulations for radionuclides in drinking water. In: Cothern R, Rebers P (eds) Radon, radium, and uranium in drinking water, pp 1–16
Mjengera H, Mkongo G (2003) Appropriate deflouridation technology for use in flourotic areas in Tanzania. Phys Chem Earth Parts a/b/ C 28(2003):1097–1104
Mohammed MN (2011) Quality assessment of Tigris river by using water quality index for irrigation purpose. Eur J Sci Res 57:15–28
Mohr PA (1962) The Ethiopian Rift system. Bull Geophys Observ Addis Ababa 5:33–62
Ocampo-Duque W, Osorio C, Piamba C, Schuhmacher M, Domingo JL (2013) Water quality analysis in rivers with non-parametric probability distributions and fuzzy inference systems: application to the Cauca River, Colombia. Environ Int 52:17–28. https://doi.org/10.1016/j.envint.2012.11.007
Oromia Water Works Design and supervision Enterprise (OWWDSE) (2016) Geological investigations for impact assessment of water resources exploitation on Lake Ziway, Addis Ababa, Ethiopia
Oyem HH, Oyem IM, Usese AI (2015) Iron, manganese, cadmium, chromium, zinc and arsenic groundwater contents of Agbor and Owa communities of Nigeria. Springer plus 4:104. https://doi.org/10.1186/s40064-015-0867-0
Parvez F, Chen Y, Brandt-Rauf PW, Bernard A, Dumont X, Slavkovich V (2008) Nonmalignant respiratory effects of chronic arsenic exposure from drinking water among never-smokers in Bangladesh. Environ Health Perspect 116(2):190–195
Pascual-Ferrer J, Pérez-Foguet A, Codony J, Raventós E, Candela L (2014) Assessment of water resources management in the Ethiopian Central Rift Valley: environmental conservation and poverty reduction. Int J Water Resour Dev 30(3):572–587. https://doi.org/10.1080/07900627.2013.843410
Peter KH (2009) Defluoridation of high fluoride waters from natural water sources by using soils rich in bauxite and kaolinite. J Eng Appl Sci 4:240–246
Piper AM (1944) A graphic procedure in the chemical interpretation of water analysis. Am Geophys Union Trans 25:914–923
Rafique T, Naseem S, Usmani HT, Bashir E, Farooque KA, Bhanger MI (2009) Geochemical factors controlling the occurrence of high fluoride groundwater in the Nagar Parkar area, Sindh. Pak J Hazard Mater 171(1–3):424–430
Rajankar PN, Tambekar DH, Wate SR (2010) Groundwater quality and water quality index at Bhandara District. Environ Monit Assess 179:619–625. https://doi.org/10.1007/s10661-010-1767-y
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ Geol 46:47–61
RamyaPriya R, Elango L (2018) Evaluation of geogenic and anthropogenic impacts on spatio-temporal variation in quality of surface water and groundwater along Cauvery River, India. Environ Earth Sci 77(2):11. https://doi.org/10.1007/s12665-017-7176-6
Rango T, Bianchini G, Beccaluva L, Ayenew T, Colombani N (2009) Hydrogeochemical study in the main Ethiopian Rift: new insights to source and enrichment mechanism of fluoride. Environ Geol 58:109–118
Rango T, Bianchini G, Beccaluva L, Tassinari R (2010) Geochemistry and water quality assessment of central main Ethiopian Rift natural waters with emphasis on source and occurrence of fluoride and arsenic. J Afr Earth Sci 57:479–491
Rango T, Kravchenko J, Atlaw B, Peter GM, Jeuland M, Merola B, Vengosh A (2012) Groundwater quality and its health impact: an assessment of dental fluorosis in rural inhabitants of the main Ethiopian Rift. Environ Int 43:37–47
Rango T, Vengosh A, Dwyer G, Bianchini G (2013) Mobilization of arsenic and other naturally occurring contaminants in groundwater of the Main Ethiopian Rift aquifers. Water Res. https://doi.org/10.1016/j.watres.2013.07.002
Rango T, Vengosh A, Jeuland M, Tekle-Haimanot R, Weinthal E, Kravchenko J, Paul C, McCornick P (2014) Fluoride exposure from groundwater as reflected by urinary fluoride and children’s dental fluorosis in the Main Ethiopian Rift Valley. Sci Total Environ 496:188–197. https://doi.org/10.1016/j.scitotenv.2014.07.048
Rango T, Vengosh A, Jeuland M, Whitford GM, Tekle-Haimanot R (2017) Biomarkers of chronic fluoride exposure in groundwater in a highly exposed population. Sci Total Environ 596:1–11
