Abstract
The Lake Togo-Lagoon of Aného complex is located in the area of the phosphorite mining which produces different types of waste that are dumped directly into the surrounding environment without treatment. In addition, the contributions of its tributaries after leaching agricultural and urban soils need to be considered. This increases the risk of contamination of the hydrosystem, especially the sediments in trace elements. The objective of this study is to evaluate the level of trace elements contamination and their spatial and seasonal variation in the sediments of the Lake Togo-Lagoon of Aného complex. The sediment physicochemical parameters were determined in line with French standards. The trace elements were determined using an Atomic Absorption Spectrometer coupled to a hydride and cold vapour generator. The spatial and seasonal variation was assessed using Ordinary Kriging Interpolation method. The average content of trace elements in sediments in mg/kg was as follow: Cd: 0.75, Pb: 13.26, Cr: 50.63, Ni: 36.05, Cu: 10.90, Hg: 0.04, As: 3.88, Zn: 47.38 and Mn: 766.74 in the dry season. In rainy season, the average concentration was: Cd: 0.46, Pb: 9.50, Cr: 27.43, Ni: 21.36, Cu: 9.61, Hg: 0.05, As: 5.23, Zn: 39.21 and Mn: 910.08. These concentrations showed a strong spatial variation. Significant seasonal variations were obtained for conductivity, organic matter as well as for Cd, Pb, Cr, Ni, Hg and As. The sediments were found to be weakly to moderately contaminated and enriched by trace elements with a higher contamination for Cd. The sediments may have low to moderate adverse effects on aquatic living organisms. This trace element contamination is of anthropogenic origin through the phosphorite mining and the agricultural and urban effluents. Therefore, this lagoon ecosystem warrants regular monitoring for better understanding to inform protection of the resource.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic ecosystems have received significant amounts of anthropogenic contaminants, such as trace elements, from industrial, mining exploitation, agricultural, and urban wastes during recent decades (Caeiro et al. 2005; Hounkpè et al. 2017; Jamshidi-Zanjani and Saeedi 2013). In aquatic ecosystems, sediments are recognized and studied as reservoirs or traps for many chemical pollutants, especially for trace elements (Bastami et al. 2012; Boucheseiche et al. 2002; Yao et al. 2009). More than 90% of the trace elements in these ecosystems are associated with suspended particulate matters and sediments (Amin et al. 2009; Saeedi et al. 2012; Zhang and Shan 2008). Any modification of the physicochemical conditions of the environment can lead to remobilization of trace elements associated with sediments (Förstner and Wittmann 1981; François et al. 2002; Soro et al. 2009). Consequently, trace elements contamination of sediments constitutes a hazard for the biocenosis of lagoon ecosystems and human health because of the phenomenon of bioaccumulation in the food chain. Thus, the management of the quality of aquatic environments and in particular their sediments has become a priority for many countries. It is a problem that preoccupies regions concerned about maintaining the health of their coastal aquatic ecosystems (Hounkpè et al. 2017; Yao et al. 2009). In aquatic ecosystems, sediments are widely used as indicators for assessing the level of trace elements contamination (Islam et al. 2015b; Soro et al. 2009; Yao et al. 2009).
In Togo, the Lake Togo-Lagoon of Aného complex is located in the Maritime Region which supports 42% of the national population (DGSCN 2010). The phosphorite mining takes place in this region with the discharge of various kinds of mining waste into coastal waters. However, these phosphorites contain high contents of trace elements (Gnandi et al. 2009; Gnandi and Tobschall 2002; Tchangbedji et al. 2003). In addition, this hydrosystem receives inputs from runoff waters, Haho and Zio rivers after leaching mining, urban and agricultural soils where agrochemicals are used in an uncontrolled manner. It is clear that this ecosystem is not immune to the problems of trace elements pollution and its consequences. Thus, it is essential to monitor regularly the state of quality of this ecosystem for its sustainable management. This study aims to evaluate the level of trace elements contamination of sediments of the Lake Togo-Lagoon of Aného hydrosystem and their spatial and seasonal variation. This will provide a good knowledge of this lagoon ecosystem for better management planning of the resource.
Materials and methods
Study area
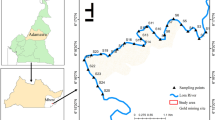
The Lake Togo-Lagoon of Aného complex is the largest part of a lagoon system in south-eastern Togo, located between the latitudes 6° 14′ 38″ and 6° 17′ 37″ North and the longitudes 1° 23′ 33″ and 1° 37′ 38″ East. With a total surface area of 64 km2, it comprises the Lake Togo (46 km2), the Lagoon of Togoville (13 km length) which is parallel to the coast with a width that varies between 150 and 900 m, the Lake Zowla (6.55 km2) in the northeast, and the Lagoon of Aného in the southeast. The entire hydrosystem interacts with the ocean at Aného and with the Mono River in the East, via the Gbaga channel which runs parallel to the coast. Phosphorite mining takes place in this watershed with the discharge of liquid and solid waste without prior treatment. The lagoon system is mainly fed by the rivers Zio (176 km, 9.9 m3/s) and Haho (140 km, 5.8 m3/s) (Millet 1986). The climate of the Lake Togo watershed is the subequatorial or Guinean type with two rainy seasons alternating with two dry seasons. The dominant economic activities around the lagoon system are agriculture, livestock farming and fishing with the illegal and abusive practice of “Acadja” which is a fishing practice using branches as a trap for fish. The study area and the sampling points were presented on Fig. 1.
The coastal basin of Togo is part of a large sedimentary basin of the Gulf of Guinea which extends from the south-east of Ghana to Cameroon. The sedimentary succession of this basin, about 600 m thick, rests in discordance on a Pan-African metamorphic substratum of age of 600 ± 50 Ma. The sedimentary sequence contains deposits of Maastrichtian, Paleocene, Lower to Middle Eocene, Upper Oligocene and Quaternary age (Da Costa et al. 2013, 2006; Johnson and Da Costa 2008; Jonhson et al. 2000). The Maastrichtian deposits consist of sandy and marly facies with the presence of sandstones, limestones and laminated argillites with kaolinite, attapulgite and glauconite. The Paleocene deposits consist mainly of a succession of sands, limestones and argillites with palygorskite. The Eocene deposits are characterized by phosphorites, laminated argillites and marls. The Upper Oligocene deposits are represented by glauconitic limestones and clayed sands. The Quaternary deposits occurring at the top of the sedimentary sequence are constituted by conglomerates, sands, sandy argillites and kaolinitic argillites.
The phosphorite deposited during Eocene is actually exploited at Hahotoe-Kpogamé in the coastal basin. Mineralogical studies of raw phosphorite indicate that apart from apatite, it also contains quartz, calcite, goethite, clay and organic matter (Slansky 1980). Chemical analysis of the phosphorite shows that, it is a Carbonate-rich fluorapatite (Gnandi and Tobschall 1999a) called commonly, francolite. It contains a large amount of trace elements through substitution phenomenon (Gnandi 1998; Kunkel 1990). Indeed, several studies have shown that the phosphorites of Hahotoe-Kpogamé contain high levels of trace elements (Gnandi 1998; Gnandi et al. 2009; Gnandi and Tobschall 2002; Kunkel 1990; Tchangbedji et al. 2003) with average concentrations in mg/kg as follow: Cd: 44, Cr: 356, Cu, 158, Ni: 109, Zn: 465, Pb: 67, Sr: 1429, Zr: 46 and V: 173. It contains major elements whose average concentrations vary from 0.02% for MnO to 33.86% for CaO (Gnandi 1998; Gnandi and Tobschall 2002). The soils around the phosphorite extraction zone have high concentrations of trace elements (in mg/kg) which are: Cd: 44, Cr: 13, Cu, 106, Ni: 138, Zn: 211, Pb: 72, Sr: 825, Zr: 234 and V: 279 (Gnandi and Tobschall 2002). Aduayi-Akue and Gnandi (2014) reported trace element concentrations in mg/kg in the soils of the villages surrounding the phosphorite pant zone which varied as follow: Cd: 21.93–42.53, Pb: 2.93–16.05, Ni: 21.08–69.46, Cu: 26.58–40.25.
Sampling and laboratory analysis
Sampling was carried out according to the standards ISO 5667-12 and NF EN ISO 5667-15, at the end of the dry season (March 2016) and at the end of the rainy season (October 2016). A total of 30 sediment samples were collected per season in clean and sterile polypropylene bags. A reference site (6° 26′ 56.97″ N; 1° 04′ 44.46″ E) was sampled far from the phosphorite mines in the Zio River to highlight the level of trace elements contamination of sediments from the hydrosystem. Samples were transported to the laboratory in a cooler and stored at 4 °C in the dark until treatment (El-Sayed et al. 2015; Yao et al. 2009).
