Abstract
Natural radionuclides activity concentrations of 226Ra, 228Ra, and 40K in soil and grass samples, statistical analysis of these radionuclides, and transfer factors (TFs) from soil to the grass were carried out from phosphate mining and processing sites in southern areas of Togo using gamma-ray spectrometry-based high-purity germanium (HPGe) detector. The average activity concentrations of 226Ra, 228Ra, and 40K were found within the range usually measured worldwide. Statistical analysis was done by investigating the skewness, the kurtosis, and the density distribution of radionuclides in the study areas. Both skewness and kurtosis displayed that the activity concentrations of soil and grass samples in the discharge waste site (Kpémé) and mining site (Hahotoé) exhibited slightly positively skewed, negatively skewed, leptokurtic, and platykurtic distributions. The TFs for 226Ra, 228Ra, and 40K from discharge waste soil to grass were evaluated. The average transfer factors of 226Ra, 228Ra, and 40K in the phosphate processing area were found to be 0.16, 0.32, and 8.42, respectively. 226Ra and 228Ra TFs values were found to be within the limit of worldwide measure values, while that of 40K was found to be larger than the values measured in other countries. The average TF value of 8.42 for 40K was eight times higher than the average value of unity, showing its large intake by vegetables. The high TFs for 40K could be explained by the extensive application of phosphate fertilizers to soils when growing vegetables.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Humans and biota are constantly exposed to natural ionizing radiations made of terrestrial, cosmic, and cosmogenic radionuclides, the main sources of natural radiation. The environmental background radiation is made of natural radiation sources and may be affected by anthropogenic radionuclides resulting from atmospheric nuclear weapon testing conducted in the past century, nuclear accidents, and any other human activities (Tagami and Uchida 2009; UNSCEAR 2017). Mining is harmful to human health because it is a natural source of radioactivity. Phosphate extraction and treatment activities in southern Togo could contribute potentially to an increase in radiation exposure to humans and its environment due to the high concentration of radionuclides. The impact of these radionuclides on human health depends on the amount of radionuclide humans are exposed to, through ingestion, inhalation, or direct exposition. It is then important to assess the pathway radionuclides move from soil or atmosphere to the human body.
An important step to estimate the intake of radionuclides in the human food chain in the form of ingestion in the human organism is known as the soil–plant transfer factor (TF), which is defined as the ratio between the activity concentration of the plant and the activity concentration of the associated soil (Tagami and Uchida 2009; Banzi et al. 2017). TF is a quantity used in investigations to estimate the impact of routine or accidental monitoring releases of radionuclides into the environment for the most important agricultural products such as cereals, tubers, and other plants grown in the same area (Tagami and Uchida 2009; Adjirackor et al. 2014). Plants are drawing their nutrient resources from their soil roots and this phenomenon is the main carrier of radioactive contamination leading to radionuclides migration in the food chains. One of the most important parameters in the assessment of the environmental safety around nuclear installations such as nuclear power plants, factories and industries, is the TF installation-soil and installation-air parameter (IAEA 1994).
The transfer factors are necessary to estimate the environmental transfer models from the soil to plants that draw their nutrients from the same soil and which is an important aspect in predicting the level of radioactivity in agricultural crops and foodstuffs for assessment of dose to humans (IAEA 1994; Adjirackor et al. 2014). The most important natural radionuclides are 226Ra (referred to as 238U), 228Ra (referred to as 232Th), and 40K, with different physical and chemical properties related to their environments (Petrinec et al. 2017). The amount of radioactive material in various food and plants according to their absorption capacity is different depending on the different geological formation and composition of the area they grew, the weathering conditions, and the chemical and physical properties of the investigated area.
The phosphate well core is a sample in the solid state of rock in the form of a cylinder and is taken inside the extraction drilled (IAEA 1994; Banzi et al. 2017). These extraction and treatment processes produce waste that must be handled with care. The uncontrolled disposal of these types of waste could lead to environmental pollution and an increase in radioactivity levels in the waste discharge site and surrounding areas (Righi et al. 2005; UNSCEAR 2008). It is therefore possible to increase the radiological exposure of the public. With the increased public concern and awareness about radioactivity in the environment, the present study aims to statistically analyze the amount of each natural radionuclide transfer from soil to grass in the phosphate processing area in southern Togo. The increasing use of phosphate in industries worldwide (milling, processing, and fertilizer manufacturing), especially in Togo, justifies the importance of investigating the potential radioactivity exposure of phosphate ores to the public and workers.
