Abstract
River heavy metal contamination (HM) is a serious growing concern across the globe. In Iran, particularly, rivers serve as the most important freshwater resource and underlie the majority of water-demanding activities, resulting in highly polluted rivers. In central Iran, the Ghareh-Aghaj River supports many industrial, municipal, and agricultural land-uses along its path to the Persian Gulf. Although there may be reciprocal HM effects, no study has yet provided supporting scientific evidence on the river’s sediment HM content that might affect the sensitive biodiversity of the river and the Persian Gulf. This study measured the concentrations of five HMs at 11 susceptible and accessible locations along the river as follows: Arsenic (41.83 mg/kg) > Nickel (38.08 mg/kg) > Zinc (24.46 mg/kg) > Copper (18.86 mg/kg) > Lead (4.97 mg/kg). The HM distributions were uniform due to insignificant correlations and high coefficient of variations. According to the results, the river was not polluted with Cu, Zn, and Pb, partly with Ni, and highly with As. The concentration of As exceeded natural values in the river midstream zone and the seawardmost station where the river is exposed to intense agriculture and extensive use of arsenical pesticides and herbicides. This study highlighted the Ghareh-Aghaj River as worthy of further HM investigation, particularly As and urged implementation of HM elimination and monitoring measures to ensure their health and well-functioning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iran is an arid and semi-arid country with very limited supplies of surface freshwater (Ashraf et al. 2019). In recent decades, the surge of Iranian population growth and the ensuing freshwater demand have put unprecedented pressure on underground and surface freshwater resources (Mombeni et al. 2013) and turned Iran into a “water bankrupt” nation (Madani et al. 2016). Rivers are the main sources of surface water across the country that underlie the majority of water-demanding activities in central Iran including municipal, agricultural, and industrial activities (Vahid et al. 2016). Despite the current leading role and importance of rivers in central Iran, their pollution exposure has been poorly involved in development plans (Madani 2014). Particularly, heavy metal-rich leaches from agricultural fields usually end up in rivers due to their close adjacency to rivers (Diagomanolin et al. 2004) and unrestricted use of pesticides (Fadaei et al. 2012). Moreover, municipal and industrial (especially mining activities) effluents are mostly discharged into rivers as a common solution, resulting in highly heavy metal polluted rivers (Salati and Moore 2010).
In global rivers, the concentration and number of heavy metals have increased considerably since the 1990s and exceeded the threshold values of the WHO and USEPA standards, especially in Africa, Asia, and South America (Zhou et al. 2020). Although the river heavy metal pollution sources are region-specific and differ significantly between continents, their sources are growing fast in number and resulted in mixed heavy metal pollution over time. Studies on the world’s aquatic ecosystems have yeilded two major findings: first, heavy metals have a high solubility and usually find ways into lakes and rivers. In other words, heavy metals remain in contact with freshwater environments (Kumar et al. 2019). Hence, heavy metal analysis in aquatic ecosystems has been the subject of many studies investigating the pollution levels of the ecosystems. Second, heavy metal sources have changed significantly over time and across regions between fertilizer and pesticide use, mining, and manufacturing (Li et al. 2019). The findings from these studies indicated the failure of the majority of heavy metal control and abatement measures and related a wide range of health problems and diseases to heavy metal pollution by measuring their levels in aquatic environments (Li et al. 2019; Kumar et al. 2019; Nafchi and Chamani 2019).
As Zhou et al. (2020) conclude, South America, Africa, and Asia are at higher risk of river heavy metal contamination. In Iran, this issue has already become a serious, growing concern and attracted wide scholarly attention in recent years. Different metals were analyzed in these studies depending on the probable source(s) of heavy metal contamination. For instance, the dominant sources of sediment heavy metal pollution in Haraz (Nasrabadi et al. 2010), Gorganrud (Bagheri et al. 2011), and Chitgar (Sayadi et al. 2010) rivers were found associating with small-scale industrial activities close to the rivers with increased concentrations of Zn, Ni, and Pb while in landscapes with extensive agricultural activities, river sediment analysis revealed high levels of Ni, Cu, Cd, and Pb in Karoon (Diagomanolin et al. 2004) and Kor (Sheykhi and Moore 2013) rivers. In some other rivers, particularly Khoshk (Salati and Moore 2010), Zayandeh Rood (Sanayei et al. 2009), and Siahroud (Charkhabi et al. 2005), a wide variety of potentially polluting activities were found to contribute to increasing levels of various heavy metals. These studies categorize the majority of Iranian rivers as heavy metal-polluted rivers, requiring further examination and implementation of monitoring plans to ensure their health and well-functioning.
