Abstract
In the present study, 3-year (1997, 2008 and 2017) satellite images as well as different hydro-chemical parameters, nitrogen and oxygen isotopic composition of nitrate were used to examine the impacts of land use and land cover change on surface and groundwater quality. Through isotopic composition, sources of surface and groundwater pollutants were also elucidated. The results showed significant land use transition whereby land use changed from forest and bare land to agricultural land and built-up areas. A slight reduction in the size of areas covered by water bodies was also observed. Results indicate differences in nitrate concentration that mirror land use changes. Samples with elevated levels of nitrate above 10 mg/L were located near agricultural fields and areas with intensive livestock keeping activities. In groundwater, ẟ15N-nitrate and ẟ18O-nitrate ranged from 3.2‰ to 20.1‰ with a mean value of 11.7 ± 1.8‰ and from 2.1‰ to 12.0‰ with mean value of 5.4 ± 1.8‰, respectively. In surface water, ẟ15N-nitrate and ẟ18O-nitrate ranged from 2.4‰ to 19.3‰ with mean value of 4.9 ± 1.4‰ and from 1.5‰ to 21.9‰ with a mean value of 13.5 ± 2.8‰, respectively. Isotopic composition data suggest sources of nitrate in both ground and surface water dominated by synthetic and organic fertilizer application and to a lesser extent a natural soil nitrate source.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrate (\({\text{NO}}_{3}^{-}\)) contamination in surface and groundwater is a global environmental issue of concern (Choi et al. 2007; Eppicha 2012). High inputs of nitrate to surface and groundwater have been attributed to intensive land use, application of N-containing organic and inorganic fertilizers, animal manure, and discharge of human untreated sewage as well as natural sources like soils, bedrock and atmospheric deposition (Xue et al. 2009; Elisante and Muzuka 2016). In Babati town, where this study was conducted, groundwater is a major source of drinking water. Previous studies conducted in areas of Manyara region, where Babati town is located, have indicated that nitrate pollution above the background levels of 10 mg/L is common, which threatens the water quality of the area (Hongoa 2014; Elisante and Muzuka 2017; Pantaleo et al. 2018).
In many communities, land use and land cover (LULC) change is inevitable, and it contributes towards pollution of water resources. The main anthropogenic drivers of LULC changes include deforestation, expansion of agriculture, conversion of wetland to pastureland and agriculture as well as urbanization (Miserendino et al. 2011). For example, in Europe, it has been observed that modifications in landscapes have been largely caused by political and socio-economic changes which took place during the first half of the 19th century (Bičík et al. 2015). Moreover, high and fast-growing population density in Africa has led to expansion of crop cultivation together with animal husbandry and consequent resettlement. These have been noticed as the major drivers of LULC changes (Tappan et al. 2004).
Integration of natural and human factors in the classification of LULC dynamics can be a complex mixture; apart from anthropogenic activities on LULC changes, natural factors such as climate change and variability have also contributed (Reid et al. 2000). A study conducted in the vicinity of Mount Meru, Tanzania, reported that many areas in Northern Tanzania have been converted to cultivated lands, grazing lands, human habitats, mining sites as well as industrial areas due to rapid increase in human population and consequent economic growth (Kitalika et al. 2017). Moreover, during the mid and late 1940s and in the late 1950s, many people migrated to Babati because of its fertile lands and easy access to the area’s natural resources. Huge land was cleared due to tsetse fly infestation, while most of the virgin land was converted into farmlands (Sandström 1995).
Land use and land cover changes are considered to be associated with changes in the quality of surface and groundwater which threatens human and environmental health (Choi et al. 2007; Namugize et al. 2018). Moreover, in most developing countries, sources of nitrate in surface and groundwater have not been well studied. Babati is an emerging town, with rapid population growth resulting into high demand for housing, crop farming, grazing lands as well as sanitation facilities; increasing the risk to water pollution (Sandström 1995; Hongoa 2014). Thus, it is imperative that sources and levels of aquatic pollutants be studied in such areas like Babati that will be the cities of tomorrow.
Intake of water with high nitrate concentration may cause several diseases including methemoglobinemia, gastric problems, thyroid gland retardation, even cancer (Tank and Chandel 2010; WHO 2011). Excessive loading of nitrate into lakes may cause eutrophication and hypoxia (Mitsch et al. 2001). According to WHO, the acceptable limit of \({\text{NO}}_{3}^{-}\) in drinking water is 50 mg/L. It is therefore important to determine the levels, distribution, and possible sources of nitrate in semi-urban aquatic environments in the developing countries of the world, especially in the rapidly growing urban areas.