Particular areas of Kpémé (phosphate processing site: 6°12′ 53" N, 01° 30′ 16" E) and Hahotoé (phosphate mining site: 6°21′ 45" N, 01°23′ 48" E) have been identified as contaminated sites earlier by heavy metals and toxic chemicals due to the constant disposal of industrial effluent (Bouka et al. 2013; Aduayi-Akue and Gnandi 2015). As vegetables are an essential part of diets and are ingested both in cooked and raw forms by humans, it is worthy to investigate the transfer of radionuclides from soil to grass used as food. Soil to vegetable transfer of radionuclides is the major pathway of human exposure to potential radioactivity contamination (Righi et al. 2005). In this study, activity concentrations in soil and grass samples are assessed, then transfer factors are calculated based on the measured activity concentrations and statistical analysis of the radionuclide distribution in the investigated area. The estimation of transfer factors of these elements to grass is an important tool used to investigate the potential health effect of humans and animals that consume the products grown in the study region. All these parameters are accessed using some tools and techniques that are described in the following section.
The results obtained from this investigation would be useful for establishing a database in this area of phosphate ore processes and represent a basis to assess any future changes in the levels of radioactivity background due to modern agricultural technologies or any artificial influences around the phosphate ore mining and discharge waste areas.
Materials and methods
The study area, equipment, procedures, and statistical analysis used in the study are presented in this section.
Description of the study area
The soil and grass sampling areas were located at Hahotoé (phosphate extraction site) and Kpémé (discharge phosphate waste site), localities of south-east Togo. These places are part of the Maritime Region, in the prefecture of Vo and geographically located between 6°12′ 53" N–6° 21′ 60" N latitude and 1°23′ 26 "E–1°31′ 41" E longitude. The Kpémé area is located on the coastal sedimentary basin of the Atlantic Ocean and extends along the Gulf of Guinea and is covered with grass, including foodstuffs (Aduayi-Akue and Gnandi 2015). The area of the Hahotoé mining site is affected by weathering and erosion processes which are created by torrential rains. The geological structure of the study areas consists of shale, phosphate shale, quartz, limestone, and phosphate limestone (Bouka et al. 2013; Hazou et al. 2019; Hazou and Patchali 2020). The Hahotoé phosphate extraction site is the site where material resources are extracted and transported to the Kpémé treatment site. It is worth investigating both sites as there is a transfer of radionuclides from the first to the second and the crops grow on the second site.
Sampling and sample preparation
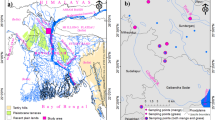
Twenty soil samples and ten grass samples were collected from the Hahotoé phosphate extraction site (10 soil samples), from the Kpémé phosphate (10 soil samples) and from the Kpémé phosphate treatment site (10 grass samples). The grass samples were considered, respectively, as P1, P2, P3, P4, P5, P6, P7, P8, P9 and P10, as shown in Fig. 1. One soil sample was made off five sub-samples taken at the four corners and the center of a square of 25 × 25 m2. A global positioning system (GPS) was used to record the geographical coordinates of the sampling points to facilitate future investigations on radiological exposures in these mining and discharge areas.
Soil samples were taken from a depth of approximately 2–15 cm from the soil after removing debris from the top layer (first centimeter) to minimize contamination (Guembou Shouop et al. 2017; Penabei et al. 2018). Composite samples were then packaged in a polythene bag and transferred to the laboratory for preparation and measurement. Grass samples were taken around the Kpémé phosphate treatment area. The grass samples were washed in distilled water, dried at about 60 °C for 24 h, and ground to a fine powder in the laboratory (Forkapic et al. 2017; Glavič-Cindro et al. 2020).
The activity concentrations’ measurement in samples was done using the formula suggested by Beretka (Beretka and Mathew 1985; Guembou et al. 2018). Each sample was prepared in beakers and closed with caps to ensure that the radon gas was confined in the volume and plastic tape was wrapped over the caps so that the daughter radionuclide would also remain in the sample before measurement. This is important to achieve secular equilibrium, as the activity of uranium and thorium series depends on their daughter’s radioactivity (Nguelem et al. 2013; Guembou Shouop et al. 2017; Hazou et al. 2019; Hazou and Patchali 2020).
Measurement
The activity concentrations of the investigated radionuclides were determined on four HPGe detectors in the Laboratory for Radioactivity Measurements (LMR) of the Jožef Stefan Institute (JSI), Slovenia. These detectors have efficiency higher than 24% and cover the energy range from 4 to 2800 keV (Korun et al. 2012). All detectors have fit for purpose in-house built and constructed shields and each shield consists of an outer shell shield, inner shell shield, and graded layers. The shields are vented by aged (radon free) nitrogen air.
The results obtained from these measurements are traceable to the French national standard at LNE-LNHB (Glavič-Cindro et al. 2016). The geometry of the sample, its matrix, its density, the coincidence summing corrections, and the counting frequency known as systematic effects in gamma-ray spectrometry are taken into account in the estimation of the activity concentration of the radionuclides (Vidmar et al. 2007; Glavič-Cindro et al. 2016). The measurement uncertainties are calculated following the Guide to the Expression of Uncertainty in Measurement rules (GUM 2008; Glavič-Cindro et al. 2020).