Studies dealing with heavy metal measurement often take advantage of sediment pollution indices as useful and simple-to-calculate tools to estimate the enrichment and contamination level of sediments-water systems according to a single or a combination of heavy metals (Memoli et al. 2019). These indices are categorized into two groups of single metal indices with most attempting to compare measurements with background values, such as geoaccumulation index and integrated metal indices that give a comprehensive view of contamination degree regardless of the heavy metal type, such as degree of contamination (Memoli et al. 2019; Nazarpour et al. 2019). To cope with the genuine deficiencies in using a limited number of indices, studies in this field adopt and compare the results achieved from a wide range of sediment pollution indices to provide a more holistic picture of contamination (see “Materials and Methods” section). In the present study, the primary objective was to measure sediment heavy metal concentrations in 11 susceptible locations along the less-studied Ghareh-Aghaj River which provides drinking water for surrounding villages and cities and is subject to extensive agricultural and mining activities. An array of sediment pollution indices was used to interpret the level of heavy metal contamination and enrichment. These results, along with the comparison of measurements with sediment quality guidelines, helped ultimately conclude whether heavy metal conamination levels of the case study river are high and may affect the functioning and biota of the river.
Materials and methods
Study sites
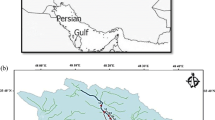
Ghareh-Aghaj (GHA) River is one of the longest perennial rivers of Iran that intersects two provinces of Fars and Bushehr with a total length of 700 km. It originates from Zagros Mountains and heads towards the coast of the Persian Gulf in the south of Iran (Fattahi and Jahangiri 2012). The width of the river ranges between 20 m in mountainous areas and over 400 m at the mouth of the river joining the Persian Gulf. The flow rate of the river ranges from 3.5 to over 4.0 m3 depending on the location and time of year and peaks at ~ 6000 m3 during flood times. The water level of the river is controlled by the upstream Salman Farsi Dam to manage water allocation for different purposes (Bahrami and Rahimi 2009).
In the present research, 11 sampling stations (S1–S11, Fig. 1) were selected along 190 km of the GHA River where the river is exposed to various anthropogenic impacts such as agriculture and mining. Moreover, the depth, width, and flow rate of the river and convenient accessibility were applied in selecting sampling stations. Sampling was carried out eight times at each station, with an interval of 45 days during the year 2019. Figure 1 illustrates the location of sampling stations along the GHA River.
Sampling protocol and heavy metal analysis
Sediment samples were collected in the middle of the river from the first 5 cm of surface sediments (n = 3) and held in polyethylene bags on dry ice for transport to the laboratory. Soil samples were passed through a series of standard sieves to determine the distribution of sediment particles. The sediment sizes were classified as clay (< 0.004 mm), silt (0.004–0.06 mm), sand (0.06–2 mm). The samples were dried in an oven for 48 h at 70 °C and passed through a 63-µm sieve and powdered with the help of an agate mortar. Next, they were digested with 20 mL nitric acid solution 65% (Merck) and 5 mL hydrogen peroxide solution, placed under a hood for 48 h and heated for 5 h at 70° using a digesting device (heater). After cooling at room temperature, the resulting solution was distilled twice by distilled water, sieved using a Whatman Grade 42 filter paper and reached a volume of 25 mL (APHA 2005). The concentrations of Cu, Zn, Cd, Pb, Fe, Ni, Hg* and As (mg/kg) were determined using the GBC Savantaa atomic absorption spectrometry. Some important heavy metals like Hg and Cd were excluded in the present study since their concentrations were almost zero at the stations.
To make the control, 20 mL of 65% nitric acid solution and 2 mL of oxygenated water were mixed with the standard heavy metal concentration in sediment (Nourouzi et al. 2018). The resulting metal concentrations were subtracted from the sample HM concentrations. Spiked samples were used to evaluate the accuracy of the analytical methods. Electrical conductivity (EC) and pH were measured in the saturated paste extract using a pH meter and EC meter, respectively. Total organic carbon (TOC) (Walkley and Black 1934), CaCo3 (Black et al. 1965), bulk density (BD), and cation exchange capacity (CEC) (Chapman 1965) and grain size (Bouyoucos 1962) were also calculated to aid interpretation of the results.
Measuring sediment pollution indices
Geoaccumulation index
The geoaccumulation index (Igeo) was proposed by Muller (1979) as a quantitative measure to account for pollution intensity and has gained recognition among researchers to quantify the quality of sediments concerning each heavy metal. The mathematical expression of Igeo is as follows (Eq. 1) where \({c}_{n}\) is the concentration of metal n and \({B}_{n}\) serves as the geochemical background value as suggested by Bowen (1979). The Igeo classes were considered as: extremely contaminated (Igeo > 5: Class (hereafter C) 1), strongly to extremely contaminated (Igeo 4–5: C2), strongly contaminated (Igeo 3–4: C3), moderately to strongly contaminated (Igeo 2–3: C4), moderately contaminated (Igeo 1–2: C5), uncontaminated to moderately contaminated (Igeo 0–1: C6), and uncontaminated (Igeo = 0: C7).