The sediments were dried at room temperature. After this, the sediments clumps were disaggregated before being sieved to 2 mm. The organic matter (OM) was determined by the loss on ignition in a furnace at 550 °C for 4 h after a 24 h stay in an oven at 105 °C. The OM was calculated according to the following equation:
where M1 and M2 are, respectively, the masses before and after calcination (Agah et al. 2016; Dean 1974; Mathieu and Pieltain 2003). Grain size analyses were carried out using mechanical sieving methods according to the standard NF EN 933-1. The conductivity, pH and salinity of sediments were measured using the methods described by Mathieu and Pieltain (2003). Indeed, the 2 mm fraction of the dry sediment was collected in a beaker to which distilled water was added in the proportions of “2:5”. This mixture was stirred for 1 min on a magnetic stirrer and left to stand for 2 h. Then, the supernatant was used for the measurement of pH, electrical conductivity and salinity using a pH-meter CRISON PH 25 and a multimeter Knick Portamess. After grinding and sieving, 2 g of the fraction less than 63 μm was digested using aqua regia method (1 HNO3:3 HCl) according to the standard NF ISO 11466, at room temperature for the determination of mercury and at 110 °C for other trace elements (Bastami et al. 2015a; Monroy et al. 2014). The elements Cd, Pb, Cr, Cu, Ni, Zn and Mn were analyzed using atomic absorption spectrometers (AAS), Varian AA 240 FS type at “Vasile Alecsandri” University of Bacau (Romania) and Thermo Electron S Series type at the University of Lomé (Togo). Concerning Hg and As, they were only analyzed at the University of Lomé using a hydride and cold vapour generator, Thermo Scientific VP 100 type coupled to the AAS Thermo Electron S Series according to French standards (NF T90-112, NF EN ISO 5961, NF EN 1233, NF EN 1483, NF EN ISO 11969). The reagents used for this purpose are from Sigma-Aldrich and Merck.
Calculation of contamination indexes and factors
The mean probable effect level quotient (mPEL-Q) was used to determine the level of toxicity to aquatic life of the combination of trace elements studied in sediments (Fairey et al. 2001; Jamshidi-Zanjani and Saeedi 2013; Long 2006) using the following equation:
where n and Ci are, respectively, the number of elements and the concentration of the element “i”. PEL represents Probable Effect Level (CCME 2001; MacDonald et al. 1996). Their interpretation was made according to the following scale: PEL-Q ≤ 0.1: low potential for adverse biological effect (10% risk); 0.1 < PEL-Q ≤ 1.5: low-medium potential for adverse biological effect (25% risk); 1.5 < Q-PEL ≤ 2.3: medium–high potential for adverse biological effect (50% of risk) and PEL-Q > 2.3 reveals the highest potential for adverse biological effect (76% of risk) (Long and MacDonald 1998; Long et al. 2000).
The enrichment factors (EF) were calculated according to the following equation (Agah et al. 2016; Jamshidi-Zanjani and Saeedi 2013; Wang et al. 2014):
where (Cx/Cn)sa and (Cx/Cn)ref = ratios of the contents of studied element “x” and the normalization element “n”, respectively, in the studied and reference samples. The normalization element used in this study is the Fe. The EF values are interpreted as follows: FE < 1 (no enrichment), 1 ≤ EF < 3 (low enrichment); 3 ≤ EF < 5 (Moderate enrichment); 5 ≤ EF < 10 (Moderate to high enrichment); 10 ≤ EF < 25 (high enrichment); 25 ≤ EF < 50 (very high enrichment); EF ≥ 50 (extremely high enrichment).
The contamination factor (CF) was calculated according to the relation (4) (Hakanson 1980; Islam et al. 205a):
where Csa: concentration of the element in the study sample and Cr: concentration of the same element in the reference sample. They were interpreted according to the following scale: CF < 1 (low contamination factor); 1 ≤ CF < 3 (Moderate contamination factor); 3 ≤ CF < 6 (high contamination factor); CF ≥ 6 (Very high contamination factor).
The pollution load index (PLI) allows to evaluate the overall contamination status of the sediments based on the combination of all the trace elements contamination factors. It is expressed according to the Eq. (5) developed by Tomlinson et al. (1980) and, widely used (Ali et al. 2016; Li et al. 2013; Rabee et al. 2011).
where “n” is the number of elements studied and CF is the contamination factor of Hakanson (1980). Thus, if PLI = 0, there is no pollution; if PLI < 1, it is a reference situation and when PLI > 1 there is a progressive deterioration of environmental quality.
Quality control and accuracy
The quality of the analytical methods has been verified by internal control. A blank sample was prepared simultaneously with the same experimental procedure (reagents and experimental conditions). The blank allowed zeroing the device and was regularly measured. This permits determination of the possible contamination and to eliminate the quantization errors. Also, standard solutions of each element were analyzed at regular intervals to verify the accuracy of the results and the internal fidelity of the method. In addition, to check the repeatability of the results, double samples were incorporated among the samples to be analyzed.
Statistical analysis and cartography
Spatial distribution maps were generated by ArcGIS 10.2.2 software using the Ordinary Kriging Interpolation method. It is a geostatistical analysis method that estimates the values of unsampled points to produce prediction maps. Ordinary Kriging Interpolation provides a better prediction and has the elements of precision measurement (Nshimiyimana et al. 2016; Wang et al. 2014; Wani et al. 2013). To determine the interrelationships between the different studied parameters and to deduce their possible origins, a principal component analysis (PCA) and a Pearson correlation analysis were performed (Ayah et al. 2015; Solitoke et al. 2018). Student’s t test was used to compare inter-season concentrations. The STATISTICA 6.1 software was used for the statistical analysis.
Results
Physicochemical parameters of sediments
The results of the physicochemical parameters of the sediments are presented in Table 1. The average values of the pH of the sediments were around the neutrality both in the dry season (7.34 ± 0.27) and rainy season (7.27 ± 0.32). These values varied from 6.55 to 7.78 and from 6.5 to 7.9 in the dry and rainy seasons, respectively. The electrical conductivities (EC) ranged from 0.81 to 12.31 mS/cm with an average of 4.55 ± 3.22 mS/cm in the dry season and from 0.15 to 8.89 mS/cm with an average of 1.31 ± 1.79 mS/cm in the rainy season. The average salinity values were 3.02 ± 2.03 g/l in the dry season and 0.96 ± 1.1 g/l in the rainy season. They varied, respectively, from 0.8 to 7.3 g/l and from 0.1 to 4.2 g/l. It appears that the values of conductivity and salinity show a gradient of concentration increasing from upstream to downstream with the highest values recorded in the lagoon of Aného.
Total organic matter (OM) ranged from 0.76 to 20.6% with an average of 7.98 ± 6.83% in the dry season and from 0.5 to 17.91% with an average of 5.26 ± 4 95% in the rainy season. In contrast to pH and OM, significant differences were found between seasonal mean values of conductivity (t = 4.82, p = 0.0023) and salinity (t = 4.88, p = 0.0016). A homogeneous variation of the pH values was noted during both seasons contrary to the conductivity, salinity and organic matter which had a heterogeneous variation.
The particle size compositions of the sediments studied are depicted in Fig. 2. The sediments of the selected sites are generally sandy muds. However, there are sediments that are mostly muddy or sandy. Muddy sediments tend to occupy the central parts and southern coast of the hydrosystem. The highest proportions of fine particles (< 63 μm) are 90% at the site S10 and 84% at the site S16 during the dry season and the rainy season, respectively, while the sandy sediments are often located at the banks of Lake Togo and in the lagoons of Togoville and Aného with proportions reaching 88% at the site S3 during the dry season and at the S4 site during the rainy season.
The lowest proportions of silts and clays (< 63 μm) were located in the Lake Togo and in the Lagoon of Aného. Coarse particles (> 200 μm) were very poorly represented and mainly constituted by bivalve and gastropods shells. Their percentages vary from 0 to 12% in dry season and from 0 to 9% in the rainy season.
Concentration of trace elements in sediments
Spatial and seasonal variation in sediment trace element contents
The statistical values of seasonal concentrations of trace elements in sediments are depicted in Table 2.
The average Cd content in the dry season (0.75 ± 0.56 mg/kg) is significantly higher (t = 2.20; p = 0.0316) than that of the rainy season (0.46 ± 0.48 mg/kg). They varied, respectively, from 0.17 to 2.24 mg/kg and from 0.10 to 2.16 mg/kg (Table 2; Fig. 3). The spatial distribution of Cd contents (Fig. 4a, d) shows that they tend to increase from upstream to downstream during both seasons with the highest concentrations recorded in the lagoons of Togoville and Aného, respectively, in the dry season and in the rainy season. The larger area of the hydrosystem presented Cd contents ranging from 0.46 to 2.24 mg/kg in the dry season and from 0.18 to 2.16 mg/kg in the rainy season.
