Abstract
This study aims to use the pollution index (PI) and integrated pollution index (IPI) to evaluate the heavy metals in the urban soil in Kirkuk governorate at north part of Iraq. The pollution indices are powerful tools for assessing the eco-toxicity which is very important issue that influences the ecosystems and human health. The heavy metals (Cr, Co, Ni, Cu, Zn, As, Ag, Cd and Pb) were investigated in soil samples collected from various land use categories including residential (RE), commercial (CM), industrial (IN), green space (GS), open space (OS) and roadside (RS). Heavy metals are distributed in the urban soils with content that has a range less than the average geochemical background, but exceeded the background by maximum values. High concentration of Ni, Cr and Co is observed in residential and open space soils. The integrated pollution index revealed that the industrial soil is highly polluted, roadside, commercial, and residential soil categories are of middle pollution level, whereas low pollution level was recorded only in the open and green space soils. Lead, silver, and zinc appear to be linked with industrial sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
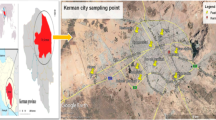
The term urban geochemistry describes the interface between environmental geochemistry and urban pollution (Thornton 1991), whereas the interactions between chemical elements and their compounds refer to urban environmental geochemistry. Soils in urban areas are affected by human activities, such as industry, transportation and housing as well as growing population growth. The urban soil represents not only as a sink of pollutants, but also as a source of heavy metals and contamination (Lu and Bai 2010). Heavy metals threaten both of human health and environmental safety (Baran and Wieczorek 2015) through the emission of chemical and toxic elements from the industrial agriculture and transportation activities (Wuana and Okieimen 2011). The behavior of heavy metals and impact on the quality of urban soil are not clear, due to the intense underground heterogeneity caused by strong anthropogenic activity and land use changes (Custodio 1997; Vidal et al. 2000; Grzebisz et al. 2002; Wong et al. 2006; Mohrlok and Schiedek 2007; Boschetti et al. 2014; Flem et al. 2017). Kirkuk city is located in the north of Iraq and considered as an oil-rich province (Abdur-Rakib and Bhuiyan 2014). The main manufacturing sectors in Kirkuk city are cement plant and oil refining plant. Airborne pollutants such as cement dust, vehicle exhausts and burning oil trash contribute to soil pollution. Most previous studies focused directly on the geochemical values for describing the pollution level. Anomalous concentrations of some heavy metals in the industrial quarter soils at Kirkuk city have been pointed out by Al-Jumaily (2009). The vehicle exhausts and oil trash burning were a source participated in rising some heavy metals in soil of Kirkuk city (Mohammed 2009). The Kirkuk Oil Refinery as source of air pollution impacted the soil of Kirkuk city with heavy metals and some organic compounds as well (Al-Dabbas et al. 2012). However, and generally, the geochemical background values of trace elements in Iraqi soils and recent sediments are high and tend to form positive anomalies (Al-Bassam and Yousif 2014). Over the past 2 decades, the population growth in Kirkuk city was increased, on an average, by 3% per annum. The population growth is expected to enhance from 1,050,000 in 2008 to 1,445,556 in 2020 (Omar et al. 2014). Consequently, the quantity of solid waste may increase with increasing population growth. In 2011, the generated daily waste is about 1000 tons, whereas may reach to 1200 tons in 2021 (Mustafa et al. 2013). These contributions may influence on soil quality. Approximately, 90% of the world’s population growth living in urban centers is estimated to occur in Africa and Asia, which currently stays mostly rural. Unplanned and rapid growth of cities has often led to unsustainable consumption of natural resources, pollution, and other toxic environmental impacts. For this reason, there is need to understand the sources, transport, transformations, human health impacts, and fate of chemicals in urban environments and their effect on the ecosystem. Six land use categories were targeted by this study: residential (RE), commercial (CM), industrial (IN), green space (GS), open space (OS) and roadside (RS) areas (Fig. 1). This research focuses on assessment the pollution level of heavy metals (chromium, cobalt, nickel, copper, zinc, arsenic, silver, cadmium and lead) in the urban soil and identifies factors controlling pollutants using pollution index (PI) and integrated pollution index (IPI).