Contamination factor and degree of contamination
The contamination factor (CF), also known as single pollution index, is a popular means of measuring the contamination status of a single toxic substance in the sediment. CF was proposed by Hakanson (1980) as a ratio of heavy metal concentration between the contaminated and background (or control) sites as given in Eq. (2) where \({M}_{x}\) and \({M}_{b}\) are the calculated concentration and the background (control) value of the given element. The classes of CF were considered as following: very high contamination CF ≥ 6 (C1), considerable contamination 3 ≤ CF < 6 (C2), moderate contamination 1 ≤ CF < 3 (C3), and low contamination 1 > CF (C4).
The degree of contamination [Cd—Hakanson (1980)] depicts the extent of heavy metal pollution based on the measurement of CF and is defined as the sum of all CF values of the metals under study (Eq. 3) where \({C}_{f}^{i}\) is the CF value of element i. According to Hakanson (1980), Cd ≥ 24 is considered as considerable degree of contamination (C1), 12 ≤ Cd < 24 as high degree of contamination (C2), 6 ≤ Cd < 12 as moderate degree of contamination (C3), Cd < 6 as low degree of contamination (C4).
Modified degree of contamination
The modified degree of contamination (mCd) is a modified version of Cd suggested by Abrahim (2005). The mCd index is a simple aggregate measure depicting the accumulation of heavy metal in the contaminated sediments and is calculated by dividing the sum of all the CFs by the number of pollutants analyzed (n) (Eq. 4) (Abrahim 2005). The mCd values are classified as: extremely contaminated (16 ≤ mCd < 32: C1), very highly contaminated (8 ≤ mCd < 16: C2), highly contaminated (4 ≤ mCd < 8: C3), moderately contaminated (2 ≤ mCd < 4: C4), low contaminated (1.5 ≤ mCd < 2: C5), and non-contaminated (mCd < 1.5: C6).
Enrichment factor
The enrichment factor (EF) was first employed by Buat-Ménard (1979) in the field of ecotoxicology and chemoecology and has gained recognition for assessing the lithogenic or anthropogenic source of heavy metals measured in soil and sediments (Dou et al. 2013). EF is defined as the heavy metal concentration ratio between soil (or sediment) and the natural (uncontaminated) local background, as suggested by Bowen (1979) (Eq. 5). In this equation, \({C}_{s}\) refers to a non-human-affected conservative detrital fraction of sediment and \({C}_{M}\) is the measured heavy metal concentration. High EF values (especially > 2) indicate the increasing share of anthropogenic sources in heavy metal contamination (2). The following classes are used to interpret EF values: extremely high enrichment (EF > 40: C1), very high enrichment (20 ≤ EF < 40: C2), significant enrichment (5 ≤ EF < 20: C3), moderate enrichment (2 ≤ EF < 5: C4), and deficiency to minimal enrichment (EF < 2: C5):
Potential ecological risk index (RI)
The potential ecological risk index (RI) is a useful index developed by Hakanson (1980) to measure the combined ecological risk of heavy metals which accounts for the sensitivity of various biological communities to heavy metals (Barkett and Akün 2018). In the RI equation (Eq. 6), \({E}_{r}^{i}\) (Eq. 7) refers to the monomial ecological risk factor of metal i that is calculated by multiplying the ratio of metal concentration between soil (\({M}_{x}^{i}\)) and the background value in the Earth’s crust (\({M}_{b}^{i}\)) by Tr: the toxic response factor of metal I, as recommended by Ra et al. (2014). RI values are categorized into four classes of very high (RI ≥ 600: C1), considerable (300 ≤ RI ≤ 600: C2), moderate (150 ≤ RI ≤ 300: C3), and low (RI ≤ 150: C4):
Pollution load index
The pollution load index (PLI) is a simple, comparative index developed by Tomlinson et al. (1980) to calculate the pollution level of heavy metals. To measure LPI, the pollution index must be computed for each metal by dividing the concentration of heavy metals in the sample by background concentration. The resulting values are then used to determine PLI by calculating the nth root of metal i where n indicates the number of metals studied (Eq. 8). The results of PLI index are categorized as extremely heavy pollution (3 < PLI: C1), heavy pollution (2 < PLI < 3: C2), moderate pollution (1 < PLI < 2: C3), and no pollution (PLI < 1: C4):
Sediment quality guidelines (SQGs) and mean ERM quotient
Sediment quality (or effect-range) guidelines (SQGs) are predictive tools helping to make an initial assessment of sediment heavy metal toxicity to aquatic biota. In other words, SQGs indicate heavy metals’ ranges within which sediment-bound contaminants exert tangible biological impacts upon aquatic biota (Simpson and Batley 2016). The US National Oceanic and Atmospheric Administration (NOAA) provide one of the most fundamental guidelines for estuarine and marine environments by sedimentary analyses performed on three seaboards of North America. According to NOAA thresholds causing different adverse biological impacts, the 10th and 50th percentiles of each criterion are regarded as effects-range-low (ERL, the initial concentration at which adverse impacts emerge) and effects-range-median (ERM) where < ERL, ≥ ERL and < ERM, and ≥ ERM are used to indicate rare, occasional, and frequent occurrence of adverse impacts (Long et al. 1995). To more convenient interpretation, the mean ERM quotient (MERMQ) was employed to calculate the mean quotient of all metals incorporated in the analysis, as given in Eq. (9) where \({C}_{i}\) is the concentration of heavy metal i, ERMi is the ERM of heavy metal i reported by Long et al. (1995) and n is the number of heavy metals. The toxicity probability of MERMQ values of 0.1, 0.11–0.5, 0.51–1.5, and > 1.51 are considered to be 9, 21, 49, and 76%, respectively (Jafarabadi et al. 2017). Table 1 shows the ERM, ERL, interim sediment quality guidelines (ISQGs), portable effect level (PEL) values used to assess the toxic risk of heavy metals in this research, adopted from Long et al. (1995).