The isotopic composition of N-nitrate (δ15N-nitrate and δ18O-nitrate) has been used to identify sources of nitrate because different sources of nitrate often have different isotopic signatures (Kendall and Aravena 2000; Chang et al. 2002; Mayer et al. 2002; Pardo et al. 2004). Synthetic fertilizers produced by fixation of atmospheric N2 such as urea, ammonium nitrate and potassium nitrate usually show δ15N values quite similar to that of atmospheric N2 i.e. levels between –4‰ and + 4‰ (Kendall and Aravena 2000). Organic fertilizers such as plant composts and animal waste have a wider range of compositions of δ15N from + 2‰ to + 30‰. Animal manure normally has high δ15N values in the range of + 10‰ to + 20‰. Widory et al. (2005) reported δ15N values for wastewater ranging from 10.3‰ to 23.5‰ and from 4.3‰ to 17.4‰. Nitrate derived from atmospheric deposition, has high positive δ18O-nitrate values ranging from + 50‰ to + 94‰ (Durka et al. 1994; Kendall et al. 2007). Nitrate derived from nitrification, for instance, in soils or derived from mineralization of aquatic phytoplankton and nitrogen fixing bacteria followed by ammonification and nitrification has δ18O-nitrate values between − 15‰ and + 15‰ that are dependent on the δ18O of water (Rock and Mayer 2004; Deutsch et al. 2006). Also, nitrate derived from manure and sewage have δ18O-nitrate values between − 15‰ and + 15‰ (Aravena et al. 1993; Wassenaar 1995). Since the values of δ15N-nitrate from different sources may overlap, then a combined use of δ15N and δ18O values of nitrate can offer a delineation tool for discriminating the sources of nitrate in surface and groundwater (Kendall et al. 2007).
This study aimed at identifying the contribution of land use practices towards nitrate contamination in surface and groundwater of a rapidly growing urban area in Tanzania. The study applied δ 15N-nitrate and δ 18O-nitrate to determine the sources of nitrate in surface and groundwater in Babati town. Samples collected from existing wells, springs, and Lake Babati were used to produce the needed information. Levels of major cations, anions and trace elements were also analyzed, and the results were compared against the WHO drinking water guidelines.
Materials and methods
Study area
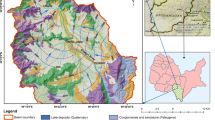
Babati town is an administrative headquarter of the Manyara region, northern Tanzania. It lies between latitudes 3°–40S and longitudes 35°–36°E (Fig. 1). One of the most conspicuous landmarks of Babati town is Lake Babati. Lake Babati has diverse species of fish, water birds, and is known to be home to a sizeable population of hippos and other wild ungulates (Pantaleo et al. 2018). The lake is a source of water for various economic and domestic activities (Pantaleo et al. 2018). In the studied area, volcanic soils are dominant and range from sandy-loamy to clay and alluvial soils (Ghiglieri et al. 2012). However, alkaline soils may also be present in the lowlands such as those around Lake Babati (Nkotagu 1996).
The climate of the study area is warm and relatively wet with a mean annual precipitation of 831 mm. The mean annual temperature is 20.2 °C. The area’s lithology was formed during the upper Mesozoic. Sedimentary rocks overlie a Karoo system and a Precambrian basement. These rocks comprise mainly of sandstone, limestone, shale, marl and local evaporates. Agriculture and livestock keeping are the primary livelihood activities in the study area. Smallholder farming includes maize, sesame, sunflower, horticultural crops and legumes. Furthermore, a recent study pointed out poor sanitation as one the major environmental and health challenges in the area (Pantaleo et al. 2018).
Water sampling
Surface water was collected from Lake Babati and a spring whereas groundwater was collected from boreholes and shallow wells. Sampling took place in the month of April 2018 and all water samples were collected during the same season. Each sampling location was geo-tagged using a handheld GPS unit and plotted on the Tanzania map using ArcGIS software. Twenty-one samples were collected from the lake, 16 from boreholes, four from shallow wells and one from a spring. All boreholes and wells were active therefore pre-pumping was not necessary during sampling. Lake samples were collected using a water sampler. Water samples for analysis of the levels of major ions and trace elements were filtered through a 0.45 µm filter paper and kept in prewashed 500 mL HDPE bottles. Samples for 15N-nitrate and δ18O-nitrate were collected in 30 mL HDPE bottles. All water samples were kept in cold condition and transported to the NM-AIST laboratory for analysis. Field measurements such as pH, temperature, EC, DO and TDS were carried out during sampling using an Eijkelkamp 18.52 SA Model (pH/mV/EC/T/sal/TDS/DO) multimeter.
Image acquisition and pre-processing
Three Landsat images were utilized in this study. Landsat 5 TM images captured on 02/01/1997, Landsat 7 ETM + images captured on 02/01/2008 and Landsat 8 OLI images captured on 17/2/2017. More information about satellite images used for the present study is shown in Table 1 below. Remote sensing imagery for the study area for 1997, 2008 and 2017 were downloaded from the USGS Earth Explorer for the Babati town. Landsat 5 data were obtained for 1997, Landsat 7 data were obtained for 2008 and Landsat 8 data were obtained for 2017 to examine land use changes over the past 20 years.
Satellite images required pre-processing to ensure that the primary object could be established into a more direct affiliation between acquired data and biophysical phenomena (Rawat and Kumar 2015). This pre-process was accomplished using ArcGIS Version 10.1 for georeferencing, mosaicking, and sub-setting of the image for the area of interest (AOI). Landsat 8 images underwent spatial sharpening using the panchromatic bands which resulted in images with a 30 m resolution. Further image processing analysis was carried out using ENVI 5.0. The image was displayed in natural color composite using a band combination of 3, 2, and 1 for Landsat 5 TM and 5, 4, 3, and 2 for Landsat 7 and Landsat 8. Maximum likelihood supervised classification was performed using several selected regions of interest (ROIs) based on defined classes which were agriculture, built-up area, water, bare lands, and forests area as shown below in Table 2.