Rango T, Jeuland M, Whitford GM, Tekle-Haimanot D, Alemayehu B, Assefa G, Wolfe A (2020) Bone quality in fluoride-exposed populations: a novel application of the ultrasonic method. Bone Rep. https://doi.org/10.1016/j.bonr.2019.100235
Ravikumar P, Aneesul Mehmood M, Somashekar RK (2013) Water quality index to determine the surface water quality of Sankey tank and Mallathahalli lake, Bangalore urban district, Karnataka, India. Appl Water Sci 3:247–261. https://doi.org/10.1007/s13201-013-0077-2
Reimann C, Bjorvatn K, Frengstad B, Melaku Z, Tekle- Haimanot R, Siewers U (2003) Drinking water quality in the Ethiopian section of the East African Rift Valley Iedata and health aspects. Sci Total Environ 311(1–3):65–80
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Agri. Handbook 60. U.S. Department of Agriculture, Washington, DC, p 160
Rodriguez de Francisco JC (2008) Preconditions for a payment for environmental services establishment at Central Rift Valley, Ethiopia. MSc. Thesis, Wageningen University
Saeedi M, Abessi O, Sharifi F, Meraji H (2010) Development of groundwater quality index. Environ Monit Assess 163:327–335
Sahu P, Sikdar PK (2008) Hydrochemical framework of the Aquifer in and around East Kolkata Wetlands, West Bengal, India. Environ Geol 55:823–835
Saleh A, Al-ruwaih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Saxena VK, Ahmed S (2003) Inferring the chemical parameters for the dissolution of fluoride in groundwater. Environ Geol 43:731–736
Schoeller H (1965) Hydrodynamicue lans lekarst. Actes du Colloque de Dubrovnik, IAHS/ UNESCO, Paris, pp 2–20
Selden AI, Lundholm C, Edlund B, Hogdahl C, Ek BM, Bergstrom BE, Ohlson CG (2009) Nephrotoxicity of uranium in drinking water from private drilled wells. Environ Res 109(4):486–494. https://doi.org/10.1016/j.envres.2009.02.002
Selvam S, Manimaran G, Sivasubramanian P, Balasubramanian N, Seshunarayana T (2013) GIS-based Evaluation of Water Quality Index of groundwater resources around Tuticorin coastal city, south India. Environ Earth Sci 716:2847–2867. https://doi.org/10.1007/s12665-013-2662-y
Singh P, Khan I (2011) Ground water quality assessment of Dhankawadi ward of Pune by using GIS. Int J Geomatics Geosci 2:688–703
Singh S, Ghosh NC, Gurjar S, Krishan G, Kumar S, Berwal P (2018) Index-based assessment of suitability of water quality for irrigation purpose under Indian conditions. Environ Monit Assess 190(1):art. no. 29. https://doi.org/10.1007/s10661-017-6407-3
Smedley P, Kinniburgh G (2017) Molybdenum in natural waters: a review of occurrence, distributions and controls. Appl Geochem 84:387–432. https://doi.org/10.1016/j.apgeochem.2017.05.008
Smedley PL, Zhan M, Zhang G, Luo Z (2003) Mobilization of arsenic and other trace elements in fluvio-lacustrine aquifers of the Huhhot Basin, Inner Mongolia. Appl Geochem 18(9):1453–1477
Smedley PL, Knudsen J, Maiga D (2007) Arsenic in groundwater from mineralized Proterozoic basement rocks of Burkina Faso. Appl Geochem 22(5):1074–1092
Smith AH, Goycolea M, Haque R, Biggs ML (1998) Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol 147(7):660–669
Srinivasamoorthy K, Chidambaram M, Prasanna MV, Vasanthavigar M, John PA, Anandhan P (2008) Identification of major sources controlling groundwater chemistry from a hard rock terrain—a case study from Mettur taluk, Salem district, Tamilnadu, India. J Earth Syst Sci 117(1):49–58
Sutadian A, Muttil N, Yilmaz A, Perera B (2016) Development of river water quality indices-a review. Environ Monit Assess. https://doi.org/10.1007/s10661-015-5050-0
Tchounwou PB, Yedjou CG, Udensi UK, Pacurari M, Stevens JJ, Patlolla AK, Noubissi F, Kumar S (2019) State of the science review of the health effects of inorganic arsenic: perspectives for future research. Environ Toxicol 34(2):188–202. https://doi.org/10.1002/tox.22673