Results
Among the grain size contents, sand was the most predominant component (67.7% ± 2.8), followed by clay (27.8% ± 2.7) and silt (4.4% ± 0.5), respectively (Fig. 2). The concentration of other metals in the surface sediments of the GHA river decreased as follows: As (41.83 ± 20.79 mg/kg) > Ni (38.08 ± 12.08 mg/kg) > Zn (24.46 ± 4.82 mg/kg) > Cu (18.86 ± 2.16 mg/kg) > Pb (4.97 ± 2.76 mg/kg).The highest coefficient of variation (CV) value was obtained for Pb (55.50%), followed by As (49.17%), Ni (31.78%), Zn (19.72%), and Cu (11.50%), respectively (As > Ni > Zn > Cu > Pb). The highest mean concentration of As (71.28 mg/kg), Zn (33.02 mg/kg) and Cu (22.57 mg/kg) was observed at S11 and Pb (9.17 mg/kg) and Ni (61.33 mg/kg) at S8 (Fig. 2). The values of other sediment parameters including total organic carbon (TOC), pH, EC (ds/m), CaCo3 (%), bulk density (g/cm3), and CEC (meq/100 g) are represented in Table 2. The correlation coefficients between each metal pair varied largely (Table 3). Arsenic had no significant association with other metals. The most significant correlation between heavy metals was related to Fe–Ni (0.826, p < 0.01), followed by Fe–Cu (0.785, p < 0.01), and Cu–Ni (0.826, p < 0.737). There were highly significant correlation coefficients between grain size percentage (> 0.93, p < 0.01). CaCo3 had a significantly positive correlation with the concentration of Ni, Cu and Zn. There was no significant correlation between CEC and heavy metals concentrations. Bulk density contributed significantly to the uptake of Ni and Fe. The association of Pb concentration was significantly positive with pH while significantly negative with EC.
All sediments were calcareous in nature with carbonate contents (as (%) CaCO3) ranging from 49 to 53.25%. The concentrations of heavy metals were found to be independent of organic carbon content due to their insignificant correlations with TOC. The significant strong negative correlation between TOC and CaCO3 indicated the dominance of calcium carbonate content in the sediments. The significantly negative correlations between heavy metals and CaCO3 seemed to be related to the alkaline pH of the environment in which the uptake of metals like Zn, Cu, Fe, and Ni decreases with increasing CaCO3 content (Kabata-Pendias 2010). Soil samples were classified as un-contaminated with Zn, Cu, and Pb at all stations (Table 4). In terms of Ni, 72% of stations were uncontaminated to moderately contaminated (Igeo of < − 0.07 at S1 and S2) and 18% were moderately contaminated (0.28 < Igeo < 0.81 at S3–S7 and S9–S11). Geoaccumulation index values ranged between 0.65 and 2.98 for As (mean of 1.99) across all stations, of which 64% of stations were found to be moderately to strongly contaminated. The results of CF, Cd, and mCd values computed for each station are tabulated in Table 5. According to the CF values, the soil samples investigated in the present research were slightly contaminated due to Zn (0.30 < CF < 0.55), Cu (0.62 < CF < 0.90), and Pb (0.00 < CF < 0.48). Around 80% of stations were classified as moderately contaminated with Ni (1.42 < CF < 2.64), except for S1 and S8 which had a low and high contamination level, respectively. The majority of soil samples fell into the highly contaminated CF level (54% of all sites: S3–S8 and S11) ranging from 6.96 to 10.25. Contamination degree (Cd) values ranged between 4.05 and 16.26 which corresponded to none (18% of all sites), low (36% of all sites), and moderate (45% of all cases) contamination classes. The mCd values also fell in the same classes as Cd, accounting for 27, < 1, and 63% of stations being classified as none, low, and moderate contamination classes.