Box-plots of trace element concentrations in sediments (Stars correspond to the maximum and minimum. The Square figures in the box-plots are the average values. The horizontal lines in the top, middle and bottom of the box-plots correspond to 75% percentile, median and 25% percentile, respectively. DS Dry season, RS Rainy season)
Concerning the Pb contents, they ranged from 2.83 to 28.48 mg/kg with an average of 13.26 ± 6.81 mg/kg in the dry season and from 1.31 to 23.99 mg/kg with an average of 9.50 ± 5.92 mg/kg in the rainy season (Table 2; Fig. 3). Seasonal averages of Pb contents were significantly different (t = 2.28; p = 0.0263). Figure 4b, e which shows that the highest Pb contents were located in southeastern Lake Togo during the dry season and in the lagoon of Aného during the rainy season. The larger area in the hydrosystem was covered by the Pb contents comprised between 10.55 and 28.48 mg/kg and between 7.33 and 23.99 mg/kg, respectively, in dry and rainy seasons.
Chromium (Cr) contents ranged from 8.26 to 165.28 mg/kg in the dry season and from 7.58 to 62.45 mg/kg in the rainy season. The mean content in the dry season (50.63 ± 39.32 mg/kg) is significantly higher (t = 3; p = 0.004) than that of the rainy season (27.43 ± 15.67 mg/kg) (Table 2; Fig. 3). The spatial distribution of Cr contents (Fig. 4c, f) shows that in the dry season, the highest concentrations were recorded in the south-western part of Lake Togo, while in the rainy season, they were located in the Southeast of the Lake Togo and in the northern part of the lagoon of Aného. Most recorded contents in the hydrosystem oscillated between 25.88 and 165.28 mg/kg in the dry season and between 19.05 and 62.45 mg/kg in the rainy season.
The average Ni content in the dry season (36.05 ± 24.91 mg/kg) was significantly higher (t = 2.8; p = 0.007) than that of the rainy season (21.36 ± 14.35 mg/kg). These values ranged between 5.31 and 100.11 mg/kg in the dry season and between 4.40 and 47.88 mg/kg in the rainy season (Table 2; Fig. 3). The larger area of the hydrosystem was covered by trace elements contents ranging from 26.37 to 100.11 mg/kg in the dry season and from 14.47 to 47.88 mg/kg in the rainy season. The highest concentrations were, respectively, obtained in the Southwest and Northwest of the Lake Togo (Fig. 5a, d).
The Cu contents ranged from 2.52 to 24.60 mg/kg with an average of 10.90 ± 6.76 mg/kg in the dry season and from 2.54 to 32.04 mg/kg with an average of 9.61 ± 6.24 mg/kg in the rainy season (Table 2; Fig. 3). These levels did not show a significant difference (t = 0.77; p > 0.05) in means. Figure 5b, e shows that the highest Cu contents were located in southeastern Lake Togo and in the Lagoon of Aného both in the dry and rainy seasons. The larger area of the lagoon system was covered by concentrations ranging from 7.80 to 24.60 mg/kg in the dry season and from 6.09 to 32.04 mg/kg in the rainy season.
Mercury (Hg) concentrations were the lowest and ranged from 0.02 mg/kg in the dry season to 0.07 mg/kg in the rainy season. The average dry season content (0.04 ± 0.01 mg/kg) is significantly lower (t = − 5.09; p = 0.0001) than that obtained during the rainy season (0.07 ± 0.01 mg/kg) (Table 2; Fig. 3). The spatial distribution of Hg (Fig. 5c, f) shows that in the dry season, the highest contents were located in the South-East of the Lake Togo and in the lagoon of Aného. During the rainy season, they were recorded in the north-west and south-east of the Lake Togo and in the lagoon of Aného. It was the concentrations between 0.028 and 0.064 mg/kg in the dry season and between 0.048 and 0.071 mg/kg which occupied the larger area of the hydrosystem.
The mean As content in the dry season (3.88 ± 1.66 mg/kg) was significantly lower (t = − 2.41; p = 0.019) than that of the rainy season (5.23 ± 2.57 mg/kg). These contents varied, respectively, from 1.20 to 7.02 mg/kg and from 2.27 to 11.91 mg/kg (Table 2; Fig. 3). The spatial variation of As contents (Fig. 6a, d) shows that the highest contents were located in the lagoons of Togoville and Aného, both in the dry and rainy seasons. In the rainy season, an increase in concentrations from upstream to downstream was observed. The larger area of the hydrosystem was covered by As contents ranging from 3.63 to 7.02 mg/kg in the dry season and from 3.82 to 11.91 mg/kg in the rainy season.
For Zn contents, they ranged from 11.42 to 124.25 mg/kg with an average of 47.38 ± 29.74 mg/kg in the dry season and from 8.58 to 72.43 mg/kg with an average of 39.21 ± 17.98 mg/kg in the rainy season (Table 2; Fig. 3). These mean contents did not differ significantly (t = 1.29; p > 0.05). The highest levels were recorded in the Northwest and South of the Lake Togo during the dry season and in the lagoon of Aného during the rainy season where an increasing gradient of concentrations from upstream to downstream was noted. The larger area of the hydrosystem was occupied by Zn concentrations comprised between 31.35 and 124.25 mg/kg and between 8.58 and 43.86 mg/kg, respectively, in the dry and rainy season (Fig. 6b, e).
The Mn was the most abundant element. Its mean content in the dry season (766.74 ± 600.01 mg/kg) was not significantly different (t = − 0.7; p > 0.05) from that of the rainy season (910.08 ± 945.75 mg/kg). These contents varied, respectively, from 109.82 to 2335.92 mg/kg and from 106.15 to 3426.05 mg/kg (Table 2; Fig. 3). The spatial distribution of Mn (Fig. 6c, f) shows that the highest concentrations occupied the Lake Togo in its South-Western and South-Eastern parts, both in the dry and rainy seasons. The lowest concentrations were only located in the Lagoon of Aného during both seasons.
Heterogeneous variations in Cd, Pb, Cr, Ni, Cu and Mn contents in both seasons and Zn contents in the dry season were noted with coefficients of variation (CV) ranging from 51.35% for Pb in the dry season and to 106.49% for Cd in the rainy season. As for the Hg and As contents during the two seasons and the Zn contents during the rainy season, their variations are fairly homogeneous with CV ranging from 17.32% for the Hg to 49.18 for the Zn in the rainy season. All trace elements except As in the dry season and Zn in the rainy season showed positive asymmetry with positive skewness ranging from 0.46 in the dry season for Pb to 2.04 in the rainy season for Cd (Table 2; Fig. 3). It appears that, most of the sites were in the zone of the lowest trace element concentrations. This asymmetry is more pronounced for Cd, Pb and Mn in the dry season and for Cd, Cu and Mn in the rainy season with skewness greater than 1. This can be confirmed by lower values of medians compared to averages (Table 2; Fig. 3). The decreasing order of seasonal abundance of trace elements in these sediments is as follows: Mn > Cr > Zn > Ni > Pb > Cu > As > Cd > Hg in the dry season and Mn > Zn > Cr > Ni > Cu > Pb > As > Cd > Hg in the rainy season.
Comparison of trace element contents with quality standards
Trace element concentrations were compared to the USA and Canadian sediment quality guidelines (Fig. 7). These standards are presented in two categories based on their toxic effects. They are the threshold effect level (TEL) and the probable effect level (PEL) for Canada and effect range low (ERL) and effect range medium (ERM) for the USA (Table 3). During the dry season, the Cd, Cr, Ni and As contents of the studied sediments were greater than the TEL in the respective proportions of 43.33, 46.67, 66.67 and 10%. Concentrations above the ERL are those of Cd (13.33%), Cr (26.67%) and Ni (56.67%). In the rainy season, concentrations greater than TEL were recorded for trace elements Cd (30%), Cr (20%), Ni (43.33%) and As (26.67%). Only one sample had concentration of Zn which is superior to the TEL during both seasons, representing 1.67% of the samples. However, contents above the ERL were observed for Cd (10%) and Ni (43.33%). Pb, Cu and Hg contents in both seasons and Zn contents in the rainy season were all below the TEL. In addition, contents of Pb, Cu, Hg and Zn in both seasons and of As in the dry season were all lower than ERL. It is noted that all the trace element contents were lower than the PEL except for the Ni in the dry season and rainy season for 43.33 and 23.33% of the sites, respectively, and Cr in the dry season for 6, 67% of the sites. However, only the Ni, presented concentrations that are higher than the ERM for 30% of the samples in the dry season.
The mean probable effect level quotients (mPEL-Q) were determined to evaluate the overall quality of the studied sediments in terms of polymetallic pollution which is a threat to aquatic life. Figure 8 shows that in the dry season, mPEL-Q values ranged from 0.09 to 0.55 with an average of 0.28 ± 0.13 while in the rainy season, they ranged from 0.09 to 0.38 with an average of 0.20 ± 0.08. In addition, it is noted that 96.67% of sediment samples studied during both seasons were considered to have low to moderate potential for adverse biological effects for living organisms (0.1 < mPEL-Q ≤ 1.5).
Multivariate analysis of trace element concentrations
The multivariate analysis was conducted through Principal Component Analysis (PCA) applied on a data matrix of 13 variables and 30 samples per season.