Location and surface geology
The urban area of Kirkuk city is located 250 km north of Baghdad, the capital city of Iraq. Geographically, the study area is located in the northwestern part of Lailan sub-basin between latitudes 35° 23.099′N–35° 30.416′N and longitude 44° 19.688′E–44° 26.289′E, spreading over an area of 101.14 km2 (Fig. 1). The height of the study area is 329 m above sea level, the highest altitude (374 m) is in the northern part and the lowest elevation (294 m) is in the southern part. It has one hydrologic feature which is an ephemeral river named Khassa River. The study area as shown in Fig. 2 is covered by several geological outcrops of ages extended from Middle Miocene to the Recent. Accordingly, the cover lithology is varied. Anhydrite, gypsum and salt, interbedded with limestone and marl, are the main lithology composed of the Fat’ha Formation (M. Miocene). Successive beds of the Injana Formation (U. Miocene) with 900 m composed of gray sandstone and red siltstone that are mostly attributed to continental source with few lenses of limestone and gypsum at the lower part of the formation (Jassim et al. 1984) are exposed at the north part of the study area. The northern part of the study area is characterized by high permeability due to the porous-exposed formation. Porous materials are exposed represented by AL-Mukdadyia Formation (L. Pliocene) which comprised of 2000 m thick sandstone, mudstone, and gravel, with sequences of large conglomerate masses (Jassim and Goff 2006), so, it is a main aquifer in the study area. Pleistocene and Recent sediments with a thickness of 120 m covered the main study area. Sheet run-off sediments comprised of clay, silt and sand and less gravels cover a flat area. Poorly cement Terraces River along the Khassa River consist of gravels, sands and clays cross the study area from the northeast to the southwest.
Geological map showing a Khassa River cuts through the study area (Bander and AL-Jumaily 1993)
Materials and methods
Systematically, soil samples were collected with a density of one sample per square kilometer and described based on land use category including 75 surface samples, 15 subsoil samples and 6 samples from rural soil. Surface soils were taken from the uppermost layer of the soil profile (0–20 cm), subsoil was taken from 35 to 50 cm depth and rural soil samples were collected taking in consideration the prevalent wind direction. The rural soil and subsurface soil denote as a reference for the comparison with the urban surface soils. Soil samples were collected using a stainless steel spade. The agglomerates of soil samples were dismantled by a plastic hammer, air dried, and then sieved through 0.5 mm. The metal content may increase with decreasing particle size of soil (Acosta et al. 2009), the samples were collected from the uppermost layer of soil in which the significant accumulation of heavy metals takes place at coarse fractions (0.22–0.5 mm) (Maslennikova et al. 2012).