The activity concentrations of the long-lived radionuclides are evaluated by determining first the activity concentrations of daughter products which have short half-lives. Thus, the activity concentrations of the uranium series (238U-series) were determined using the activity concentration of 234Th and 234mPa, the activity concentration of 228Ra is calculated from the activity concentration of 228Ac, and the activity concentration of 226Ra is calculated from the activity concentration of 214Pb and 214Bi by taking into account the radon exhalation from the sample matrix and the sample container (Korun and Kovačič 2011; Bučar et al. 2012; Hazou and Patchali 2020). The emission line at 1460.8 keV (10.7%) of 40K is used to determine directly its activity concentration, while the activity concentrations of 226Ra and 228Ra were calculated based on the weighted average value of their decay products at equilibrium, respectively. The secular equilibrium of the daughter products of the U series (226Ra) and Th series (228Ra) is generally assumed for soil activity concentration; however, it is not true for vegetable samples as they are not direct earthly products with connection to emanation or exhalation. Plants are at least 10 cm from the ground level. All samples were packed in the same geometries, while the counting time for measurement process was 24 h. Therefore, the 226Ra, 228Ra, and 40K activity concentrations were used for both soil and vegetation to determine the transfer factors (TFs).
The activity concentrations of each radionuclide in the samples were evaluated using the following expression (Guembou Shouop et al. 2017; Joel et al. 2017a):
where \(A({\text{Bq kg}}^{{ - 1}} \, )\) is the activity concentration of the measured radionuclide, Ns/ts is the count rate of each radionuclide present in the sample, NB/tB is the count rate of each radionuclide present in the background, Ms is the dry weight of the sample, \(\varepsilon\) is the efficiency of the full energy peak, Pγ represents the emission probability, KSC is the factor for cascade summing correction, KSA is the correction factor for self-attenuation, and KDC is the factor for decay correction for each radionuclide.
Parameters that influence the level of radioactivity in the soil and grass samples were evaluated by studying the density distribution of 40K, 226Ra, and 228Ra (Xhixha 2012). The concepts of skewness and kurtosis were used to determine the distribution function of the naturally occurring radionuclides using R version 3.6.2 software (R Core Team 2019).
Statistical analysis
-
Skewness
The skewness is about the average measure of the asymmetry of the distribution of the activity concentration of a given radionuclide. The skewness (S) of a random variable xi (the activity concentration in our case) with the data average value \(\overline{x }\) is the third standardized moment, defined by the following equation (R Core Team 2019):
-
Kurtosis
The kurtosis indicates the degree of “flatness” or “peakedness” in the same distribution of the activity concentration of a given radionuclide relative to the normal distribution or describes how the dataset is clustered or spread around the average. Kurtosis can either be mesokurtic, platykurtic, or leptokurtic. The kurtosis (ku) is the fourth standardized moment, defined as (R Core Team 2019):
Transfer factors
The transfer factor (TF) defines the correlation between the activity concentration found in the soil sample matrix and the grass matrix for the same geographical location and the same radionuclide. It is expressed as a function described in Eq. (4) (Rosén et al. 1999). For natural radionuclides, instead of considering deposition on the ground, the activity concentration in plant matter is divided directly by the activity concentration in dry soil matter (Rosén et al. 1999):
In this study, dry weight of samples was preferred because the quantities of radioactivity per kilogram of dry weight are much less variable than the quantities per unit of fresh weight. This observation reduces uncertainties in the measurements performed (IAEA 1994). This formula is appropriate as the radionuclides investigated are telluric radionuclides that use the specific activity in grass and in soil to evaluate the factor.
Results and discussion
Activity concentrations
-
Soil samples
In the soil samples of phosphate discharge waste areas (Kpémé) from where the grass samples were collected, the activity concentration of 226Ra, 228Ra and 40K ranged from 182 ± 16 to 919 ± 91 Bq.kg−1, 27 ± 1 to 67 ± 3 Bq.kg−1, and 37 ± 2 to 89 ± 5 Bq.kg−1, respectively, with an average of 442 ± 74 Bq.kg−1, 41 ± 4 Bq.kg−1, and 69 ± 6 Bq.kg−1 for phosphate discharge waste (Kpémé) soil. For phosphate mining fields (Hahotoé), the activity concentration of these radionuclides in the soil samples were found to be within 63 ± 6–559 ± 51 Bq.kg−1 for 226Ra, 20 ± 1–61 ± 3 Bq.kg−1 for 228Ra, and 14 ± 2–78 ± 4 Bq.kg−1 for 40K, with an average of 242 ± 42 Bq.kg−1 for 226Ra, 48 ± 5 Bq.kg−1 for 228Ra, and 38 ± 7 Bq.kg−1 for 40K.