Accuracy assessment
This is a quantitative assessment of how effectively the pixels were sampled into the correct land cover classes. The accuracy assessment of an image classification was performed by creating the classification error matrix. In this confusion matrix, classification results were compared with ground truth data acquired during fieldwork. This was done by picking the menu item classifier > accuracy assessment, then importing the ground coordinates of the ground truth samples from an Excel file which was already saved in text format. According to Coppin and Bauer (1996), the accepted classification results should normally be > 70%. Table 3 shows the accuracy assessment results for the year 1997, 2008 and 2018 for image classification.
Analysis of water samples
Anionic concentration of \({\text{NO}}_{3}^{-}\) and \({\text{SO}}_{4}^{2-}\) was determined using a spectrophotometer while determination of the concentration of Cl−, \({\text{CO}}_{3}^{2-}\) and \({\text{HCO}}_{3}^{-}\) was done using titration method (APHA 2017). Determination of major cations (Na+, Mg2+, K+ and Ca2+) and trace elements (Fe2+, Pb2+, Zn2+, Cu2+, Mn2+, and Cd2+) was done using atomic absorption spectrophotometer. Trace elements and ionic concentration results were compared against the WHO guidelines. Water samples were randomly selected for detection of δ15N-nitrate and δ18O-nitrate from various water sources within the area. The removal of nitrite was done by using sulfamic acid (Granger and Sigman 2009) and kept in 30 mL HDPE bottles and shipped to the University of Waterloo, Ontario, Canada, for analysis. The δ15N–nitrate and δ18O-nitrate in 12 groundwater samples and 7 surface water samples were detected through conversion of nitrate to nitrite (Granger and Sigman 2009) using cadmium catalyst then chemically converted to N2O which was then analyzed on a Trace Gas–GVI IsoPrime-IRMS (TG-IRMS).
Statistical analyses
Paleontological Statistics (PAST, Version 3.08), MS Excel, and SigmaPlot software were used to perform all statistical analyses. Mean and standard errors were calculated for various parameters and significant relationships between various parameters were studied by using relevant correlations.
Results and discussion
Physico-chemical parameters
Understanding the physico-chemical quality of surface and groundwater is essential as it is an important factor in determining water suitability for human and environmental applications. Tables 4, 5 contain results of the physico-chemical laboratory and onsite analyses. Temperature of groundwater varied from 22.9 °C to 28.1 °C with mean value of 25.2 ± 0.3 °C whereby BH5 showed the highest temperature of 28.1 °C (elevation 1294 m.a.s.l.) and the lowest temperature in groundwater was 22.9 °C (elevation 1383 m.a.s.l.). This slight variation in water temperature may be attributable to the differences in altitude rather than aquifer type. The EC values in the present study indicated similar magnitude to findings of e.g. Yusuf (2007) (EC: 120–1860 µS/cm) who investigated the groundwater quality of Lagos city; Saana et al. (2016) (EC: 131–873 μS/cm) in borehole water samples from seven districts of Ghana; and those of Nkotagu (1996) (EC: 301–4740 µS/cm) in groundwater of a semi-arid region (Dodoma), Tanzania. In the present study, the relatively low EC values, EC: 701–2370 μS/cm for groundwater and 750–1068 μS/cm for surface water, may be attributed to low mineral content—thus water in the present study may be classified as freshwater (Saana et al. 2016).
Furthermore, when raw data in Tables 4, 5 were used to plot correlations between EC and TDS, the R2 values were high—0.96 and 0.93, respectively (Fig. 2). This means that, generally, a high linear correlation existed between water conductivity and total ions’ concentrations. The Excel file containing raw datasets has been included as Supplementary Materials.
The concentration of major cations in groundwater and surface water was in the following order Ca2+ > Mg2+ > Na+ > K+ (Tables 4, 5). Figure 3 is a Piper diagram that shows major ion composition in surface and groundwater of Babati. Both surface and groundwater indicated a calcium–magnesium bicarbonate water type. The chemical properties of groundwater showed hardness in all samples and were dominated by alkaline earth elements (Ca2+ and Mg2+), which exceeded the alkalis (Na+ and K+). During infiltration, groundwater may dissolve the CaCO3 and MgCO3 found in water rocks to add up to the concentration of Ca2+ and Mg2+ in water (Dehnavi et al. 2011). A significant negative correlation between Ca2+ and \({\text{HCO}}_{3}^{-}\) in surface water (Table 7; r = − 0.51, p = 0.018) may indicate that Ca2+ was constantly being replaced by Mg2+ meaning that MgCO3 or Mg(HCO3)2 were more dominant species rather than CaCO3 or Ca(HCO3)2 (Jahnen-Dechent and Ketteler 2012). This phenomenon is further proved by a significant negative correlation between Mg2+ and Ca2+ in surface water; with r = − 0.52 and p = 0.016 (Table 7). It further follows that the solubility of MgCO3 probably decreased with increasing surface water temperature and that is probably why Mg2+ was negatively correlated with temperature (r = − 0.58, p = 0.047; Table 7) (Dong et al. 2008).