The results of EF ranged from 1.42 to 10.73 for Ni, 0.73 to 2.00 for Zn, 1.25 to 4.17 for Cu, 0 to 1.63 for Pb, and 4.72 to 50.25 for As (Table 6). All soil samples were categorized as minimal enrichment due to Pb and Zn at all stations. Soil samples showed a moderate enrichment for Ni at S2, S3, and S11 (mean of 4.12) and for Cu at S4–S7 and S9–S10 (mean of 3.36) and minimal enrichment for the remaining samples. Mean EF for As was 25.02 with 54% of stations being equally classified as very high and extremely high enrichment classes. In terms of PLI, none of the stations were categorized as heavily and extremely heavily contaminated while stations S3–S5, S7, and S8 (54% of all stations) had a moderate and the remaining 46% had no contamination according to PLI. RI values ranged from 30.63 to 138.50 (mean of 85.22). According to the RI classification scheme presented in the material and methods section, samples were not polluted with the studied heavy metals due to obtaining RI values lower than 150. The MERMQ results are given in Table 5. In nine samples, toxicity probability was 76% due to being ranged between 0.51 and 1.5 (mean MERMQ of 1.76) while this probability was found to be 49% at the remaining two stations of S9 and S10.
Discussion
In some Iranian rivers, such as Haraz (Nasrabadi et al. 2010), Jajrood (Saeedi et al. 2011), and Kor rivers (Sheykhi and Moore 2013), the spatially densified pattern of anthropogenic activities along the rivers has raised the possibility of statistically significant relationships between metals. These activities, however, are dispersedly distributed along the GHA river due to traversing through mountainous areas, thus helping better identify the probable sources of heavy metal contamination. In other words, different and mostly insignificant correlation coefficients among the studied heavy metals indicated their different heavy metal sources and migration pathways along the river. The spatial distribution of Pb and As also varied significantly among the stations, suggesting that they may originate from different sources in locations with high concentration levels, particularly at S7 and S8 for Pb and S11 for As while Ni, Zn, and Cu showed uniform distributions across the study stations with CV values of less than 36% (Zhang et al. 2019). The concentration of Ni, the highest among the analyzed HMs, showed a significant rise at S6 and thereafter (up to 61.31 mg/kg) where sand mining activities begin to occur. In Haraz River (Nasrabadi et al. 2010), similar Ni concentrations were found in stations surrounded by limestone, sand, and gravel mines. In some other rivers, such as Koshk (Salati and Moore 2010), the high concentration of Ni (> 100 mg/kg) was attributed to the geochemical composition of parent rock where no significant anthropogenic activity was found close to the study stations. Globally, the mean concentration of Ni in water ecosystems is greater than the WHO and USEPA standards, especially in Africa and Europe. From the 1970s onwards, moreover, the concentration of this metal increased largely from 1.43 (µg/L) to over 80 (µg/L) up to the 2010s (Zhou et al. 2020). The concentration of Zn was also high at S7 and S8 (alongside Pb), the two closest stations to mining activities and at S11 after the Kavar City whose wastewater effluent is partly delivered to the River. The highest concentration of Cu (22.57 mg/kg) was observed at S11. Similar to our results, Sheykhi and Moore (2013) also found higher levels of Cu and Zn in stations receiving domestic sewage and leachates from municipal waste (Table 7). Currently, the threat of Zn overexposure is highest in Europe, followed by Africa and lowest in North America. In Asia, the mean Zn concentration in river and lake ecosystems is about its threshold [~ 890 (µg/L) compared to the WHO and USEPA standard limit of 1000 (µg/L)] (Zhou et al. 2020).
The results of this study indicate high concentrations of As beyond the universally acceptable limits which could be ascribed to large-scale agricultural activities along the river length, particularly at S4–S8 and S11 where the landscape is dominated by agricultural activities. In 2015, the total agricultural area of three Shiraz, Kavar, and Frioozabad counties supplied with the river water was 51,419, 10,917, and 25,149 ha, with the total crop production of 577,035, 126,579, 121,523 tons, respectively. During the same year, the fruit tree area (and the volume of production) in the counties were 31,653 ha (202,323 tons), 7194 ha (111,798 tons), and 8302 ha (14,474 tons), respectively (Office of Planning and Economy 2015). The Plant Protection Organization of Fars Province in 2015 performed chemical control of agricultural pests (287,780 ha), disease (33,345 ha), and weed (552,250 ha), and chemical control of horticultural pests (343,600 ha), disease (82,690 ha), and weed (111,400 ha) (Office of Planning and Economy 2015). Additionally, the open system greenhouse agriculture is being rapidly established and developed in the province. A recent study showed significant residues of four pesticides Stamipride, Diazinon, imidacloprid, and Pirimicarb in tomato crops of the Fars Province (Yazdanpak et al. 2020). Despite the dominance of agricultural activities and the extensive utilization of pesticides, waters and sediments receiving their outflows are not yet monitored nor controlled. Mirzaee Mahmoodabadi (2019) reported increasing levels of Chromium, Zinc, Nickel, Lead, Copper, and Cadmium in the sediments of Ghareh-Aghaj River in Kahfr Watershed, southern parts of the studied region. Likewise, in Zarrin-Gol River, Malvandi (2017) and Karimian et al. (2020) reported high sediment As concentration at stations located in agricultural landscapes. As Sheykhi and Moore (2013) discuss, high river sediment contamination with As is attributable to traditional Iranian farmers who make use of arsenical pesticides and herbicides more extensively than other types due to their high effectiveness (see Table 7). High As concentrations measured in the stations may not be acutely toxic but chronic exposures can pose serious dangers to the biotic component of aquatic environments. For instance, Kazemi et al. (2018) found that high As concentrations in river sediments degrade the food web of the ecosystem and affect human health. More corroborating results were also found by Luo et al. (2010). They revealed that As has a high percentage of bioaccessibility from soil to plants and other organisms. In Asia, water contamination to As has also been a challenging issue. The mean concentration of As in Asian rivers was found to be by far higher than those of other continents (178.30 µg/L), related to various sources including mining, weathering, and agricultural activities (Zhou et al. 2020). The Plant Protection Office (PPO) of the Fars Province has no As-containing pesticide in its list of official distribution and management (Noorbakhsh et al. 2011); however, a large black market provides traditional less-aware farmers with contraband pesticides such as As-containing compounds (PPO officials, personal communication). Recent research emphasized the probability of the polluting influence of horticultural and agricultural fertilizers and pesticides in lower parts of the Ghare Aghaj river (Mirzaee Mahmoodabadi 2019).