When phosphate discharge waste and phosphate mining soils were compared taking into account their natural radionuclide activity concentrations, it was found that phosphate discharge waste soils contain slightly higher activity concentrations than phosphate mining soils, as shown in Table 1. This could be explained by the phosphate processing activities that increase the level of radionuclides by concentrating them in the areas where activities are undertaken. For comparison with the present work in Table 2, values of activity concentration for naturally occurring radionuclides in phosphate discharge wastes and phosphate mining soils in some studies in other countries around the world are shown (Table 2). Table 2 shows that the activity concentrations of natural radionuclides in phosphate discharge waste and phosphate mining soils of the investigated areas were higher than the activity concentrations of phosphate mining soils and phosphate discharge waste soil obtained by Bolca (Bolca et al. 2007) in Turkey, but lower than the activity concentrations of soil obtained by Uosif (Uosif et al. 2008) in Egypt (Abu-Tartur).
-
Mixed grass samples
The activity concentrations of 226Ra, 228Ra, 40K, and 7Be radionuclides in grass samples from ten different fields of phosphate discharge waste (Kpémé) are presented in Table 3. In the grass collected from agricultural fields, the activity concentrations of 226Ra, 228Ra, 40K and 7Be ranged 26 ± 7 Bq.kg−1–132 ± 20 Bq.kg−1, 5 ± 1–27 ± 1 Bq.kg−1, 395 ± 19–736 ± 36 Bq.kg−1 and 31 ± 6–167 ± 27 Bq.kg−1, with an average of 54 ± 10 Bq.kg−1, 11 ± 3 Bq.kg−1, 547 ± 36 Bq.kg−1 and 78 ± 12 Bq.kg−1, respectively. The 7Be was detected because the grass samples were measured directly without storage procedure like soil samples.
The highest activity concentrations of 226Ra, 228Ra, 40K, and 7Be were observed in grass sample P3, followed by grass sample P1 and P6, while the lowest activity concentrations of 226Ra, 228Ra, 40K, and 7Be were observed in grass sample P10, grass sample P7 and grass sample P2, respectively. The samples with high activity concentrations (P1, P3, and P6) were found to be closer to the processing plant than the samples with low activities (P2, P7, and P10) as can be seen in Fig. 1. Table 4 shows the average activity concentrations obtained in the present study [phosphate discharge waste (Kpémé)] compared to the results of other studies in other countries. It revealed that the activity concentrations of 226Ra, 228Ra, 40K, and 7Be in grass of the areas studied in phosphate discharge waste (Kpémé, Togo) was higher than the activity concentrations reported worldwide by the UNSCEAR (2000) and by Ahmed (Ahmed et al. 2005) in Egypt, but similar to those reported in Malaysia by Asaduzzaman (Asaduzzaman et al. 2014) and in Turkey by Bolca (Bolca et al. 2007).
Statistical analysis
-
Skewness and kurtosis for 226 Ra in dry weight soil and grass samples
Statistics using R Core Team (2019) from Table 5 shows that the distribution of activity concentrations of 226Ra in both phosphate mining sites (Hahotoé) and discharge waste site (Kpémé) soil and grass samples is positively skewed (1.15, 0.73, and 1.55, respectively) at 5% level of significance; thus, the average activity concentration of 226Ra is greater than the modal activity concentration of measured soil samples (as shown in Fig. 2). The negative kurtosis (− 0.74) of discharge waste (Kpémé) soil shows that the distribution of activity concentrations of 226Ra is platykurtic. The term platykurtic describes a statistical distribution with extremely dispersed activity concentrations of 226Ra in discharge waste soil, and the distribution will tend to have a flat top near the average rather than a sharp peak (as shown in Fig. 2). The positive kurtosis (0.79 and 1.52) of phosphate mining soil and grass from the processing area shows that the distribution of activity concentrations of 226Ra is leptokurtic (as shown in Fig. 2). Leptokurtic describes a statistical distribution where the activity concentrations of 226Ra are clustered around the average activity concentration in the soil, and the distribution will tend to have a distinct peak near the average, which declines rapidly, and a heavy tail (as shown in Fig. 2).
-
Skewness and kurtosis for 228Ra in dry weight soil and grass samples
Statistics from Table 5 also shows that the distribution of activity concentrations of 228Ra in discharge waste site (Kpémé) soil and grass from the same area is positively skewed (0.54 and 1.07, respectively); thus, the average activity concentration of 228Ra in discharge waste soil and grass is greater than the modal activity concentration, or the soils and grass that measured relatively lower activity concentrations of 228Ra are more than those that measured higher activity concentrations (as shown in Fig. 3). The activity concentrations of 228Ra in phosphate mining soil is negatively skewed (− 0.94); thus, the average activity concentration of 228Ra in phosphate mining soil is less than the modal activity concentration, or soils that measured a relatively higher activity concentration of 228Ra are more than those that measured a lower activity concentration (as shown in Fig. 3). The negative kurtosis of both discharge waste (soil and grass) and mining soil (− 1.32, − 0.66, and − 0.82, respectively) shows that the distribution of activity concentrations of 228Ra is platykurtic, describing a statistical distribution with extremely dispersed activity concentrations of 228Ra in phosphate soils and grass samples and the distribution will tend to have a flat top near the average rather than a sharp peak (as shown in Fig. 3).