Usually, the Piper diagram (Fig. 3) reveals the analogies, dissimilarities and different types of waters (Utom et al. 2013). It shows the variation or domination of cations and anion concentration in water. It is made up with three parts, the lower left triangle showing the cations, the lower right triangle that shows anions and the diamond plot at the middle that shows the combination of the two. Results from the present study indicated that alkaline earth type of water (Ca2+, Mg2+) exceeded alkalis (Na+, K+) whereas weak acid (\({\text{HCO}}_{3}^{-}\)) exceeded the strong acids \({\text{SO}}_{4}^{2-}\) and Cl− which may be indicative of slight hardness in all groundwater samples. This was further corroborated by the Pearson correlation values in Tables 6, 7.
Surface water temperature varied from 22.9 °C to 23.8 °C with a mean value of 23.4 ± 0.1 °C (Table 5). The pH of groundwater ranged from 7.1 to 7.8 with a mean value of 7.4 ± 0.1 (Table 4) while for surface water the pH varied from 7.3 to 7.7 with mean value of 7.5 ± 0.1 (Table 5). These pH values fell within the normal pH of the natural aquatic environments. The EC of groundwater varied from 657 to 2370 µS/cm, with a mean value of 1301.18 ± 119.74 µS/cm (Table 4). For surface water, the EC ranged from 750 to 1063 µS/cm with a mean value of 1027.43 ± 15.13 µS/cm (Table 5). The values of TDS and EC correlated well in both surface and groundwater (Fig. 2). For surface water, the correlation coefficient valued indicated that EC significantly correlated with Ca2+ (r = 0.71, p = 0.009) indicating that calcium was probably the dominant major cation (Table 7). This finding is further corroborated by the Piper diagram (Fig. 3). Moreover, in surface water, the EC was negatively but significantly correlated with \({\text{SO}}_{4}^{2-}\) (r = − 0.84, p = 0.0007); this would probably mean that \({\text{SO}}_{4}^{2-}\) was not the major anion and decreased with increasing dissolved solids in surface water (Table 7).
Nitrate concentration in groundwater ranged from 1.7 to 54.5 mg/L, with an average value of 11.5 ± 3.1 mg/L (Table 4). Only one groundwater sample (BH3) exceeded the WHO drinking water guideline of 50 mg/L (WHO 2011). Higher levels of nitrate in drinking water systems are known to cause health and aesthetic problems. Furthermore, nitrate did not have strong correlation with other anions or cations for both groundwater and surface water (Tables 6, 7). This may suggest that nitrate in the analyzed samples did not share the source with other ions. Surface water showed relatively low concentrations of nitrate ranging from 1.2 to 4.4 mg/L with an average value of 2.5 ± 0.2 mg/L (Table 5). The main nitrogen species in surface water are ammoniacal nitrogen and organic nitrogen. The concentration of sulfate in both surface and groundwater samples was low compared to the WHO guideline (250 mg/L). The highest concentration of sulfate detected in groundwater was 46 mg/L and the lowest was 1.0 mg/L with the mean value of 18.0 ± 4.1 mg/L (Table 4), whereby in surface water the mean value was 1.7 ± 0.2 mg/L; highest concentration was 4.0 mg/L and the lowest was 1.0 mg/L (Table 5). Chloride concentration was higher in groundwater samples than in surface water samples whereby the maximum chloride value in groundwater was 1706.79 mg/L, and the lowest was ~ 7 mg/L with a mean value of 447.54 mg/L (Table 4). The origin of the observed chloride in groundwater was probably linked to the application of inorganic fertilizers in the study area (Kelly et al. 2012). In surface water, chloride ranged from 0.5 to 34 mg/L with an average value of ~ 7 mg/L (Table 5). Bicarbonate in groundwater ranged from 648 to 1927 mg/L with an average value of 1171.57 ± 376.58 mg/L (Table 4) whereas in surface water it ranged from 939.6 to 1479.1 mg/L with an average value of 1257.2 ± 135.9 mg/L (Table 5).