Correlation matrix
The Pearson correlation matrices between variables in the dry season (A) and rainy season (B) are presented in Table 4 with the significant correlations marked in bold. During the dry season, correlations were obtained between Cd and As, Mn. Lead (Pb) presented a correlation with Cd and As. Chromium (Cr), is correlated with Ni, Cu, Zn and Mn. Correlations were observed between Ni and each of the following elements Cu, Zn, Mn. For Cu, it was correlated with Hg, Zn, and Mn. Good correlations were also found between As and Zn and between Zn and Mn. The pH is negatively correlated with Cu, Zn and Mn. Conductivity (EC) is positively correlated with Cd and negatively with Ni and Mn. Only Cd is correlated with salinity. Organic matter (OM) is correlated with Cr, Ni, Cu, Hg and Mn. Clay and silt (C/S) were positively correlated with Cr, Ni, Cu, and Mn.
In the rainy season, Cd is positively correlated with Pb, As, and Zn. For Pb, it is correlated with Cr, Cu, As and Zn. Positive correlations were obtained between Cr and Ni, Cu, Zn, Mn. The Ni was positively correlated with Cu, Zn and Mn. Also, correlations were also obtained between Cu and Zn (positive), between Hg and Mn (negative) and between As and Zn (positive). Clays and silts are positively correlated with the elements Cr, Ni, Mn and OM. The pH is positively correlated with As. Concerning conductivity, it is positively correlated with Cd, Pb, Cu, As, and Zn. The salinity is positively correlated with Cd, Pb, As and Zn. Organic matter shows a positive correlation with Cr, Ni, Cu, Zn and Mn then a negative correlation with Zn.
Eigenvalues and projection of variables and cases (sites) in the factorial plan F1 × F2
The Eigenvalues and the percentages of variances explained as well as the correlation coefficients between the variables and the factorial axes are presented in Table 5. According to this table, the four factors observed in the dry season explain 77.42% of the total information with F1: 37.29; F2: 19.77%; F3: 11.59% and F4: 8.77%. In the rainy season, the four factors observed explain 78.38% of the total variance with F1: 34.50%; F2: 29.20%; F3: 7.87%; and F4: 6.81%. The factorial plan F1 × F2 explains 57.06 and 63.70% of the total information, respectively, in the dry season and rainy season. Thus, the process of trace elements distribution in sediments can be illustrate explained by the information contained in these factorial axes.
The values in bold indicate the highest and significant correlation coefficients between factorial axes and variables
The projection of the variables in the F1 × F2 factorial plan (Fig. 8) shows that in the dry season (Fig. 9a), the F1 axis (37.29%) is determined in its negative part by Cr, Ni, Cu, Zn, Mn, OM and C/S. This indicates an organic matter (OM) enrichment gradient associated with Cr, Ni, Cu, Zn, Mn contamination. The F2 axis (19.77%) is positively defined by Cd, As, EC and Sa representing a gradient of salinity and contamination by Cd and As. Table 5 indicates that the F3 axis (11.59%) is negatively determined by Pb and pH while the only contributor of the F4 axis (8.77%) is Hg. Also, the conductivity (EC) is positively correlated well with the F1 factorial axis. During the rainy season (Fig. 9b), the F1 axis (34.50%) presents a trace element contamination gradient and is defined in its negative part by the trace elements Pb, Cr, Cu, Ni, Zn, OM and C/S. The main contributors of the F2 factorial axis (29.20%) are Mn in its positive part and the variables Cd, As, EC and Sa in its negative part. This axis describes a gradient of Mn contamination in opposition to the gradient of salinity, mineralization and contamination of sediments by Cd and As. Table 5 indicates that the F3 axis (7.87%) is negatively defined by the pH while the Hg is the only contributor of the F4 axis (6.81%). In addition, the Mn is also negatively correlated with the axis F1 while the Ni and the OM are also correlated with the positive part of the axis F2.
The projection of sites in the factorial plan F1 × F2 (Fig. 10) shows that three types of sediments can be distinguished during both seasons. Indeed, during the dry season, the first type (G1) is constituted by sites S1, S2, S7–S10, S13–S17, S19 and S20 which were all located in Lake Togo. Their sediments are characterized by a greater enrichment of organic matter and a higher contamination by Cr, Ni, Cu, Zn and Mn. The second type (G2) is composed of sites S21–S24, S26–S30 whose sediments are less enriched in organic matter. They are more contaminated by Cd and As with higher salinities and electrical conductivities. The third type (G3) comprises the sites S3–S6, S11 and S25 whose sediments have low salinities and conductivities and are poorly enriched in organic matter. In addition, they are marked by low contents of trace elements.
In the rainy season, the first type (G1) is composed of sites S2, S7, S9, S10, S13, S15, S16, S19 whose sediments are highly enriched in organic matter associated with a greater contamination by elements Cr, Ni and Mn. The second type (G2) of sediment comprises S20–S23, S25, S28–S30. They are less enriched in organic matter. They have high salinities and conductivities with a higher contamination by elements Cd, Pb, Cu, As and Zn. The third type of sediment (G3), composed of sites S1, S3–S6, S8, S11, S12, S14, S17, S18, S24, S26, S27, is the poorest in organic matter with low values of salinity and conductivity. In addition, it has the lowest contents of trace elements studied.
Assessment of trace elements contamination level of sediments
Enrichment factor (EF)
Table 6 indicates that the highest enrichment factor (EF) values ranged from 2.22 for Hg to 18.35 for Cd in the dry season and from 2.51 for Hg to 15.60 for Cd in the rainy season. The lowest values ranged from 0.25 for Mn to 1.62 for Cd and from 0.19 for Mn to 1.31 for Hg, respectively, in the dry season and rainy season. Sites with moderate to high enrichment (5 ≤ EF < 10) were observed for the Cd (23.33%), Pb and Mn (3.33% each element), Cr (16.67%), Ni (13.37%) in the dry season and for the Cd and Mn (16.67%), Pb, Cu and As (3.33% each element), in the rainy season. In addition, high enrichments (10 ≤ EF < 25) were recorded for Cd and Cr in the dry season, respectively, in 10 and 3.33% of the sites studied and for Cd in the rainy season for 10% of the sites.
The average EF values show that in the dry season, sediments of the Lake Togo-Lagoon of Aného hydrosystem showed moderate to high enrichment only for Cd with an average EF of 5.42 and a moderate enrichment for Cr with an average EF of 3.03. The other elements (Pb, Ni, Cu, Hg, As, Zn and Mn) had mean EF between 1 and 3, indicating their low enrichment in sediments. During the rainy season, only Cd exhibits moderate sediment enrichment with an average EF of 3.60. The average EF of the other trace elements (Pb, Cr, Ni, Cu, Hg, As, Zn and Mn) was between 1 and 3 and indicates their low enrichment in the sediments of the hydrosystem. However, all trace elements showed EF slightly above 1.5 with the exception of Hg, As and Zn in the dry season and Zn in the rainy season. The order of trace elements enrichment in the sediments for each season is as follows: Cd > Cr > Ni > Pb > Mn > Cu > Zn > As > Hg in the dry season and Cd > Mn > As > Pb > Ni > Cr > Hg > Cu > Zn in the rainy season. Cd is therefore the most enriched element in these sediments.
Contamination factor and PLI
The statistical values for contamination factors (CF) and pollution load indices (PLI) are reported in Table 7. The lowest CF values range from 0.28 for Mn to 1.42 for Cd in the dry season and from 0.27 for Pb and Mn to 1.24 for Hg in the rainy season. The highest values vary from 2.25 for Hg to 18.74 for Cd in the dry season and from 2.49 for Hg to 18.09 for Cd in the rainy season.
During the dry season, the average CF values show that only Cd had a very high contamination factor (CF ≥ 6) in the sediments of the hydrosystem Lake Togo-Lagoon of Aného with an average CF of 6.31. In addition, a high contamination factor (3 ≤ CF < 6) was obtained for Cr with an average CF of 3.25. The average CF of the other trace elements (Pb, Ni, Cu, Hg, As, Zn and Mn) was between 1 and 3, indicating moderate contamination. In the rainy season, only Cd had a high contamination factor (3 ≤ FC < 6) while all the other elements were classified as moderate contamination factors (1 ≤ CF < 3). The decreasing order of sediment contamination for each season is as follow: Cd > Cr > Ni > Pb > Mn > Cu > Zn > As > Hg in the dry season and Cd > Mn > As > Pb > Hg > Cr > Ni > Cu > Zn in the rainy season. These sediments were therefore more contaminated by Cd during both seasons.
To evaluate the levels of contamination caused by the combination of all the trace elements studied, the pollution load indices (PLI) were calculated. Table 7 shows that in the dry season, PLI values ranged from 0.79 to 3.20 with an average of 2.04 ± 0.73 and from 0.77 to 2.90 with an average of 1.70 ± 0.69 in the rainy season. A gradual deterioration of the sediments quality (PLI > 1), considered as moderate contamination, was noted for 90% and 70% of sites, respectively, in the dry and rainy seasons. Almost all of the hydrosystem was occupied by PLI values > 1 with the highest values observed in the south-west and south-eastern areas of the Lake Togo during the dry season (Fig. 11a) and in the Lagoons of Togoville and Aného during the rainy season (Fig. 11b).