-
Skewness and kurtosis for 40K in dry weight soil and grass samples
Statistics from Table 5 shows that the activity concentrations of 40K in the discharge waste site (Kpémé) soil is negatively skewed (− 0.36); thus, the average activity concentration of 40K in phosphate mining soil is less than the modal activity concentration, or soils that measured a relatively higher activity concentration of 40K are more than those that measured a lower activity concentration (as shown in Fig. 4). The distribution of activity concentrations of 40K in phosphate mining site (Hahotoé) soil and in grass samples from discharge waste site is positively skewed (1.02 and 0.18 respectively) (as shown in Fig. 4); thus, the average activity concentration of 40K in discharge waste grass samples and in phosphate mining soil is greater than the modal activity concentration, or the soils and grass samples that measured relatively lower activity concentrations of 40K are more than those that measured higher activity concentrations. The negative kurtosis values (− 0.56, − 1.43, and − 1.50, respectively) of both phosphate mining site (Hahotoé) soil and discharge waste site (Kpémé) soil and grass samples show that the distribution of activity concentrations of 40K is platykurtic. The term platykurtic describes a statistical distribution with extremely dispersed concentration of 40K in these soils and grass samples and the distribution will tend to have a flat top near the average rather than a sharp peak (as shown in Fig. 4).
Transfer factors of 226Ra, 228Ra, and 40K from soil to grass in discharge waste (Kpémé)
The TFs of 226Ra, 228Ra, and 40K from the investigated phosphate discharge waste soil (Kpémé) to grass using Eq. (4) are presented in Table 6. The average values of TF were 0.16 ± 0.04, 0.32 ± 0.09, and 8.42 ± 0.80, respectively, for 226Ra, 228Ra, and 40K. The highest TF of (0.44) for 226Ra was found in the grass sample from P3, followed by grass sample from P5 with TF equal to (0.36). The average TF of (0.16) is higher than the result published by Hasan (Hasan et al. 2010). The highest TF values of (0.87) and (13.32) for 228Ra and 40K, respectively, were found in grass samples from P3 and P10, respectively. The average TFs for all grass samples for 228Ra and 40K were 0.32 and 8.42, respectively. These results are higher than the results published by Hasan (Hasan et al. 2010) in Pakistan.
The obtained results display the highest transfer factor for potassium. This is probably explained by the use of potassium as the crop’s fertilizer in the investigated area. However, high values of higher TF for potassium do not pose a risk to human health because 40K has low contribution to internal dose, as 40K content in the human body is homeostatically controlled (IAEA 1994; Banzi et al. 2017)). TFs for 226Ra were found to be less than those of 228Ra and 40K in all grass samples in this study. This indicates that 226Ra is less effectively transported from soil to grass than 228Ra and 40K. The variation of soil-to-plant transfer factors depends on the soil’s properties as the nutrient standard, the exchangeable K content, and the content of moisture in the soil (Frissel et al. 2002; Banzi et al. 2017). For the investigated radionuclides, the literature usually presents the same range of TFs values obtained in the present study except for the 40K that are slightly lower than those got in this work.
The observed variation of transfer factors for radium can be explained by its non-uniform distribution in various lands where crops grow, even for closest sampling points. This non-uniform distribution is also displayed in the results of statistical analysis as can be seen in Figs. 2 3, 4. Investigations of grass samples showed that the most abundant radionuclide in grass is potassium 40, followed by radium 228 and radium 226, respectively. This explains the low absorption of radium isotopes by grass, with values of TFs less than the unity. The uptake of different radionuclides from the soil by grass depends on various properties, including soil texture and clay content, also taking into account the dominant clay minerals in the soil (IAEA 1994). The soil in these areas would be more inclined to the transport of potassium than other radionuclides.
The present study’s results were compared to what has been done in similar regions in the world with mining and processing activity of phosphate. We can assume that the average values of the soil to grass transfer factors have been found to be in close agreement with those published by the IAEA (1994) except those for potassium which are higher than the IAEA’s values as shown in Table 7. The TF of 228Ra in our study was found to be close to the result found by Harb (Harb et al. 2014) in Egypt, but less than the value found by Mohannad (Mohannad et al. 2014) in Palestine and much higher than that found by Hasan (Hasan et al. 2010) in Pakistan as shown in Table 7. This variation might be due to different soil’s physical and chemical properties, the climate in the area the study had been carried out, and some extra practices at operational processing sites.
This high transfer coefficient from soil to plant compared to other parts of the world can affect the animal grazing such as cows that are later used for milk and meat production. Therefore, the transport of radionuclides from soil to grass (40K > 228Ra > 226Ra decrease in this order) affects animals that consume grass. Furthermore, by accumulating those radionuclides in the animal’s body, the radiological risk of people who take milk or meat from those animals increases. The variations of TFs values from each country depend strongly on variability in contamination of soil due to non-homogeneous deposition of radionuclides into associated soil and can also be due to seasonal variations in diet and/or feeding behavior in dry or rainy season (IAEA 2010).