In groundwater, calcium values varied from 7.0 to 314.2 mg/L with a mean value of 146.3 ± 90.9 mg/L (Table 4) while in surface water, calcium varied from 10.7 to 89.0 mg/L with a mean value of 50.1 ± 15.7 mg/L (Table 5). High levels of Ca in groundwater systems may be attributed to the dissolution of carbonate and gypsum rocks (Chenini et al. 2010). The desirable limit of calcium in drinking water as specified by WHO is 75 mg/L; it was observed that in five groundwater samples Ca levels had exceeded the limit set by WHO. Magnesium concentration in groundwater varied from 2.6 to 203.4 mg/L with a mean value 67.1 ± 65.2 mg/L while in surface water it varied from 19.0 to 80.0 mg/L with mean value of 50.8 ± 17.2 mg/L. According to WHO, the desirable limit of Mg is 50 mg/L meaning that eight groundwater samples, equivalent to 47%, exceeded the WHO limit. High levels of Ca and Mg are indicative of water hardness and imply that some water sources in the studied area may not be potable. Similar to Ca, high levels of Mg in groundwater may also be linked the dissolution of carbonate minerals and gypsum rocks and, most probably, the dissolution of nesquehonite (MgCO3 • 3H2O) (Dong et al. 2008; Chenini et al. 2010). Sodium concentration in groundwater varied from 5.4 to 21.9 mg/L with a mean value of 10.5 ± 4.2 mg/L, while in surface water it varied from 23.4 to 38. 4 mg/L with a mean value of 27.7 ± 4.2 mg/L. Potassium concentration in groundwater varied from 0.06 to 1.3 mg/L with a mean value of 0.6 ± 0.4 mg/L while in surface water it varied from 0.3 to 1.0 mg/L with a mean value of 0.7 ± 0.2 mg/L. A similar study was conducted in Chad Basin in Nigeria, whereby Ca–Mg–HCO3 was traced to water recharge and its sources were connected to rainfall and dissolution of silicate minerals (Nur et al. 2012). Thus, high levels of Ca2+ and Mg2+ in the present study could be linked to silicate minerals leached by weathering due to the action of weak carbonic acid (Nur et al. 2012). Interestingly, in groundwater samples of the present study, Mg was significantly correlated to bicarbonate (r = 0.58, p = 0.014) whereas Ca had a good correlation with bicarbonate (r = 0.47, p = 0.05) (Table 6). This is probably an indication that the groundwater of Babati and its suburbs are of Ca–Mg–HCO3 type. This could also be the reason why water samples collected from Lake Babati indicated a significant correlation between Mg and bicarbonate (r = 0.54, p = 0.011) (Table 7).
Trace elements
Iron (Fe) concentration in groundwater varied from 0.01 to 1.93 mg/L with a mean value of 0.28 ± 0.52 mg/L whereby BH2 and BH5 showed higher concentration of Fe than the aesthetic value set by WHO (Table 4). In surface water, Fe varied from 0.06 to 0.16 mg/L with a mean value of 0.095 ± 0.03 mg/L (Table 5). All the analyzed surface water samples had Fe concentration within the aesthetic value set by WHO—0.3 mg/L (Table 5). The concentration of Cu in groundwater ranged from 0.01 to 0.62 mg/L with a mean value of 0.17 ± 0.2 mg/L, while that of surface water varied from 0.001 to 0.06 mg/L with a mean value of 0.028 ± 0.016 mg/L. All the water samples were within the recommended WHO standard for Cu levels (Tables 4, 5).
For Zn, the concentration in groundwater ranged from no detection to 0.12 mg/L with a mean value of 0.047 ± 0.037 mg/L, while that of surface water ranged from no detection to 0.12 mg/L with a mean value of 0.028 ± 0.028 mg/L (Tables 4, 5). The concentration of lead (Pb) in groundwater samples ranged from no detection to 0.12 mg/L with mean value of 0.028 ± 0.033 mg/L while that of surface water ranged from no detection to 0.040 mg/L with a mean value of 0.017 ± 0.009 mg/L (Tables 4, 5). Because Pb is a known neurotoxin, especially to children aged < 5 y, the levels of Pb in some of the samples seem to be alarming. Manganese concentration in groundwater varied from no detection to 0.02 mg/L with a mean value of 0.01 ± 0.003 mg/L, and that of surface water varied from 0.01 to 0.2 mg/L with a mean value of 0.04 ± 0.05 mg/L (Tables 4, 5). All the water samples were within the WHO indicative aesthetic value for Mn. The concentration of Cd in groundwater ranged from no detection to 0.05 mg/L with a mean value of 0.02 ± 0.003 mg/L while that of surface water varied from no detection to 0.030 mg/L with a mean value of 0.02 ± 0.006 mg/L (Tables 4, 5).
In groundwater and surface water systems, in most cases, trace elements originate from the parent rock and sediments but may, sometimes, originate from anthropogenic loadings e.g. mining, industry, transport and agriculture (Ramesh et al. 1995; Molina et al. 2009). The levels of trace elements found in the present study were lower than the levels found in the drinking water from wells in small scale mining in Ghana (Cobbina et al. 2015) and were comparable to the levels reported by Ilyas and Sarwar (2003) in groundwater from Peshawar in Pakistan.
Isotopic studies
The values of ẟ15N-nitrate in groundwater ranged from 2.4‰ to 20.1‰ with the mean value of 11.8 ‰ ± 1.8 ‰ while in surface water ẟ15N-nitrate ranged from 2.1‰ to 12.6 ‰ with the mean value of 4.9‰ ± 1.4‰. High concentration of nitrate (> 10 mg/L) in groundwater BH3, BH5, SW2, and SW3 may be indicative of enriched values of ẟ15N-nitrate, which varied between 2.3‰ and 19.4‰ (Fig. 4). These values bear isotopic signatures of synthetic fertilizer (− 4‰ to + 4‰), manure (+ 10‰ to 20 ‰) and sewage (+ 9‰ to 19‰) (Aravena and Mayer 2009). For the present study, intensive agricultural activities were probably the major source of nitrate. In surface water, there was low concentrations of nitrate (Fig. 4) as well as low ẟ15N-nitrate and ẟ18O-nitrate values. This may be indicative of nitrate derived from soil N (Kendall 1998).