Discussion
The pH values recorded in sediments are generally neutral, contrary to those obtained in the Bou Regreg estuary in Morocco (7.77 < pH < 8.04), which are slightly basic (Nadem et al. 2015). The pH may be related to the nature of the rocks or soils crossed by the runoff in the watershed. In addition, the decomposition of aquatic plants releases organic acids into the water and causes a decrease in pH (Razaie-Boroon et al. 2011). It is one of the most important factors in aquatic ecosystems concerning chemical behavior and distribution process of chemical elements (Chapman and Kimstach 1996). The conductivity values are much higher than those usually observed in freshwater sediments (100–1000 μS/cm). These high values of conductivities are associated with high salinity and can be explained by a marine influence with intrusion of salt water loaded with effluents from the phosphorite treatment plant during the dry season and low tide period (Kambiré et al. 2014; Millet 1986; Nadem et al. 2015). This can be confirmed by increasing conductivity and salinity values from upstream to downstream. The decrease in sediment conductivity and salinity values in rainy season is due to the dilution effect during floods followed by the massive influx of continental fresh water and non-salty sediments (Kambiré et al. 2014; Yao et al. 2009). Indeed, the soils crossed by Zio and Haho rivers, which are the main contributors of the hydrosystem sediments, are mainly modal ferralitic and tropical ferruginous (Lamouroux 1969).
The recorded organic matter contents are higher than those obtained in the Lake Togo by Millet (1986), in the Lere Lake in Chad by Ekengele et al. (2016) and in Lagos Lagoon in Nigeria (Ekaete et al. 2015). These contents are not generally different from those observed in the Lake Ahémé and lagoon of Ouidah in Bénin (Dedjiho et al. 2014; Hounkpè et al. 2017). The classification of the state of ecological quality of sediments in three levels, according to the contents of organic matter (OM) was proposed by Marin et al. (2008) as follows: high-good (less than 5%), moderate (between 5 and 10%) and poor-bad (more than 10%). Thus, the sediments of this hydrosystem are of good quality for 53.33 and 60% of the sites, respectively, in the dry season and in the rainy season. Poor-quality sediments were recorded for 40 and 16.67% of the sites, respectively, in the dry season and in the rainy season. Organic matter and grain size are one of the important factors affecting the distribution of trace elements in sediments (Aloupi and Angelidis 2001; Bastami et al. 2012; Liaghati et al. 2003). In fact, fine grains have the largest specific surface and are therefore more amenable to the adsorption of contaminated organic and inorganic materials (Horowitz and Elrick 1987; McCave 1984). In general, fine-grained sediments containing a large amount of organic matter are more contaminated than coarse-grained sediments (De Mora and Sheikholeslami 2002). This affinity between trace element content, fine grain of sediment, and organic matter was confirmed in this study by the results of principal component analysis (PCA) (Table 5; Fig. 9) and correlation analysis (Table 4), which showed positive relationships between trace elements (Cr, Ni, Cu, Mn) and fine grains (C/S) and between trace elements (Cr, Ni, Cu, Hg, Zn, Mn) and organic matter (OM). Thus, it is noted that, in contrary to coarse particles, fine particles are the largest carriers of trace elements (Lin et al. 2002). However, the co-precipitation phenomenon must not be neglected in the process of these trace elements accumulation in sediments. Indeed, trace elements in water as dissolved form can co-precipitate in new minerals, such as hydrated oxides, according to the physicochemical conditions (Singer and Stumm 1970). The sediments of the central part and the southern coasts of the Lake Togo-Lagoon of Aného hydrosystem are richer in fine particles (clays and silts) and are in agreement with the results obtained by Millet (1986).
The results of this study compared to those obtained by other authors show that the average Cd content is higher than those recorded in Anzali Wet Zone, Aghien Lagoon, Weija Reservoir, Lake Togo for previous studies (Table 8). Most of the lagoons and lakes listed in Table 8, have higher average Pb, Cr, Cu and Zn concentrations than those observed in the hydrosystem Lake Togo-Lagoon of Aného. The average concentration of As is much higher than those of the lagoons listed in Table 8 except for Owalla Reservoir in Ghana. With regard to Hg and Mn, only the average contents, recorded in the lagoon system of Lomé in Togo for Hg, Mn, Aghien Lagoon and Weija Reservoir for Hg and Wet zone of Anzali for Mn are higher than those obtained in the present study (Table 8). These variations in trace element concentrations between study areas may be related to the petrographic nature of the rocks crossed by runoff, the types of anthropogenic activities in the area, and the sample digestion methods in each study. It should be noted that the sediments of this study underwent partial digestion with aqua regia method.
The increase in concentrations from upstream to downstream during both seasons for Cd (Fig. 4a, d) and for As (Fig. 6a, d) is a good illustration of the marine influence in the contamination process of the hydrosystem by effluents from the Kpémé phosphorite plant. These effluents contain finely suspended particles of phosphorites and clays which settle as soon as hydrodynamic conditions allow. However, it is known that the effluents discharged by the processing plant into the sea at Kpémé are rich in phosphorites and still have high concentrations of trace elements, particularly the Cd (Bawa et al. 2007; Gnandi et al. 2009). In addition, dissolved forms of trace elements may precipitate during favorable physico-chemical conditions. In addition, the passage of the train loaded with raw phosphorites over the lagoon of Togoville resulting in phosphorite falls in the lagoon for decades and the leaching of mining soils can be quoted. Indeed, natural phosphorites can contain up to 20 mg of As/kg of phosphorite (Rodier et al. 2009) and average Cd contents of 49 (Tchangbedji et al. 2003) and 71 mg/kg (Gnandi et al. 2009) were obtained in Hahotoé-Kpogamé phosphorites. Gnandi and Tobschall (1999b) showed that these phosphorites are highly enriched in Cr, Ni, Cu and Zn. However, these elements can also come from the leaching of agricultural soils. Indeed, phosphate, nitrogenous and potash fertilizers can contain up to 6.3 mg of Cd/kg of fertilizer (Raven and Leoppert 1997) and 83.3 mg of Zn/kg of fertilizer (Mermut et al. 1996). The Mn of these sediments originates from the mainland and may derive from the natural geological processes of rock alteration and particle transport associated with the phenomenon of Fe/Mn hydrated oxide precipitation in an aerobic environment (Singer and Stumm 1970). In fact, the waters of the hydrosystem were oxygenized with averages of dissolved oxygen ranging from 7.16 to 8.01 mg/l in the same period (Ouro-Sama et al. 2018) and the sediments had a good to moderate quality (5.26–7.98%) as far as organic matter contents are concerned, preventing a high oxygen consumption by the oxidation of organic matter. In addition, Mn is able to substitute Fe, Ca and Mg in silicate structures and can lead to their abundance in ferralitic and ferruginous soils. This may explain the high concentration of Mn given that the soils traversed by the main contributor rivers of the hydrosystem, are mainly modal ferralitic and ferruginous tropical soils with concretions and cuirasses on a granito-gneissic and quartzitic base rocks (Lamouroux 1969). However, an anthropogenic origin cannot be excluded due to the fact that the Mn contents remain highest in this hydrosystem with a decrease in concentrations from upstream to downstream. In fact, agrochemicals (fertilizers and pesticides) and fuels contain Mn (Biney et al. 1994) which can reach the lagoons as a result of agricultural soil leaching and atmospheric deposition. The North-West of Lake Togo is in the same direction as the Lomé airport runway and is thus permanently over-flown by planes before landing. This may be a possible source of Mn by deposition following fuel combustions (Fang et al. 2010, 2007; Gnandi et al. 2011). It is known that Mn is increasingly used as a substitute for Pb in octane and other high energy fuels and is thus sprayed by atmospheric deposition around airports (Boyes 2010; Butcher 2002; Kaiser 2003). The other trace elements (Pb, Cr, Ni, Cu, Hg and Zn) generally have a heterogeneous distribution with no particular orientation. Their concentrations vary according to metal type, sampling point (site) and sediment type with the high concentrations distributed sporadically. This can be explained by less abundant inputs of these elements. This could allow them to engage in different biogeochemical processes depending on the local physicochemical conditions that can modify their spatial distribution. Indeed, Cu and Hg are known to have a strong affinity for organic matter. Copper (Cu) is intimately related to aerobic degradation of organic matter (Das and Nolting 1993; Neculita et al. 2005; Pietrzak and Mcphail 2004). This is confirmed by the results of the PCA (Table 5; Fig. 9) and correlation analysis (Table 4) for Cu. Copper (Cu) is strongly related to the oxidizable fraction of sediments (Lee et al. 2005; Stead-Dexter and Ward 2004) and more specifically to humic substances for which it has a high affinity (Pempkowiak et al. 1999). As for Hg, the fraction bound to organic matter and sulphides is solubilized while that bound to inorganic oxides and hydroxides is precipitated (Thomassin et al. 2003). Significant strong and positive correlations were obtained between the trace elements on the one hand and between these trace elements and the physicochemical parameters (Table 4) on the other. This implies that they originate from the same natural or anthropogenic source, are controlled by the same physicochemical processes and have the same behavior during transport (Agah et al. 2016; Ali et al. 2016; Bastami et al. 2015b; Chen et al. 2012; Yao et al. 2009). Thus, the positive and significant correlations obtained between Cd, conductivity (EC) and salinity (Sa) during both seasons are illustrated by correlation analysis (Table 4) and PCA (Table 5; Fig. 9). These results confirm that the main source of Cd in the hydrosystem is anthropogenic and comes from phosphorite via the intrusion of marine waters laden with effluents from the processing plant of Kpémé.