Tekle-Haimanot R (1990) Neurological complication of endemic skeletal fluorosis with special emphasis on rediculo-myelopathy. Paraplegia 28:244–251
Tekle-Haimanot R, Fekadu A, Bushra B (1987) Endemic fluorosis in the Ethiopian rift valley. Trop Geogr Med 39:209–217
Tekle-Haimanot R, Melaku Z, Kloos H, Reimann C, Fantaye W, Zerihun L, Bjorvatn K (2006) The geographic distribution of fluoride in surface and groundwater in Ethiopia with an emphasis on the Rift Valley. Sci Total Environ 367:182–190
Todd DK (1980) Groundwater hydrology, vol 10016, 2nd edn. Wiley, New York, pp 267–315
UNDP (1973) Geology, geochemistry and hydrology of hot springs of the East African Rift system within Ethiopia. UNDP report DD/SF/ON-11, NY; 300
US Environmental Protection Agency (USEPA) (2003) Drinking water standards. Office of Drinking Water. US Environmental Protection Agency, Washington, DC
Vartanian JP, Sala M, Henry M, Wain-Hobson S, Meyerhans A (1999) Manganese cations increase the mutation rate of human immune deficiency virus type 1 ex vivo. J Gen Virol 80:1983
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Rajiv Ganthi R, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2010) Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environ Monit Assess. https://doi.org/10.1007/s10661009-1302-1
Wada Y, Wisser D, Bierkens MFP (2014) Global modeling of withdrawal, allocation and consumptive use of surface water and groundwater resources. Earth Syst Dyn 5(1):15–40. https://doi.org/10.5194/esd-5-15-2014
Wilcox LV (1955) Classification and use of irrigation waters. U.S. Department of Agriculture. Circ, Washington, DC, p 969
WoldeGabriel G, Aronson JL, Walter RC (1990) Geology, geochronology, and Rift basin development in the central sector of the main Ethiopian Rift. Geol Soc Am Bull 102:439–458
WoldeGabriel G, Heiken G, White TD, Asfaw B, Hart WK, Renne PR (2000) Volcanism, tectonism, sedimentation, and the paleoanthropological record in the Ethiopian Rift System. In: McCoy FW, Heiken G (eds) Volcanic hazards and disasters in human antiquity. Geol Soc Am Spec Pap, vol 345, pp 83–99
World Health Organization (WHO) (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva, p. 541
WRI, UNEP, UNDP, World Bank (1998) World resources 1998–99: a guide to the global environment—environmental change and human health (English). The World Bank, Washington, DC. http://documents.worldbank.org/curated/en/545461468744003084/World-resources-1998-99-a-guide-to-the-global-environment-environmental-change-and-human-health
Wu J, Sun Z (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expos Health 8:311–329. https://doi.org/10.1007/s12403-015-0170-x
Wu Y, Wang YX, Xie XJ (2014) Occurrence, behavior and distribution of high levels of uranium in shallow groundwater at Datong basin, northern China. Sci Total Environ 472:809–817
WWDSE (2016) Impact assessment of water resources exploitation on Lake Ziway, Oromia Water Works Design and Supervision Enterprise (WWDSE), Addis Ababa, Ethiopia
Yadav SK, Ramanathan AL, Kumar M, Sabarathinam C (2020) Assessment of arsenic and uranium co-occurrences in groundwater of central Gangetic Plain, Uttar Pradesh, India. Environ Earth Sci 79:154. https://doi.org/10.1007/s12665-020-8892-x
Yirgu G, Dereje A, Peccerillo A, Barberio MR, Donati C, Donato P (1999) Fluorine and chlorine distribution in the volcanic rocks from the Gedemsa volcano, Ethiopian Rift Valley. Acta Vulcanol 11:169e176
Acknowledgements
The authors would like to acknowledge Adama Science and Technology University (ANSD/02/1252/09), for financial support to conduct this research. The authors also acknowledge Muhammed Haji for support with liquid analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mechal, A., Shube, H., Godebo, T.R. et al. Application of multi-hydrochemical indices for spatial groundwater quality assessment: Ziway Lake Basin of the Ethiopian Rift Valley. Environ Earth Sci 81, 25 (2022). https://doi.org/10.1007/s12665-021-10135-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-10135-5