The obtained results demonstrate that each primordial radionuclide follows complex dynamics phenomena. These processes lead those radionuclides to be transported from soil to vegetables in different manner as described by Mohanned et al. (2014) and in IAEA (1994 and 2010). The portion of these radionuclides, which is in the soil matrix, can be drawn into vegetables through their roots. In some cases, this is facilitated by their chemical similarity with other elements that the vegetables normally use for their growth. This explains why there are differences between values of TFs observed between different vegetables in different locations around the world as shown in Tables 6, 7. Furthermore, radionuclides belonging to physiologically regulated elements, or their analogs, may be selectively adsorbed, whereas others may be excluded. In general, the highest activity concentration in vegetables was found in samples collected from areas with the highest radioactivity concentration in soil substrate (IAEA 2010; UNSCEAR 2017). This observation on the level of the activity concentration in vegetables is not linearly correlated to the activity in soils. Several authors reported that this nonlinear and non-uniform correlation in phosphate and mining sites is due to adsorption phenomena and the clayey nature of the phosphate soils (Frissel et al. 2002; Uosif et al. 2008; Alharbi et al. 2013; Asaduzzaman et al. 2014; Banzi et al. 2017). These two properties are the main reasons for the retention of radionuclides on the top layer of the soil. This situation decreases the mobility of radionuclides at depths. Therefore, the uptake of all radionuclides depends not only upon the mining and processing activities, but also on their chemical metabolism which defines the mobility of these radionuclides from soil to plant.
Conclusion
The present study investigated the activity concentration of natural radionuclides in soils and grass samples from phosphate exploitations sites in Togo, discharge waste site of Kpémé and phosphate mining site of Hahotoé. 226Ra (238U-series), 228Ra (232Th-series), and 40K were found to be the main radiological constituents of soil and grass samples in the studied areas and 7Be was found in small concentration but in the range measured in other countries worldwide. Statistical analysis was performed by assessing the skewness, kurtosis, and the density distribution of the radionuclides in the investigated samples. The transfer factors of radionuclides from soil to grass obtained were within the range measured in other parts of the world except for that of 40K. The high TFs for 40K could be explained by the extensive application of phosphate fertilizers to soils when growing vegetables. Even though the fertilizer is not made of 100% 40K, it may slightly increase the activity concentrations of natural radionuclides. Its values are not a sign of significant radiological alarm as 40K is part of human/animal body and contribute to maintaining their life on Earth. The level of TFs for 40K highlighted the necessity of performing further investigation about its transfer from soil to grass and the characteristic of the discharge waste site.
Considerable variability was observed in the activity concentrations of 226Ra, 228Ra, and 40K from all samples in both sites and all grass samples investigated. A high level of activity concentrations in discharge waste soil (Kpémé) in this study confirmed the previously reported values by Hazou et al. (Hazou et al. 2019). This leads us to conclude that phosphate treatment and waste storage increase the level of radioactivity in the processing area and therefore affect crops and pasture on the surrounding soils. TFs are statistically uncertain, thus for further investigations, there is a need to replicate TFs values for individual combinations of each radionuclide, soil type and crop, necessary to obtain reliable average values based on radiological assessment models.
References
Adjirackor T, Darko EO, Kpeglo DO et al (2014) Radiological study of soil, fertilizer and foodstuffs in some selected farming communities in the greater Accra region, Ghana. Nucl Radiat Phys 77:29112–29118
Aduayi-Akue A, Gnandi K (2015) Evaluation de la pollution par les métaux lourds des sols et de la variété locale du maïs Zea mays dans la zone de traitement des phosphates de Kpémé (Sud du Togo). Int J Biol Chem Sci 8:2347. https://doi.org/10.4314/ijbcs.v8i5.37
Ahmed NK, El-Arabi AG (2005) Natural radioactivity in farm soil and phosphate fertilizer and its environmental implications in Qena governorate, Upper Egypt. J Environ Radioact 84:51–64
Alharbi A, El-Taher A (2013) A study on transfer factors of radionuclides from soil to plant. Life Sci J. http://www.lifesciencesite.com