Seasonal variations in trace element contents of sediments have also been observed in other tropical lagoons (Doamekpor et al. 2018; Gouin et al. 2016; Hounkpè et al. 2017; Wognin et al. 2017). The increase in Cd, Pb, Cr, Ni, Cu and Zn concentrations obtained during the dry season (low water) could be explained by the low flow of water which can favor the accumulation of trace elements in the sediments from the phosphorite effluents transported by seawater (Ali et al. 2016; Islam et al. 2015a). The low contents of Hg, As and Mn observed in the dry season may be due to their diffusion in the dissolved phase due to the physico-chemical conditions (high salinity, anoxia, high temperature) of the environment (Ouro-Sama et al. 2018; Van Den Berg 1993). Their high contents recorded during the rainy season can be due to a higher input of Mn, Hg and As rich particles related to a high erosion and removal of material from rocks and agricultural soils. In the rainy season, low contents of trace elements (Cd, Pb, Cr, Ni, Cu, Zn) may linked to the dilution phenomena that occur through less or uncontaminated sediment inputs and to the resuspension of fine particles which are richer in trace elements (Lin et al. 2002; Yao et al. 2009). Since this phenomenon can be reversible, the accumulation of trace elements in sediments can cause ecotoxicological problems for aquatic life (Soro et al. 2009). Indeed, there are risks of the release of trace elements to the liquid phase and of their absorption by the living organisms when the physicochemical conditions allow as a result of the seasonal conditions (Förstner and Wittmann 1981; François et al. 2002).
According to Canadian sediment quality guidelines, it can be concluded that sediments of this studied hydrosystem are likely to cause adverse biological effects, related to Cd, Cr, Ni and As, on living organisms (CCME 2001; MacDonald et al. 1996, 2000). This is confirmed by the mean PEL-Q values, which revealed low to moderate risks of adverse biological effects, related to all trace elements, on at least 25% of aquatic life (Long and MacDonald 1998). In addition, EF and CF values calculated relative to the reference site indicate that the sediments are most contaminated with Cd followed by Cr and Ni. The main origin of these elements in this lagoon environment is the exploitation of phosphorites (Gnandi 2002) while the other trace elements come mainly from other sources, such as agricultural activities and urban effluents. Indeed, according to Zhang et al. (2007), EF values between 0.5 and 1.5 (0.5 ≤ EF ≤ 1.5) suggest that trace elements would be derived entirely from the earth’s crust materials or natural processes of rock alteration. However, when EF values are greater than 1.5 (EF ≥ 1.5), it assumes that a significant portion of the trace elements derive from human activities. These results are confirmed by the EF values, which are, for the most part, higher than 1.5 and the pollution load indices (PLI), which show a progressive deterioration of the metallic quality of the sediments from the hydrosystem Lake Togo-Lagoon of Aného. The Cd, therefore, has an influence on the overall state of trace elements contamination of the sediments of this hydrosystem. This strong contamination by Cd was observed by (Wognin et al. 2017) in the Ebrié lagoon in Côte d’Ivoire.
Conclusion
This study has shown that the sediments of the hydrosystem Lake Togo-Lagoon of Aného are of the saline type with high conductivities, especially during periods of low flow under a strong marine influence. These sediments are rich in organic matter in some locations with rates reaching 20.6% in the dry season. The content of trace elements is quite high and heterogeneously distributed over the hydrosystem. The most abundant element is Mn with average levels reaching 766.74 in the dry season and 910.08 mg/kg in the rainy season. Mercury (Hg) is the least represented element with average concentrations of 0.04 mg/kg in the dry season and 0.05 mg/kg in the rainy season. The trace element concentrations showed strong spatial variations. In addition, significant seasonal differences were found for the parameters (EC and OM) and the trace elements (Cd, Pb, Cr, Ni, Hg and As). Sediments of this body of water are weakly to moderately contaminated by trace elements that are mainly of anthropogenic origin due to agricultural activity in the watershed and the phosphorite mining activities. Given the fishing activities and ecotourism around this body of water, other studies on the effects of contaminants on aquatic life are necessary to inform better management of this ecosystem.
References
Aduayi-Akue AA, Gnandi K (2014) Evaluation de la pollution par les métaux lourds des sols et de la variété locale du maïs Zea mays dans la zone de traitement des phosphates de Kpémé (Sud du Togo). Int J Biol Chem Sci 8:2347–2355
Aduwo AI, Adeniyi IF (2018) The heavy metals/trace elements contents of sediments from Owalla reservoir Osun state, southwest Nigeria. Adv Oceanogr Limnol 9:68–78
Agah H, Saleh A, Bastami KD, Fumani NS (2016) Ecological risk, source and preliminary assessment of metals in the surface sediments of Chabahar Bay, Oman Sea. Mar Pollut Bull 107:383–388
Ali MM, Ali ML, Islam MS, Rahman MZ (2016) Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh. Environ Nanotechnol Monit Manag 5:27–35
Aloupi M, Angelidis MO (2001) Geochemistry of natural and anthropogenic metals in the coastal sediments of the island of Lesvos Aegean Sea. J Environ Poll 113:211–219
Amin B, Ismail A, Arshad A, Yap CK, Kamarudin MS (2009) Anthropogenic impacts on heavy metal concentrations in the coastal sediments of Dumai Indonesia. Environ Monit Assess 148:291–305
Ansah E, Nukpezah D, Hogarh JN (2018) Levels and Distribution of Heavy Metals in Weija Reservoir Accra, Ghana West African. J Appl Ecol 26:74–88
Ayah M, Grybos M, Tampo L, Bawa LM, Bril H, Djaneye-Boundjou G (2015) Qualité et pollution des eaux d’un hydrosystème littoral tropical: cas du système lagunaire de Lomé Togo. Eur Sci J 11:95–119
Bai J, Cui B, Chen B, Zhang K, Deng W, Gao H, Xiao R (2011) Spatial distribution and ecological risk assessment of heavy metals in surface sediments from a typical plateau lake wetland, China. Ecol Modell 222:301–306
Bastami KD, Bagheri H, Haghparast S, Soltani F, Hamzehpoor A, Bastami MD (2012) Geochemical and geo-statistical assessment of selected heavy metals in the surface sediments of the Gorgan Bay, Iran. Mar Pollut Bull 64:2877–2884
Bastami KD, Afkhami M, Mohammadizadeh M, Ehsanpour M, Chambari S, Aghaei S et al (2015a) Bioaccumulation and ecological risk assessment of heavy metals in the sediments and mullet Liza klunzingeri in the northern part of the Persian Gulf. Mar Pollut Bull 94:329–334
Bastami KD, Neyestani MR, Shemirani F, Soltani F, Haghparast S, Akbari A (2015b) Heavy metal pollution assessment in relation to sediment properties in the coastal sediments of the southern Caspian Sea. Mar Pollut Bull 92:237–243
Bawa LM, Djaneye-Boundjou G, Boyode BP, Assih BT (2007) Water quality evaluation from Lomé’s lagoon: Effects on heavy metals contamination on fishes. J Appl Sci Environ Manag 11:33–36
Biney C, Amzu AT, Calamari D, Kaba N, Mbome IL, Naeve H et al. (1994) Etude des métaux lourds. In: Clamari D, Naeve H (eds) Revue de la pollution dans l’environnement aquatique africain. Document technique du CPCA No. 25, FAO, Rome, pp 37–54. http://www.fao.org/docrep/005/V3640F/V3640F3604.htm#ch3644.3641
Boucheseiche C, Cremille E, Pelte T, Pojer K (2002) Pollution toxique et écotoxicologie: notion de base, guide technique no 7. Bassin Rhône-Méditerranée-Corse Agence de l’Eau Rhône-Méditerranée-Corse, Lyon
Boyes WK (2010) Essentiality, toxicity, and uncertainty in the risk assessment of Manganese. J Toxicol Environ Health A 73:159–165
Butcher DJ (2002) Speciation of methylcyclopentadienyl manganese tricarbonyl and its derivatives: a review. Appl Spectrosc Rev 37:1–17