According to Igeo results, the quality of superficial sediments was unpolluted by Zn, Cu, and Pb across the entire length of the river and Ni in the river upstream zone (stations 1 and 2). The Igeo values of As, however, were alarmingly high across the study stations which raises concerns regarding the contribution of its anthropogenic sources (mostly agricultural activities) and potentially deleterious impacts. These levels of As contamination are unprecedented in river surface sediments of Iran. The Igeo values of this heavy metal were categorized as uncontaminated to moderately contaminated in the majority of Iranian rivers like Tajan (Alahabadi and Malvandi 2018), Haraz (Nasrabadi et al. 2010) and Kor (Sheykhi and Moore 2013). A similar pattern of classification was found for CF in which eight stations fell into considerable contamination level with Pb while minimal concern was noted about the other investigated heavy metals. Taking all CF values into single measures (Cd and mCd), stations S4, S6–S8 and S11 were prioritized as high-risk locations impacted by an array of anthropogenic sources including sand mining, agricultural and municipal activities.
According to EF results, there are limited or no anthropogenic sources of Zn, Cu, and Pb along the studies river while a combination of natural and anthropogenic sources are likely to contribute to Ni, particularly at midstream stations that are affected by sand mining operations. The river midstream zone (stations 4–8) and station 11 were found to receive significantly higher quantities of As content from anthropogenic sources as compared to natural background levels, especially from agricultural activities. The pollution load index also categorized S11 and the midstream zone of the river as moderately polluted due particularly to high As concentrations, whereas the findings from other Iranian rivers such as Zarrin Gol (Malvandi 2017) and Tajan (Alahabadi and Malvandi 2018) rivers indicated no ecological risk with PLI values of > 1.
The concentration of Ni and As at S11 was slightly higher than the ERL values given in Table 1, suggesting that these two heavy metals seem to exert detrimental impacts on biota in the river downstream whereas no impact is expected to jeopardize living organisms in the remaining stations with the investigated heavy metals. In comparison with PEL, however, As concentrations in the studied stations (except for S1) were found to be biologically meaningful. Ultimately, the probability of toxicity at the study stations was found to be high, ranging from 49 to 76%, which urges adoption of heavy metals at-source reduction, elimination and monitoring measures, particularly at midstream stations and S11 where the river is affected by various anthropogenic activities ranging from mining to agricultural operations. Of note is that the sediments analyzed in this research had large-sized particles (nearly 60% of sand) whose heavy metal adsorption and binding ability are weaker than small sediment particles, resulting in lower heavy metal content per unit sediment. Further investigation is also needed to identify the causes of high Pb temporal variation in the study area with very high concentrations from September to December (up to 18 mg/kg).
Conclusion
The present study investigated heavy metal contamination of one of the longest rivers in the central arid zone of Iran. The mean concentration of five heavy metals measured at 11 susceptible and accessible stations was as follows: As > Ni > Zn > Cu > Pb with mostly insignificant correlations and uniform temporal and spatial distribution along the river. Among the studied heavy metals, the most significant threat was associated with As which was grouped into highly contaminated classes at the midstream zone where the landscape is dominated by extensive traditional agricultural activities with high utilization of arsenical pesticides and herbicides as well as at S11 in the downstream zone where municipal and agricultural wastewater effluents are discharged into the river. Drawing from these findings, the study river merits further heavy metal investigations and can be regarded as worthy of implementing heavy metal elimination and monitoring measures. Currently, the river can be classified as single-metal pollution with As, but further development especially those contributing to Ni contamination may change the river ecosystem to mixed metal pollution. Hence, limited use of pesticides, pretreatment of contaminated waste from mining and municipal sections are recommended to control the river heavy metals pollution. The results of this research have practical implications for natural resources managers to better evaluate and protect this river which has strategic national importance.