The range of signatures of ẟ18O-nitrate in ground and surface water was low, indicating that probably nitrate was derived from nitrification (− 10‰ to + 10‰), fertilizers, manure, and soil N (0.8–5.8‰) (Fig. 5). However, mixing of water of different concentration and sources of nitrate affect the nitrate concentration as well as changes the isotopic composition of nitrate (Kendall et al. 2007; Aravena and Mayer 2009). Surface water can receive groundwater inflow, recharge groundwater or both. Such movements of water between groundwater and surface water systems may lead to mixing of water qualities. Hence nitrate and other dissolved nutrients in surface water can be transferred to the connected groundwater system and vice versa.
Land use and cover change vs. nitrate enrichment
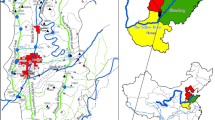
The LULC changes data show that the land use type in 1997 for built-up area was 65 km2 (14.4%), agriculture was 78 km2 (17.3%), water was 8 km2 (1.8%), forest was 73 km2 (16.2%) and bare land was 227 km2 (50.3%) (Figs. 6, 7). In 2008, the built-up area had doubled to 130.4 km2 (28.9%) whereby agriculture land also doubled up to 182.9 km2 (40.6%), respectively. However, the area covered by water had reduced 7.5 km2 (1.7%); forest cover also reduced to 57.9 km2 (13.2%) and bare land reduced by a factor of three to 70.2 km2 (15.6%). Data from 2017, indicate that the built-up area and agriculture land maintained the highest proportion, occupying 228.8 and 98.7 km2 (50.7% and 21.9%), respectively. The area occupied by water bodies has been reduced to 7.4 km2 (1.6%) whereas the forest area seems to have slightly increased to 63.1 (14%) km2 probably due to recent forest conservation efforts run by both the government and private sector. On the other hand, bare land has shrunk by a factor of four from its 228.8 km2 of 1997 to a mere 53.2 km2 (11.8%) probably due to increasing population and demand for settlement areas. These changes may have negative impacts on the quality of water and water sources.
The presence of nitrate and other nutrients at high levels in natural waters usually indicates that there is anthropogenic influence on the water quality (Salvado et al. 2006). In the studied area, agriculture is a major economic activity followed by animal keeping. These activities may have contributed to the pollution risk on water resources in the area because of the possibility of pollutants being released from both point and nonpoint sources (Camara et al. 2019). Elevated levels of nitrate were detected in some boreholes and shallow wells located near agricultural and piggery project areas. Similar cases of groundwater pollution with high levels of nitrate have been reported in Babati and other parts of Tanzania (Nkotagu 1996; Elisante and Muzuka 2017; Pantaleo et al. 2018). The sampling point BH5 with a nitrate concentration of 54.5 mg/L was located in a lowland area surrounded by maize farms where the application of fertilizers was intensive. However, water samples from Lake Babati revealed levels of nitrate close to background values, indicating less influence of anthropogenic activities on the lake’s water quality.
Conclusions
Generally, for the present study, the analyzed ground and surface water samples revealed relatively low levels of nitrate and trace elements. Five groundwater samples had nitrate concentration higher than the background levels and one sample exceed the WHO nitrate limit of 50 mg/L. Such levels may be associated with human activities such as application of synthetic fertilizers and animal manure in farms around the study area. Chemical composition of ground and surface water from the study area is largely influenced by the aquifer lithology than anthropogenic activities. Interpretation of hydro-chemical results showed that calcium carbonate dissolution and ion exchange processes controls the major cations chemistry of Ca2+, Mg2+, Na+, and K+ as well as the anionic chemistry of \({\text{HCO}}_{3}^{-}\), Cl−, \({\text{NO}}_{3}^{-}\) and \({\text{SO}}_{4}^{2-}\).
The present study has observed land use change in the study area whereby huge proportion of the land has been converted into human settlement and a shrinkage in the agricultural lands due to rapid urbanization and population growth. Farming activities and animal husbandry seem to be a lead cause in nitrate enrichment in ground and surface water of Babati town whereby enriched values of δ 15N-nitrate were mostly found at points close to the agricultural fields and in areas characterized by livestock keeping.
References
APHA 2017 Standard methods for the examination of water and wastewater, American Public Health Association, American Water Works Association, Water Pollution Control Federation, and Water Environment Federation, USA.
Aravena R., Mayer B. 2009. Isotopes and processes in the nitrogen and sulfur cycles. Environ Isotopes Biodegrad Bioremed 203–246.
Aravena R, Evans M, Cherry JA (1993) Stable isotopes of oxygen and nitrogen in source identification of nitrate from septic systems. Groundwater 31(2):180–186
Bičík I., Kupková L., Jeleček L., Kabrda J., Štych P., Janoušek Z., Winklerová J. 2015 Land use changes in the Czech Republic 1845–2010: socio-economic driving forces, Springer.