Caeiro S, Costa MH, Ramos TB, Fernandes F, Silveira N, Coimbra A et al (2005) Assessing heavy metal contamination in Sado Estuary sediment: an index analysis approach. Ecol Indic 5:151–169
CCME (2001) Canadian sediment quality guidelines for the protection of aquatic life: Summary tables. Updates. In: Canadian Council of Ministers of the Environment (CCME) (ed) Canadian environmental quality guidelines. Environment Canada, Winnipeg, Canada, pp 1–5
Chapman D, Kimstach V (1996) Selection of water quality variables. In: Chapman D (ed) Water quality and assesments-a guide to use of biota, sediments and water in environmental monitoring, 2nd edn. E. & F.N. Spon, London, pp 56–133
Chen B, Liang X, Xu W, Huang X, Li X (2012) The changes in trace metal contamination over the last decade in surface sediments of the Pearl River Estuary south China. Sci Total Environ 439:141–149
Da Costa PYD, Flicoteaux R, Affaton P, Seddoh K, Tairou MS, Johnson AKC (2006) Le Continental terminal du bassin côtier du togolais: un témoin d’altération pédogénétique sous climat tropical depuis l’oligocène supérieur. Afr Geosci Rev 13:267–288
Da Costa PYD, Affaton P, Salaj J, Johnson AKC, Seddoh K (2013) Biozonation des formations sédimentaires du bassin côtier du Togo (Afrique de l’ouest). Rev Ivoir Sci Technol 21–22:45–73
Das JD, Nolting RF (1993) Distribution of trace metals from soils and sewage sludge’s abay refluxing with aqua regia. Analyst 108:277–285
De Mora SD, Sheikholeslami MR (2002) ASTP: Contaminant screening program: Final report: interpretation of Caspian Sea sediment data. Caspian Environment Program (CEP), pp 1–27
Dean WE (1974) Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: comparison with other methods. J Sediment Petrol 44:242–248
Dedjiho CA, Akpo AB, Noumon CJ, Agbahoungbata MY, Hounsinou P, Mama D et al (2014) Evaluation of the state of pollution of complex Aheme Lake -lagoon of Ouidah by trace metals Zn Cu, Cd, Pb and Cu speciation in sediment. Res J Chem Sci 4:33–41
DGSCN (2010) Quatrième recensement général de la population et de l’habitat, Résultats définitifs. Volume spéciale. Bureau Central du Recensement (BCR), Direction Générale de la Statistique et de la Comptabilité Nationale (DGSCN), Lomé, Togo
Doamekpor LK, Abusa Y, Ketemepi HK, Klake RK, Doamekpor MEAM, Anom PA, Obeng J (2018) Assessment of heavy metals in water and sediments of Sakumo II, Chemu and Kpeshie Lagoons, Ghana West African. J Appl Ecol 26:56–71
Ekaete AG, Ibironke OK, Olatunde PS, Olaoye OP (2015) Heavy metal pollutions and its associated ecological risks in Lagos Lagoon sediments, south-western Nigeria. Am Chem Sci J 9:1–13
Ekengele LN, Apelbo B, Jung MC (2016) Accumulation of heavy metals in surface sediments of Lere Lake, Chad. Geosci J. https://doi.org/10.1007/s12303-016-0047-4
El-Sayed SA, Moussa EMM, El-Sabagh MEI (2015) Evaluation of heavy metal content in Qaroun lake, El-Fayoum, Egypt Part I: bottom sediments. J Radiat Res Appl Sci 8:276–285
Fairey R, Long ER, Roberts CA, Anderson BS, Phillips BM, Hunt JW et al (2001) An evaluation of methods for calculating mean sediment quality guideline quotients as indicators of contamination and acute toxicity to amphipods by chemical mixture. Environ Toxicol Chem 20:2276–2286
Fang GC, Wu YS, Lee WJ, Chou TY, Lin IC (2007) Ambient air particulates, metallic elements, dry deposition and concentrations at Taichung Airport, Taiwan. Atmos Res 84:280–289
Fang GC, Huang YL, Huang JH (2010) Study of atmospheric metallic elements pollution in Asia during 2000–2007. J Hazard Mater 180:115–121
Förstner U, Wittmann GTW (1981) Metal pollution in the aquatic environment, 2nd edn. Springer, Berlin
François M, Li D, Dubourguier HC, Douay F (2002) Facteurs déterminants de la mobilité des métaux (Pb, Cd et Zn) dans les sols contaminés autour de deux usines métallurgiques du Nord de la France. Paper presented at the Journées Nationales de l’Etude des sols, Orléans, France, 22–24 Octobre 2002
Gnandi K (1998) Cadmium et autres polluants inorganiques dans les sols et sédiments de la Région côtière du Togo: une étude géochimique. Thèse de Doctorat, Université Friedrich-Alexander d’Erlangen-Nurenberg
Gnandi K (2002) L’impact de l’exploitation des phosphates sédimentaires de Hahotoé-Kpogamé sur la pollution chimique des sédiments du fleuve Haho et du lac (Sud Togo). J Rech Sci Univ Lomé A 6:95–105
Gnandi K, Tobschall HJ (1999a) Heavy metal release from phosphorite tailings into seawater: a simulated laboratory study. Sci Total Environ 236:181–190
Gnandi K, Tobschall HJ (1999b) The pollution of marine sediments by trace elements in the coastal region of Togo caused by dumping of cadmium-rich phosphorite tailing into the sea. Environ Geol 38:13–24
Gnandi K, Tobschall HJ (2002) Heavy metals distribution of soils around mining sites of cadmium-rich marine sedimentary phosphorites of Kpogame and Hahotoe (southern Togo). Environ Geol 41:593–600
Gnandi K, Rezaie B, Edorh AP (2009) The geochemical characterization of mine effluents from the phosphorite processing plant of Kpémé (southern Togo). Mine Water Environ 28:65–73
Gnandi K, Han S, Rezaie-Boroon MH, Porrachia M, Deheyn DD (2011) Increased bioavailability of mercury in the lagoons of Lomé, Togo: the possible role of dredging. Ambio 40:26–42
Gouin CA, Aka N, Adiaffi B, Bamba BS, Soro N (2016) Pollution saisonnière des sédiments de lagune par des métaux lourds (Cu, Pb et Zn) en zone tropicale humide: cas de la lagune Mondoukou (Sud-Est de la Côte d’Ivoire). Int J Biol Chem Sci 10:835–845
Hakanson L (1980) Ecological risk index for aquatic pollution control a sedimentological approach. Water Res 14:975–1001
Horowitz AJ, Elrick KA (1987) The relation of stream sediment surface area, grainsize and composition to trace element chemistry. Appl Geochem 2:437–451
Hounkpè JB, Kélomè NC, Adèchina R, Lawani RN (2017) Assessment of heavy metals contamination in sediments at the lake of Ahémé in southern of Benin (West Africa). J Mater Environ Sci 8:4369–4377
Islam MS, Ahmed MK, Habibullah-Al-Mamun M, Hoque MF (2015a) Preliminary assessment of heavy metal contamination in surface sediments from a river in Bangladesh. Environ Earth Sci 73:1837–1848
Islam MS, Ahmed MK, Raknuzzaman M, Habibullah-Al-Mamun M, Islam MK (2015b) Heavy metal pollution in surface water and sediment: a preliminary assessment of an urban river in a developing country. Ecol Ind 48:282–291
Jamshidi-Zanjani A, Saeedi M (2013) Metal pollution assessment and multivariate analysis in sediment of Anzali international wetland. Environ Earth Sci 70:1791–1808
Johnson AKC, Da Costa YD (2008) Le passage Paléocène-Eocène dans les bassins sédimentaires côtiers du golfe du Benin: Togo Benin et Nigeria. Rev Ivoir Sci Technol 11:193–205
Jonhson AK, Rat P, Lang J (2000) Le bassin sédimentaire à phosphate du Togo (Maastrichtien-Eocène): stratigraphie, environnements et évolution. J Afr Earth Sci 30:153–200
Kaiser J (2003) Manganese: a high-octane dispute. Science 300:926–928
Kambiré O, Adingra A, Eblin S, Aka N, Kakou A, Koffi-Nevry R (2014) Caractérisation des eaux d’une lagune estuarienne de la Côte d’Ivoire: la lagune Aby. Larhyss J 20:95–110
Kunkel H (1990) Über die Cadmiumverteilung in den Phosphoritlagerstätten von Kpogamé/Hahotoé. PhD Dissertation, Universtät Erlangen-Nuremberg
Lamouroux M (1969). Notice explicative de la carte pédologique du Togo au 1/1000.000. Notice explicative No 34. Office de la Recherche Scientifique et Technique Outre Mer (ORSTOM), Centre ORSTOM de Lomé, Lomé, Togo
Lee PK, Yu YH, Yun ST, Mayer B (2005) Metal contamination and solid phase partitioning of metals in urban roadside sediments. Chemosphere 60:672–689
Li G, Hu B, Bi J, Leng Q, Xiao C, Yang Z (2013) Heavy metals distribution and contamination in surface sediments of the coastal Shandong Peninsula (Yellow Sea). Mar Pollut Bull 76:420–426
Liaghati T, Preda M, Cox M (2003) Heavy metal distribution and controlling factorswithin coastal plain sediments, Bells Creek catchment, southeast Queensland, Australia. Environ Int 29:935–948