Asaduzzaman KH, Mayeen UK, Amin YM, Bradley DA, Mahat RH, Nor RM (2014) Soil-to-root vegetable transfer factors for 226Ra, 232Th, 40K, and88Y in Malaysia. J Environ Rad 135:120–127. http://www.elsevier.com/locate/jenvrad
Banzi F, Msaki P, Mohammed N (2017) Assessment of radioactivity of 226Ra, 232Th and 40K in soil and plants for estamation of transfer factors and effective dose around Mkuju river project. Tanzania Min Mineral Dep 11(3):93–100
Beretka J, Mathew PJ (1985) Natural radioactivity of australian building materials, industrial wastes and by-products. Health Phys 48:87–95. https://doi.org/10.1097/00004032-198501000-00007
Bolca M, Saç MM, Çokuysal B et al (2007) Radioactivity in soils and various foodstuffs from the Gediz River Basin of Turkey. Radiat Meas 42:263–270. https://doi.org/10.1016/j.radmeas.2006.12.001
Bouka E, Lawson PE, Eklu KG et al (2013) Heavy metals concentration in soil, water, Manihot esculenta tuber and Oreochromis niloticus around phosphates exploitation area in Togo. Res J Environ Toxicol 7:18–28. https://doi.org/10.3923/rjet.2013.18.28
Bučar K, Korun M, Vodenik B (2012) Influence of the thorium decay series on the background of high-resolution gamma-ray spectrometers. Appl Radiat Isot Incl Data Instrum Methods Use Agric Ind Med 70:1005–1009. https://doi.org/10.1016/j.apradiso.2012.03.014
Forkapic S, Vasin J, Bikit I et al (2017) Correlations between soil characteristics and radioactivity content of Vojvodina soil. J Environ Radioact 166:104–111. https://doi.org/10.1016/j.jenvrad.2016.04.003
Frissel MJ, Deb DL, Fathony M, Lin YM, Mollah AS, Ngo NT, Othman I, Robinson WL, Skarlou-Alexiou V, Topcuoglu S, Twining JR, Uchida S, Wasserman MA (2002) Generic values for soil-to-plant transfer factors of radiocesium. J Environ Radioact 58:113–128
Glavič-Cindro D, Korun M, Nečemer M et al (2016) Evaluation of comparison and proficiency test results of gamma ray spectrometry at Jožef Stefan Institute from 1986 to 2014. Appl Radiat Isot 109:54–60. https://doi.org/10.1016/j.apradiso.2015.12.025
Glavič-Cindro D, Hazou E, Korun M et al (2020) Measurement uncertainty arising from sampling of environmental samples. Appl Radiat Isot. https://doi.org/10.1016/j.apradiso.2019.108978
Guembou SCJ, Moyo MN, Mekongtso EJN, Motapon O, Strivay D (2018) Monte Carlo method for Gamma spectrometry based on GEANT4 toolkit: efficiency calibration of BE6530 detector”. J Environ Radioact 189:109–119. https://doi.org/10.1016/j.jenvrad.2018.03.015
Guembou Shouop CJ, Ndontchueng Moyo M, Chene G et al (2017) Assessment of natural radioactivity and associated radiation hazards in sand building material used in Douala Littoral-Region of Cameroon, using gamma spectrometry. Environ Earth Sci. https://doi.org/10.1007/s12665-017-6474-3
Guimond RJ (1990) Radium in fertilizers, The environmental behavior of radium. International atomic energy agency) report. Vienna Tech 310:113–128
GUM (2008) Evaluation of measurement data–Guide to the expression of uncertainty in measurement. JCGM 100:2008
Harb S, El-Kamel AH, Abd El-Mageed AI, Abbady A, Rashed W (2014) Radioactivity levels and soil-to-plant transfer factor of natural radionuclides from Protectorate Area in Aswan, Egypt. World J of Nuclear Science and Technology 4: 7–15. http://www.scirp.org/journal/wjnst. (Published Online January)
Hasan MK, Zahid SC, Muhammad I, Khalid K (2010) Assessment of radionuclides, trace metals and radionuclide transfer from soil to food of Jhangar valley (Pakistan) using gamma-ray spectrometry. Water Air Soil Pollut 213:353–362
Hazou E, Patchali TE (2021) Assessment of radiological hazards in the phosphate mining area of Kpogamé. Togo Case Stud Chem Environ Eng 3:100077. https://doi.org/10.1016/j.cscee.2020.100077
Hazou E, Guembou Shouop CJ, Nguelem Mekongtso EJ et al (2019) Preliminary assessment of natural radioactivity and associated radiation hazards in a phosphate mining site in southern area of Togo. Radiat Detect Technol Methods. https://doi.org/10.1007/s41605-018-0091-x
IAEA (1994) Handbook of parameter values for the prediction of radionuclide transfer in temperate environments. A Guide Book Technical Report Series, Vienna
IAEA (2010) Handbook of parameter values for the prediction of radionuclide transfer in terrestrial and freshwater environments Technical reports series no. IAEA
Joel GSC, Penabei S, Ndontchueng MM et al (2017) Precision measurement of radioactivity in gamma-rays spectrometry using two HPGe detectors (BEGe-6530 and GC0818-7600SL models) comparison techniques: application to the soil measurement. MethodsX 4:42–54. https://doi.org/10.1016/j.mex.2016.12.003
Khan K, Khan HM, Tufail M, Khatibeh AJAH, Ahmad N (1998) Radiometric analysis of Hazara phosphate rock and fertilizers in Pakistan. J Environ Radioact 35(1):7–84