References
Abrahim G (2005) Holocene sediments of Tamaki Estuary: characterisation and impact of recent human activity on an urban estuary in Auckland, New Zealand. ResearchSpace@ Auckland
Alahabadi A, Malvandi H (2018) Contamination and ecological risk assessment of heavy metals and metalloids in surface sediments of the Tajan River, Iran. Mar Pollut Bull 133:741–749
APHA (2005) Standard methods for the examination of water and wastewater. APHA Washington DC, USA
Ashraf S et al (2019) Compounding effects of human activities and climatic changes on surface water availability in Iran. Clim Change 152:379–391
Bagheri H, Alinejad S, DarvishBastami K (2011) Heavy metals (Co, Cr, Cd, Ni, Pb and Zn) in sediment of Gorganrud River, Iran. Res J Environ Toxicol 5:147–151
Bahrami M, Rahimi A (2009) Calculation of the transpotation of the total sediment yield of Ghareh-Aghaj River at Karar Dam site using hydraulic methods. Iran J ApplGeol 5:220–229
Barkett MO, Akün E (2018) Heavy metal contents of contaminated soils and ecological risk assessment in abandoned copper mine harbor in Yedidalga, Northern Cyprus. Environ Earth Sci 77:378
Black CA, Evans D, White J, Ensminger L, Clarke F (1965) Methods of soil analysis. American Society of Agronomy. Madison, Wisconsin, part I, pp 1–770
Bouyoucos GJ (1962) Hydrometer method improved for making particle size analyses of soils 1. Agron J 54:464–465
Bowen HJM (1979) Environmental chemistry of the elements. Academic Press, Cambridge
Buat-Ménard P (1979) Influence de la retombée atmosphérique sur la chimie des métaux en trace dans la matière en suspension de l’Atlantique Nord. Thèse de Doctorat d'Etat, Sciences, Paris 7
Chapman HD (1965) Cation exchange capacity. In: Black CA (ed) Methods of soil analysis. American Society of Agronomy, Madison, pp. 891–901
Charkhabi AH, Sakizadeh M, Rafiee G (2005) Seasonal fluctuation in heavy metal pollution in Iran s Siahroud River-a preliminary study (7 pp). Environ Sci Pollut Res 12:264–270
Diagomanolin V, Farhang M, Ghazi-Khansari M, Jafarzadeh N (2004) Heavy metals (Ni, Cr, Cu) in the karoon waterway river, Iran. ToxicolLett 151:63–67
Dou Y, Li J, Zhao J, Hu B, Yang S (2013) Distribution, enrichment and source of heavy metals in surface sediments of the eastern Beibu Bay, South China Sea. Mar Pollut Bull 67:137–145
Fadaei A, Dehghani MH, Nasseri S, Mahvi AH, Rastkari N, Shayeghi M (2012) Organophosphorous pesticides in surface water of Iran. Bull Environ ContamToxicol 88:867–869
Fattahi M, Jahangiri H (2012) The relations between the fractal properties of the river networks and the river flow time series. Int J Geol Environ Eng 6:111–116
Goorzadi M, Vahabzadeh G, Ghanbarpour M, Karbassi A (2009) Assessment of heavy metal pollution in Tilehbon River sediments, Iran. J ApplSci 9:1190–1193
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach . Water Res 14:975–1001
Jafarabadi AR, Bakhtiyari AR, Toosi AS, Jadot C (2017) Spatial distribution, ecological and health risk assessment of heavy metals in marine surface sediments and coastal seawaters of fringing coral reefs of the Persian Gulf, Iran. Chemosphere 185:1090–1111
Kabata-Pendias A (2010) Trace elements in soils and plants, 4th edn. CRC Press/Taylor & Francis Group, Boca Raton
Karimian S, Chamani A, Shams M (2020) Evaluation of heavy metal pollution in the Zayandeh-Rud River as the only permanent river in the central plateau of Iran. Environ Monit Assess 192:316–316
Kazemi M, Chamani A, Agh N (2018) The ssessment of arsenic contamination in Urmia lake sediments and its effect on human health. J Environ Stud 45:485–497
Kumar V, Parihar RD, Sharma A, Bakshi P, Singh Sidhu GP, Bali AS, Karaouzas I, Bhardwaj R, Thukral AK, Gyasi-Agyei Y, Rodrigo-Comino J (2019) Global evaluation of heavy metal content in surface water bodies: a meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 236:124364. https://doi.org/10.1016/j.chemosphere.2019.124364
Li Y, Zhou Q, Ren B, Luo J, Yuan J, Ding X, Bian H (2019) Trends and health risks of dissolved heavy metal pollution in global river and lake water from 1970 to 2017. Rev Environ ContamToxicol 251:1–24
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97
Luo W, Lu Y, Wang T, Hu W, Jiao W, Naile J, Khim JS, Giesy J (2010) Ecological risk assessment of arsenic and metals in sediments of coastal areas of northern Bohai and Yellow Seas, China. Ambio 39:367–375
Madani K (2014) Water management in Iran: what is causing the looming crisis? J Environ Stud Sci 4:315–328
Madani K, AghaKouchak A, Mirchi A (2016) Iran’s socio-economic drought: challenges of a water-bankrupt nation. Iran Stud 49:997–1016