Lin S, Hsieh IJ, Huang KM, Wang CH (2002) Influence of the Yangtze River and grain size on the spatial variations of heavy metals and organic carbon in the East China Sea continental shelf sediments. Chem Geol 182:377–394
Long ER (2006) Calculation and uses of mean sediment quality guideline quotients: a critical review. Environ Sci Technol 40:1726–1736
Long ER, MacDonald DD (1998) Recommended uses of empirically derived, sediment quality guidelines for marine and estuarine ecosystems. Hum Ecol Risk Assess 4:1019–1039
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97
Long ER, MacDonald DD, Severn CG, Hong CB (2000) Classifying probabilities of acute toxicity in marine sediments with empirically derived sediment quality guidelines. Environ Toxicol Chem 19:2598–2601
MacDonald DD, Carr S, Clader FD, Long ED, Ingersoll CG (1996) Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 5:253–278
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Marin V, Moreno M, Vassallo P, Vezzulli L, Fabiano M (2008) Development of a multistep indicator-based approach (MIBA) for the assessment of environmental quality of harbours. ICES J Mar Sci 65:1436–1441
Mathieu C, Pieltain F (2003) Analyse chimique des sols: méthodes choisies, Tec & Doc. Lavoisier, Paris, France
McCave IN (1984) Size spectra and aggregation of suspended particles in the deep ocean. Deep-Sea Res 31:329–352
Mermut AR, Jain JC, Song L, Kerrich R, Kozak L, Jana S (1996) Trace element concentrations of select soils and fertilizers in Saskatchewan. Can Environ Qual 25:845–853
Millet B (1986) Hydrologie et hydrochimie d’un milieu lagunaire tropical: le lac Togo, Collection Etudes et Thèses. ORSTOM, , Paris
Monroy M, Maceda-Veiga A, De Sostoa A (2014) Metal concentration in water, sediment and four fish species from Lake Titicaca reveals a large-scale environmental concern. Sci Total Environ 487:233–244
Nadem S, El Baghdadi M, Rais J, Barakat A (2015) Evaluation de la contamination en métaux lourds des sédiments de l’estuaire de Bou Regreg (Côte atlantique, Maroc). J Mater Environ Sci 6:3338–3345
Neculita C-M, Zagury GJ, Deschenes L (2005) Mercury speciation in highly contaminated soils from chlor-alkali plants using chemical extractions. J Environ Qual 34:255–262
Nshimiyimana F-X, Faciu M-E, El Blidi S, El Abidi A, Soulaymani A, Fekhaoui M, Lazar G (2016) Seasonal influence and risk assessment of heavy metals contamination in groundwater Arjaat village, Morocco. Environ Eng Manag J 15:579–587
Ouro-Sama K, Tanouayi G, Solitoke HD, Badassan TE-E, Ahoudi H, Nyametso AY, Gnandi K (2018) Seasonal variation, quality and typology of waters’ abiotic parameters of a tropical lagoon: the hydrosystem Lake Togo-Lagoon of Aného (south-east of Togo). Int J Innov Appl Stud 24:656–673
Pempkowiak J, Sikora A, Biernacka E (1999) Speciation of heavy metals in marine sediment vs their bioaccumulation by mussels. Chemosphere 39:313–321
Pietrzak U, Mcphail D (2004) Copper accumulation, distribution and fractionation in vineyard soils of Victoria. Aust Geoderma 122:151–166
Rabee AM, Al-Fatlawy YF, Abd own AAHN, Nameer M (2011) Using pollution load index (PLI) and geoaccumulation index (I-Geo) for the assessment of heavy metals pollution in Tigris river sediment in Baghdad region. J Al-Nahrain Univ Sci 14:108–114
Raven KP, Leoppert RH (1997) Trace element composition of fertilizers and soil amendments. Environ Qual 26:551–557
Razaie-Boroon MH, Gnandi K, Tomety-Mensah KF (2011) Presence and distribution of toxic trace elements in water and sediments of the southern Togo Rivers watershed, West Africa. Fresenius Environ Bull 20:1853–1865
Rodier J, Legube B, Merlet N (2009) L’analyse de l’eau, 9th edn. Dunod, Paris
Saeedi M, Li LY, Karbassi AR, Zanjani AJ (2012) Sorbed metals fractionation and risk assessment of release in river sediment and particulate matter. Environ Monit Assess 185:1737–1754. https://doi.org/10.1007/s10661-012-2664-3
Singer PC, Stumm W (1970) Acidic mine drainage-the rate limiting step. Science 167:1121–1123
Slansky M (1980) Géologie des phosphates sédimentaire. Éditions du BRGM, Mémoire du BRGM no. 114
Solitoke HD, Ouro-Sama K, Tanouayi G, Badassan TE-E, Ahoudi H, Nyametso AY et al (2018) Etude de la contamination métallique des sédiments d’un hydro système tropicale: Cas du système Lac Zowla-Lagune d’Aného (Sud - Togo). Environ Water Sci Public Health Territorial Intell J 2:41–51
Soro G, Métongo BS, Soro N, Ahoussi EK, Kouamé FK, Zadé SGP, Soro T (2009) Métaux lourds (Cu, Cr, Mn et Zn) dans les sédiments de surface d’une lagune tropicale africaine: cas de la lagune Ebrie (Côte d’Ivoire). Int J Biol Chem Sci 3:1408–1427
Stead-Dexter K, Ward NI (2004) Mobility of heavy metals within freshwater sediments affected by motorway stormwater. Sci Total Environ 334–335:271–277
Tchangbedji G, Kili KA, Savariaul MJ, Lacout JL (2003) Chemical and structural characterization of natural phosphate of Hahotoé (Togo). Bull Chem Soc Ethiopia 17:139–147
Thomassin JF, Touze S, Baranger P, Freyssinet P (2003). Le mercure et ses composés. Comportement dans les sols, les eaux et les boues de sédiments. Rapport final BRGM/RP-51890-FR. Bureau de Recherche Géologique et Minière (BRGM), France
Tomlinson DL, Wilson JG, Harris CR, Jeffney DW (1980) Problems in the assessment of heavy metal levels in estuaries and the formation of a pollution index. Helgol Mar Res 33:566–572
Traoré A, Soro G, Ahoussi KE, Bamba BS, Soro N, Biemi J (2014) Niveau de contamination en métaux lourds des sédiments d’une lagune tropicale: la lagune Aghien (Sud-Est de la Côte d’Ivoire). Afr Sci: Rev Int Sci Technol 10:73–88
Van Den Berg CMG (1993) Complex formation and the chemistry of selected trace elements in estuaries. Estuaries 16:512–520
Wang J, Liu R, Zhang P, Yu W, Shen Z, Feng C (2014) Spatial variation, environmental assessment and source identification of heavy metals in sediments of the Yangtze River Estuary. Mar Pollut Bull 87:364–373
Wani MA, Wani J, Bhat M, Kirmani N, Wani ZM, Bhat SN (2013) Mapping of soil micronutrients in Kashmir agricultural landscape using ordinary kriging and indicator approach. J Indian Soc Remote Sens 41:319–329
Wognin AV, N’guessan YM, Assalé FJP, Aka AM, Coulibaly AS, Monde S, Aka K (2017) Les éléments traces métalliques dans la lagune Ebrié: distribution saisonnière, niveau de contamination et qualité environnementale des sédiments. Int J Biol Chem Sci 11:911–923
Yao KM, Metongo BS, Trokourey A, Boka Y (2009) Assessment of sediments contamination by heavy metals in a tropical lagoon urban area (Ebrié lagoon, Côte d’Ivoire). Eur J Sci Res 34:280–289
Zhang H, Shan B (2008) Historical records of heavy metal accumulation in sediments and the relationship with agricultural intensification in the Yangtze-Huaihe region, China. Sci Total Environ 399:113–120
Zhang L, Ye X, Feng H, Jing Y, Ouyang T, Yu X et al (2007) Heavy metal contamination in western Xiamen Bay sediments and its vicinity, China. Mar Pollut Bull 54:974–982
Acknowledgements
This study was co-funded by the International Foundation for Science (IFS) in Sweden, the organization for the prohibition of chemical weapons (OPCW) in (Netherland) (IFS Scholarship: I-2-A-6056-1) and the Romanian Government through “Eugen Ionescu” Scholarship 2016 entirely managed by the Francophone University Agency [Agence Universitaire de la Francophonie (AUF)]. We also wish to express our gratitude to the Faculty of Engineering of “Vasile Alecsandri” University of Bacau (Romania) and to the Laboratory of Management, Treatment and Valorization of Waste of the University of Lomé (Togo).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no potential conflict of interest with this research or in the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ouro-Sama, K., Solitoke, H.D., Tanouayi, G. et al. Spatial and seasonal variation of trace elements contamination in the sediments of a tropical lagoon ecosystem: the Lake Togo-Lagoon of Aného complex (southern Togo). Environ Earth Sci 80, 154 (2021). https://doi.org/10.1007/s12665-021-09390-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-09390-3