Camara M, Jamil NR, Abdullah AFB (2019) Impact of land uses on water quality in Malaysia: a review. Ecological Processes 8(1):10
Chang CC, Kendall C, Silva SR, Battaglin WA, Campbell DH (2002) Nitrate stable isotopes: tools for determining nitrate sources among different land uses in the Mississippi river Basin. Can J Fish Aquat Sci 59(12):1874–1885
Chenini I, Farhat B, Ben MA (2010) Identification of major sources controlling groundwater chemistry from a multilayered aquifer system. Chem Speciat Bioavailab 22(3):183–189
Choi W-J, Han G-H, Lee S-M, Lee G-T, Yoon K-S, Choi S-M, Ro H-M (2007) Impact of land-use types on nitrate concentration and δ15N in unconfined groundwater in rural areas of Korea. Agr Ecosyst Environ 120(2–4):259–268
Cobbina S, Duwiejuah A, Quansah R, Obiri S, Bakobie N (2015) Comparative assessment of heavy metals in drinking water sources in two small-scale mining communities in northern Ghana. Intern J Environ Res Public Health 12(9):10620–10634
Coppin PR, Bauer ME (1996) Digital change detection in forest ecosystems with remote sensing imagery. Remote Sensing Rev 13(3–4):207–234
Dehnavi AG, Sarikhani R, Nagaraju D (2011) Hydro geochemical and rock water interaction studies in East of Kurdistan, NW of Iran. Int J Environ Sci Res 1(1):16–22
Deutsch B, Mewes M, Liskow I, Voss M (2006) Quantification of diffuse nitrate inputs into a small river system using stable isotopes of oxygen and nitrogen in nitrate. Org Geochem 37(10):1333–1342
Dong M, Cheng W, Li Z, Demopoulos GP (2008) Solubility and stability of nesquehonite (MgCO3·3H2O) in NaCl, KCl, MgCl2, and NH4Cl solutions. J Chem Eng Data 53(11):2586–2593. https://doi.org/10.1021/je800438p
Durka W, Schulze E-D, Gebauer G, Voerkeliust S (1994) Effects of forest decline on uptake and leaching of deposited nitrate determined from 15N and 18O measurements. Nature 372(6508):765
Elisante E, Muzuka AN (2016) Assessment of sources and transformation of nitrate in groundwater on the slopes of Mount Meru. Tanzania Environ Earth Sci 75(3):277
Elisante E, Muzuka AN (2017) Occurrence of nitrate in Tanzanian groundwater aquifers: a review. Appl Water Sci 7(1):71–87
Eppicha G. R. (2012).Source determination of anthropogenic NO3 in groundwater by analysis of δ 15 N, δ 18 O, and δ 11 B: a case study from San Diego county, California. Groundwater Resources Association of California, Fresno, CA, 13(3).
Ghiglieri G., Pittalis D., Cerri G., Oggiano G. 2012. Hydrogeology and hydrogeochemistry of an alkaline volcanic area: the NE Mt. Meru slope (East African Rift-Northern Tanzania). Hydrol Earth Sys Sci 16(2).
Granger J, Sigman DM (2009) Removal of nitrite with sulfamic acid for nitrate N and O isotope analysis with the denitrifier method. Rapid Commun Mass Spectrom 23(23):3753–3762
Hongoa PS (2014) The impact of population increase around Lake Babati. The Open University of Tanzania, Tanzania
Ilyas A, Sarwar T (2003) Study of trace elements in drinking water in the vicinity of Palosi drain, Peshawar. Pak J Biol Sci 6:86–91
Jahnen-Dechent W, Ketteler M (2012) Magnesium basics. Clin Kidney J 5(Suppl 1):i3–i14. https://doi.org/10.1093/ndtplus/sfr163
Kelly W, Panno S, Hackley K (2012) The sources, distribution, and trends of chloride in waters of Illinois. Bulletin (Illinois State Water Survey) no. 74.
Kendall C. 1998. Tracing nitrogen sources and cycling in catchments. Isotope tracers in catchment hydrology. Elsevier.
Kendall C., Aravena R. 2000. Nitrate isotopes in groundwater systems. Environmental tracers in subsurface hydrology. Springer.