Korun M, Kovačič K (2011) Determination of (238)U in ground-water samples using gamma-ray spectrometry. Appl Radiat Isot 69(3):636–640
Korun M, Maver Modec P, Vodenik B, Zorko B (2012) Uranium-induced background of germanium gamma-ray spectrometers. Appl Radiat Isot 70:1480–1484. https://doi.org/10.1016/j.apradiso.2012.05.001
Manigandan PK, Manikandan NM (2008) Migration of radionuclide in soil and plants in the Western Ghats environment Iran J Radiat Res. J Phys Sci 24(1):95–113
Mohannad MJ, Khalil M (2014) Transfer of natural radionuclides from soil to plants and grass in the western north of west bank environment- palestine. Internat J Environ Monitoring Analy 2(5):252–258. https://doi.org/10.11648/j.ijema.20140205.14
Nguelem EM, Njinga R, Simo A et al (2013) Preliminary investigation of naturally occurring radionuclide in some six representative cement types commonly used in Cameroon as building material. Radiat Prot Environ 36:71. https://doi.org/10.4103/0972-0464.128871
Penabei S, Bongue D, Maleka P et al (2018) Assessment of natural radioactivity levels and the associated radiological hazards in some building materials from Mayo-Kebbi region, Chad. Radioprotection 53:265–278. https://doi.org/10.1051/radiopro/2018030
Petrinec B, Tišma I, Šoštarić M et al (2017) Current radiological situation in Slavonski brod, Croatia: Is there a reason for concern? Arh Hig Rada Toksikol 68:206–211. https://doi.org/10.1515/aiht-2017-68-2992
R Core Team (2019) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria, R version 3.6.2. http://www.R-project.org/. Accessed 12 Dec 2019
Righi S, Lucialli P, Bruzzi L (2005) Health and environmental impacts of a fertilizer plant–Part I. Assessment of radioactive pollution. J Environ Radioact 82:167–182. https://doi.org/10.1016/j.jenvrad.2004.11.007
Rosén K, Öborn I, Lönsjö H (1999) Migration of radiocaesium in Swedish soil profiles after the Chernobyl accident, 1987–1995. J Environ Radioact 46:45–66. https://doi.org/10.1016/S0265-931X(99)00040-5
Sam AK, Ahmad MMO, El Khngi FA, El Nigumi YO, Holm E (1999) Radiological and assessment of Uro and Kurun rock phosphates. J Environ Radioact 24:65–75
Tagami K, Uchida S (2009) Radium-226 transfer factor from soils to crops and its simple estimation method using uranium and barium concentrations. Chemosphere 77:105–114. https://doi.org/10.1016/j.chemosphere.2009.05.012
UNSCEAR (2000) Sources and effects of ionizing radiation united nations scientific committee on the effects of atomic radiation. Report to the general assembly with scientific annexes, Newyork, USA. p 111–125.
UNSCEAR (2008) United nations scientific committee on the effect of atomic radiation: sources and effects of ionizing radiation. Report to the general assembly scien annexes 1. NewYork.
UNSCEAR (2017) Sources, effects and risks of ionizing radiation: Annex B epidemiological studies of cancer risk due to low-dose-rate radiation from environmental sources.
Uosif MAM, El-Taher A (2008) Radiological assessment of abu-tartur phosphate, Western Desert Egypt. Radiat Prot Dosimetry 130(2):228–235
Vidmar T, Korun M, Vodenik B (2007) A method for calculation of true coincidence summing correction factors for extended sources. Appl Radiat Isot 65:243–246. https://doi.org/10.1016/japradiso200607012
Xhixha G (2012) PhD: advanced gamma-ray spectrometry for environmental radioactivity monitoring. University of Ferrara
Acknowledgements
The authors wish to express their great appreciation and gratitude to the ICTP-IAEA for awarding financially the fellowship, without which this work would have not been possible; and the Director-General of the Jožef Stefan Institute (JSI) of Slovenia, Prof. dr. Jadran Lenarčič for the laboratory support. The authors also appreciate the community of Hahotoé-Kpémé for their great cooperation during the sample collection period. They also wish to thank Dr. Michel WARNAU, PMO from IAEA for Togo, for his availability to this work. We also wish to address special thanks to Prof. Mazamaesso TCHAOU, NLO of Togo, without forgetting my colleagues from the Master of Medical Physics (2020/2021) for all their support!
Funding
This work was supported by International Atomic Energy Agency, under Sandwich Training Educational Programme, STEP (ICTP/IAEA) 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hazou, E., Zorko, B., Dzagli, M.M. et al. Transfer from soil to grass and statistical analysis of naturally occurring radionuclides in soil from phosphate mining and processing sites in Maritime Region of Togo. Environ Earth Sci 80, 626 (2021). https://doi.org/10.1007/s12665-021-09931-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-09931-w