Samples ware digested at the laboratory of Soil Mechanics in the Kirkuk Construction Lab. The microwave-assisted aqua regia digestion method based on the method 3051A designed by U.S. EPA (2007) was used for sample digestion. For this reason, samples were weighed to 0.1 g and transferred to digestion vessels, which consisted of 10 mL of aqua regia (HCl:HNO3, 3:1, v/v) (McGrath and Cunliffe 1995), and left to sit under reflux conditions (Abbruzzini et al. 2014). The block was gradually heated from ambient temperature to 180 °C and the samples were processed until 1 mL of acid remained. The digests were diluted by 10 mL of distilled water and filtered by cellulose filter paper (pore diameter of 0.45 µm). The average recovery rates of heavy metals in the samples are Cr (89%), Co (90%), Ni (96%), Cu (91%), Zn (94%), As (90%), Cd (89%) and Pb (96%), and within the acceptable limits (Nemati et al. 2010). The concentration of heavy metals (Cr, Co, Ni, Cu, Zn, As, Ag, Cd and Pb) were measured using Inductively Coupled Plasma Mass Spectrometry (ICP-MS). The pH value of soil samples was measured by adding 2 g of soil in a 50 mL polycarbonate centrifuge tube contained 20 mL of deionized water and shacked for 16 h (Howard et al. 2013), 20 °C, on rolling table. Soil suspension was separated by high-speed centrifugation for 30 min at 10,000 rpm. An electrode of pH meter was inserted into the sample solution and taking the measurement. Chemical analyses including soil pH were conducted in the laboratory of Water Chemistry, Department of Hydrogeology-TU Bergakademie Freiberg, Germany. For the analytical accuracy, ICP multi-element standard solutions IV (23 elements in diluted nitric acid) 1000 mg/l: Ag, Al, B, Ba, Bi, Ca, Cd, Co, Cr, Cu, Fe, Ga, n, K, Li, Mg, Na, Ni, Pb, Sr, Tl, and Zn were prepared with the same acid mixture used for sample dissolution (U.S. EPA 2007; EPA 3051), and the analytical precision is reliable and is acceptable as pH (96.2), Cr (95.3), Co (94.2), Ni (98.6), Cu (97.2), Zn (97.2), As (96.1), Ag (94.1), Cd (92.8), and Pb (95.9)% (Table 1). Maps in Figs. 1 and 2 were drawn using Arc GIS.
The upper thresholds are calculated according to median values, standard deviation (STD) and median absolute deviation (MAD) for all studied heavy metals. Upper threshold values are determined using the formula of median + 2MAD, which is more suitable for estimating geochemical background ranges (Reimann et al. 2005). The assessment of the soil quality was done by calculating the pollution index (PI) for each heavy metal and an integrated pollution index (IPI) for each sample. The PI was defined according to the following formula:
where Cn (mg/kg) is the measured concentration of the analyzed metal and Bn is geochemical background for the analyzed metal. The PI of each metal was calculated and compared as either low if it has PI ≤ 1, moderate if it has 1 < PI ≤ 3 or high contamination, if it has PI > 3. The IPI, which is a mean value of the PIs, is classified as low (IPI ≤ 1), middle (1 < IPI ≤ 2) and high (IPI > 2) pollution levels (Chen et al. 2005). The climatic dataset over 30 years for the period 1983–2013 were collected from the Kirkuk Meteorological Station.
Wind direction helps to interpret the pollutant transporting. The predominant wind direction was defined through the wind rose diagram, by which the suitable sites for sampling rural soil far from the impact of the oil trash burning was determined.
Results and discussion
Pollutants and soil quality
The statistical results including minimum, maximum, mean and median are inserted in Table 2. The median values were used to compare the content of heavy metals in each soil category with the global averages.
It is noted that median values of chromium in the urban top soils are high (× 1.4) compared to the global average and ordered as RE > IN > GS > OS > CM > RS, the marginal enrichment in RE areas is of anthropogenic impact. Cobalt concentrations in the urban soil show marginal elevated relative to the global soils (× 1.1). The soil categories are ordered as OS > GS > RE > IN > CM > RS showing high content of cobalt exists mainly in OS and GS relative to the other soil categories. Fossil fuel combustion products are the source of cobalt in the OS areas (Smith and Carson 1981); therefore, the burning of oil trash in the Kirkuk oil refinery is one source of soil pollution. The addition of the cobalt-bearing sludge and phosphate fertilizers to the soil are potential sources for further cobalt contamination in the GS, particularly close to the Khassa River (Smith and Carson 1981), where the agricultural area is irrigated by wastewater originated from the surrounding RE area. The median value of nickel in the urban area is 4.8 times compared to the global soils. The high content of nickel in OS and GS is resulted from emissions of the oil trash burning and the irrigations of local agricultural area using wastewater, particularly, the areas close to the Khassa River. Regarding the copper content, the soil categories have been arranged as IN > OS > RS > CM > RE > GS indicating that the soil of IN category has a higher content, while the low content has been recorded in the GS soil. The highest content of copper detected in the IN category is attributed to the anthropogenic action originated from the automobile workshops. The sources of Zn in the urban environment particularly in IN and RS areas are mainly sourced from the automobile tires that are lost to the road surfaces by abrasion (Councell et al. 2004), and partly attributed to the combustion exhaust (Hildemann