Kendall C, Elliott EM, Wankel SD (2007) Tracing anthropogenic inputs of nitrogen to ecosystems. Stable Isotopes Ecol Environ Sci 2:375–449
Kitalika AJ, Machunda RL, Komakech HC, Njau KN (2017) Land-use and land cover changes on the slopes of Mount Meru-Tanzania. Current World Environment 13(3):331
Mayer B, Boyer EW, Goodale C, Jaworski NA, Van Breemen N, Howarth RW, Seitzinger S, Billen G, Lajtha K, Nadelhoffer K (2002) Sources of nitrate in rivers draining sixteen watersheds in the northeastern US: isotopic constraints. Biogeochemistry 57(1):171–197
Miserendino ML, Casaux R, Archangelsky M, Di Prinzio CY, Brand C, Kutschker AM (2011) Assessing land-use effects on water quality, in-stream habitat, riparian ecosystems and biodiversity in Patagonian northwest streams. Sci Total Environ 409(3):612–624
Mitsch WJ, Day JW, Gilliam JW, Groffman PM, Hey DL, Randall GW, Wang N (2001) Reducing nitrogen loading to the gulf of Mexico from the Mississippi river Basin: strategies to counter a persistent ecological problem: ecotechnology—the use of natural ecosystems to solve environmental problems—should be a part of efforts to shrink the zone of hypoxia in the Gulf of Mexico. Bioscience 51(5):373–388
Molina M, Aburto F, Calderón R, Cazanga M, Escudey M (2009) Trace element composition of selected fertilizers used in Chile: phosphorus fertilizers as a source of long-term soil contamination. Soil Sediment Contamination 18(4):497–511
Namugize JN, Jewitt G, Graham M (2018) Effects of land use and land cover changes on water quality in the uMngeni river catchment, South Africa. Physics and Chemistry of the Earth, Parts A/B/C 105:247–264
Nkotagu H (1996) Origins of high nitrate in groundwater in Tanzania. J Afr Earth Sc 22(4):471–478
Nur A, Ishaku JM, Yusuf SN (2012) Groundwater flow patterns and hydrochemical facies distribution using geographical information system (GIS) in Damaturu, Northeast Nigeria. Intern J Geosci 3(05):1096
Pantaleo P, Komakech H, Mtei K, Njau K (2018) Contamination of groundwater sources in emerging African towns: the case of Babati town. Tanzania Water Pract Technol 13(4):980–990
Pardo LH, Kendall C, Pett-Ridge J, Chang CC (2004) Evaluating the source of streamwater nitrate using δ15N and δ18O in nitrate in two watersheds in New Hampshire, USA. Hydrol Process 18(14):2699–2712
Ramesh R, Kumar KS, Eswaramoorthi S, Purvaja G (1995) Migration and contamination of major and trace elements in groundwater of Madras City. India Environ Geol 25(2):126–136
Rawat J, Kumar M (2015) Monitoring land use/cover change using remote sensing and GIS techniques: a case study of Hawalbagh block, district Almora, Uttarakhand, India. Egyptian J Remote Sensing Space Sci 18(1):77–84
Reid RS, Kruska RL, Muthui N, Taye A, Wotton S, Wilson CJ, Mulatu W (2000) Land-use and land-cover dynamics in response to changes in climatic, biological and socio-political forces: the case of southwestern Ethiopia. Landscape Ecol 15(4):339–355
Rock L, Mayer B (2004) Isotopic assessment of sources of surface water nitrate within the Oldman River basin, Southern Alberta, Canada. Biogeochemical investigations of terrestrial, freshwater, and wetland ecosystems across the globe. Springer.
Saana SBBM, Fosu SA, Sebiawu GE, Jackson N, Karikari T (2016) Assessment of the quality of groundwater for drinking purposes in the Upper West and Northern regions of Ghana. SpringerPlus 5(1):2001
Salvado V, Quintana X, Hidalgo M (2006) Monitoring of nutrients, pesticides, and metals in waters, sediments, and fish of a wetland. Arch Environ Contam Toxicol 51(3):377–386
Sandström K (1995) The recent lake Babati floods in semi-arid Tanzania—a response to changes in land cover? Geogr Ann 77(1–2):35–44
Tank DK, Chandel CS (2010) Analysis of the major ion constituents in groundwater of Jaipur city. Nature Sci 8(10):1–7
Tappan GG, Sall M, Wood EC, Cushing M (2004) Ecoregions and land cover trends in Senegal. J Arid Environ 59(3):427–462
Utom AU, Odoh BI, Egboka BC, Egboka NE, Okeke HC (2013) Estimation of subsurface hydrological parameters around Akwuke, Enugu, Nigeria using surface resistivity measurements. J Geophys Eng 10(2):025016
Wassenaar LI (1995) Evaluation of the origin and fate of nitrate in the Abbotsford aquifer using the isotopes of15N and18O in NO3−. Appl Geochem 10(4):391–405
WHO. 2011. Guidelines for drinking-water quality. World Health Organization: Geneva, Switzerland. Available: https://www.who.int/water_sanitation_health/dwq/chemicals/nitratenitrite2ndadd.pdf [216].
Widory D, Petelet-Giraud E, Négrel P, Ladouche B (2005) Tracking the sources of nitrate in groundwater using coupled nitrogen and boron isotopes: a synthesis. Environ Sci Technol 39(2):539–548
Xue D, Botte J, De Baets B, Accoe F, Nestler A, Taylor P, Van Cleemput O, Berglund M, Boeckx P (2009) Present limitations and future prospects of stable isotope methods for nitrate source identification in surface-and groundwater. Water Res 43(5):1159–1170
Yusuf K (2007) Evaluation of groundwater quality characteristics in Lagos-City. J Appl Sci 7(13):1780–1784
Acknowledgements
Professor Alfred N. N. Muzuka, who died during the course of this work—Alfred, you will always be remembered for your diligence and adherence to standards. We also thank the African Development Bank (AfDB) for a support given to the Nelson Mandela African Institution of Science and Technology (NM-AIST) from which this research benefited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mallya, C.L., Rwiza, M.J. Influence of land use change on nitrate sources and pollutant enrichment in surface and groundwater of a growing urban area in Tanzania. Environ Earth Sci 80, 111 (2021). https://doi.org/10.1007/s12665-021-09386-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-09386-z