Malvandi H (2017) Preliminary evaluation of heavy metal contamination in the Zarrin-Gol River sediments, Iran. Mar Pollut Bull 117:547–553
Memoli V, Esposito F, Panico SC, De Marco A, Barile R, Maisto G (2019) Evaluation of tourism impact on soil metal accumulation through single and integrated indices. Sci Total Environ 682:685–691
MirzaeeMahmoodabadi R (2019) Evaluation of water quality and bed load sediment contamination of Qareaqhaj River to heavy metals, Kahfr Watershed, Fars province. J Environ Sci Stud 4(3):1696–1706
Mombeni HA, Rezaei S, Nadarajah S, Emami M (2013) Estimation of water demand in Iran based on SARIMA models. Environ Model Assess 18:559–565
Muller G (1979) Schwermetalle in den sediments des Rheins-Veran-derngrenseitt 1971. Umschan 79:778–783
Nafchi MA, Chamani A (2019) Physiochemical factors and heavy metal pollution, affecting the population abundance of Coenobitascaevola. Mar Pollut Bull 149:110494
Nasrabadi T, Bidhendi GN, Karbassi A, Mehrdadi N (2010) Evaluating the efficiency of sediment metal pollution indices in interpreting the pollution of Haraz River sediments, southern Caspian Sea basin. Environ Monit Assess 171:395–410
Nazarpour A, Watts MJ, Madhani A, Elahi S (2019) Source spatial distribution and pollution assessment of Pb, Zn, Cu, and Pb, isotopes in urban soils of Ahvaz City, a semi-arid metropolis in southwest Iran. Sci Rep 9:1–11
Noorbakhsh S, Sahraiian H, Soroosh MJ, Rezaii V, Fotoohi A (2011) Inventory of important pests, diseases, and weeds of the major agricultural crops: pesicides and recommended methods of application (in Persian). Ministry of Agriculture, Plant Protection Organization. p 197
Nourouzi M, Chamani A, Shirani M, Malekpouri P, Chuah A (2018) Effect of Cd and Pb pollutions on physiological growth: wavelet neural network (WNN) as a new approach on age determination of Coenobita scaevola. Bull Environ ContamToxicol 101:320–325
Office of Planning and Economy (2015) Agricultural statistics of Fars province. Publication of Fars Agricultural Organization, 197 pages
Ra K et al (2014) Assessment of pollution and ecological risk of heavy metals in the surface sediments of Ulsan Bay, Korea. Ocean Sci J 49:279–289
Saeedi M, Hosseinzadeh M, Rajabzadeh M (2011) Competitive heavy metals adsorption on natural bed sediments of Jajrood River, Iran. Environ Earth Sci 62:519–527
Salati S, Moore F (2010) Assessment of heavy metal concentration in the Khoshk River water and sediment, Shiraz, Southwest Iran. Environ Monit Assess 164:677–689
Sanayei Y, Ismail N, Talebi S (2009) Determination of heavy metals in Zayandeh Rood river, Isfahan-Iran. World ApplSci J 6:1209–1214
Sayadi M, Sayyed M, Kumar S (2010) Short-term accumulative signatures of heavy metals in river bed sediments in the industrial area, Tehran, Iran. Environ Monit Assess 162:465–473
Shanbehzadeh S, Vahid Dastjerdi M, Hassanzadeh A, Kiyanizadeh T (2014) Heavy metals in water and sediment: a case study of Tembi River. J Environ Public Health 2014:858720
Sheykhi V, Moore F (2013) Evaluation of potentially toxic metals pollution in the sediments of the Kor river, southwest Iran. Environ Monit Assess 185:3219–3232
Simpson S, Batley G (2016) Sediment quality assessment: a practical guide. Csiro Publishing, Clayton
Tomlinson D, Wilson J, Harris C, Jeffrey D (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländermeeresuntersuchungen 33:566
Vahid HD, Noori M, Heydari M, Sheikhi M (2016) An investigation into the qualitative and quantitative effects of climate change on rivers in Iran. Int J Rev Life Sci 6:6–13
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Yazdanpak A, Ostovan H, Hesami S, Gheibi M (2020) Determination of residue levels of pesticides (acetamipride, diazinon, imidacloprid, primicarb) in greenhouse tomato (Solanum lycopersicum) var. Izmir in Fars. J Anim Environ 11(4):289–296
Zhang M, He P, Qiao G, Huang J, Yuan X, Li Q (2019) Heavy metal contamination assessment of surface sediments of the Subei Shoal, China: spatial distribution, source apportionment and ecological risk. Chemosphere 223:211–222
Zhou Q, Yang N, Li Y, Ren B, Ding X, Bian H (2020) Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob EcolConserv 22:9–25
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahradnia, H., Chamani, A., Zamanpoore, M. et al. Heavy metal pollution in surface sediments of Ghareh-Aghaj River, one of the longest perennial rivers in Iran. Environ Earth Sci 80, 91 (2021). https://doi.org/10.1007/s12665-021-09384-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-09384-1