et al. 1991), galvanized parts and railings (Legret and Pagotto 1999), fuel and oil and brake linings (Pierson and Brachaczek 1974). Zinc in the urban soil has concentration (52 mg/kg) lower than those in the global soil (70 mg/kg) indicating no pollution in the soil categories that are accordingly ordered as IN > RS > RE > CM > OS > GS. Arsenic median values of the urban soil categories are slightly lower than that in the world soils (× 0.9). However, the OS soil category has high arsenic content as clearly shown compared to the other soil categories (OS > GS > RE > IN > CM > RS). The reason of the presence of arsenic in OS is a geogenic and derived from the parent materials rather than anthropogenic sources. The presence of arsenic in GS and RE arises from arsenic-containing pesticides and herbicides that are added to the agricultural areas. The content of silver in the urban soil is 0.6 time lower than that in the global soils. Its highest content was recorded in IN soil category, whilst the lowest was recorded in GS soil category. The soil categories are arranged sequentially as IN > RE = CM > OS = RS > GS according to the silver content in the study. The cadmium content is low by x0.6 time compared to the world averages. The RE and OS soil categories have the highest cadmium content relative to other soil categories that are ordered (RE = OS > CM > IN = GS > RS). Cadmium is derived from domestic wastewater and the Kirkuk oil refinery (Al-Dabbas et al. 2012). The land use soils are arranged as IN > RS > CM > RE > OS > GS in terms of lead which recorded in the urban soils as 0.6 times lower than that in the world averages. The relative high lead concentration in the IN soil categories is attributed to be sourced from the industrial activities. Metal workshops, garages and waste dumps are potential sources of lead (Al-Jumaily 2009). In the RS soil category, the automobile exhausts from heavy traffic and highway, as well as oil trash burning of Kirkuk oil refining are the most potential sources (Al-Dabbas et al. 2012).
Climatic effect on pollutants
Climate indicates an average state of weather conditions of selected location over a long period of time (Houghton et al. 2001). The climate of Iraq, in general, is characterized by a hot summer that lasts for 4 months or more, and a cold winter lasts for 3 months, while spring and autumn are two transitional seasons almost short. The period of 30 years (1984–2014) was considered sufficient to understand and interpret the behavior of heavy metals in the urban environment, and accordingly, the climate dataset was collected from the Kirkuk Meteorological Station (IMOS 2014) The monthly averages of climatic elements are listed in Table 3 and plotted as shown in Fig. 3. The air temperature ranged from 9.6 °C in January to 36.2 °C in July, while rainfall of 66 mm occurs in January and 158 mm in November of 1992. The chemical weathering act in winter is due to the seasonal precipitation; weathering causes breakdown and alteration the materials on surface. The disintegration by physical weathering increases the reactive surface area and thus, facilities the decomposition by chemical reaction with abundant water, oxygen and CO2. As the study area is semi-flat terrain, the physical weathering is not active. In contrast with physical processes, the chemical agents are of powerful attack on rock and minerals, and the precipitation controls the water amount available for chemical weathering, while temperature influences the rate of chemical reaction (Rose et al. 1979). The lack of activity of chemical processes due to lack of water in the dry seasons with the alkaline pH of the soil contributed effectively to the accumulation of heavy metals in the top soil and no leached. The scarcity of precipitation with the rise in temperature effected as an attenuation agent in dilution groundwater ions and consequently, heavy metals may accumulate in soil and leaches partially for a short subsurface depth (Kumar et al. 2013). Evaporation exceeds to 420 mm in July, while the lowest average is 50.3 mm in January. The results came harmonized with both of temperature and rainfall. The relative humidity ranges from 23% in July to 72% in January. The wind speed is positively correlated to air temperature and evaporation, and negatively to relative humidity, characterizing by the lowest value in December (1.3 m/s), whist increase in May and reach to 2.1 m/s. The average prevailing wind direction in the study area duration in the period 1980–2013 is NW, followed by NE and SW, and the detailed seasonal wind direction is illustrated in Fig. 4. The role of the wind speed and its direction is an example for transferring the pollutants as suspended particulates, dust and smoke of automobile exhausted that may play a significant role in the transfer of pollutants and change the soil chemistry. Some areas of the OS and GS have been affected by the materials transferred from the oil refinery and cement factory by the wind, and here, emerged the role of speed and direction of the wind in the redistribution of the heavy metals and change the soil chemistry. The study area was regionally affected by drought and locally by land uses. The mineral solubility in the wet climate is typically active, resulting in a redistribution of the elements after their dissolving. As a result, the elements may be leached from the top soil leaving low concentration. The increase in temperature results in the speed up of evaporation and concentration of solutions, which has a significant role in precipitation processes.
Soil pH effect on pollutants
The pH plays a major role in the process of redistribution of heavy metals because it has an important role in the processes of precipitation and dissolution as well (Al-Hamdani et al. 2016; Sun and Chen 2016). The acidity–alkalinity of the soil varied to include a wide range of pH ranging from 6.63 to 9.27. Based on the pH value, the soil acidity–alkalinity is classified into five classes including natural, slightly alkaline, moderately alkaline, strongly alkaline and very strong alkaline (Table 4). It was shown that soil in different study areas mostly tend to be of a slightly and moderately alkaline. The RE soil category is mainly characterized by slightly and moderately alkaline; a 73% of the RE soil category is slightly alkaline and 27% is moderately alkaline (Fig. 5). The CM soil category consists of 55% as a slightly alkaline and 36% as a moderately alkaline and 9% as a strongly alkaline. The IN soil category is composed only of 63% as a slightly alkaline and 38% as a moderately alkaline, whereas the GS soil category consists of a 4% as neutral, 29% as a slightly alkaline and 68% as a moderately alkaline. The OS category is composed of 67% slightly alkaline and 33% moderately alkaline. The different category is RS in which 7% neutral, 21% slightly alkaline, 64% moderately alkaline and 7% very strong alkaline. The soil categories studied have a wide range of pH, but are often ranging from 7.4 to 8.4, so as the leaching process expected very low, where the heavy metals tend to be precipitated. The pH of the soil system is a very important parameter, directly influencing sorption/desorption, precipitation/dissolution, complex formation, and oxidation–reduction reactions. The maximum dissolution of cationic metals occurs at acidic pH (less than 7), and maximum precipitation of anionic metals occurs at pH less than 7 (Bołzan 2017). All heavy metals are precipitated from only under alkaline conditions in the form of hydroxide, oxide, carbonate, and phosphate (Lindsay 1979). For example, copper is removed from solution at pH > 7, but released to the solution at acidic conditions. Here, we can deduce that the alkaline soils retain heavy metals as opposed to acidic and neutral soils that may lose such ions. In general, running water may find its way into aquifer, particularly, in the north part of the study area due to the high permeability, and a lot of heavy metal contents are adsorbed to the soil in response to the alkaline pH. Twenty-eight groundwater wells distributed in 630 Km2 at Kirkuk area are characterized by heterogeneity of water chemistry due to the run-off influences. Running water on the surface is directly recharged the underneath aquifer in the study area (Awadh and Al-Kelabi 2016).
Geochemical background and soil quality
The geochemical background values represent the concentration of a given element in a given area which are completely dependent on the compositional and mineralogical characteristics of the parent source geological material. Threshold is the peak of the anomaly that locates spatially limited areas characterized by extremely high concentration values due to a point source which could be mineralization or pollution (Albanese 2014). The threshold and geochemical background values of heavy metals are calculated and presented in Table 5. The calculation of the geochemical background has followed the method stated by Rose et al. (1979). The results of PI and IPI are tabulated in Table 6 and presented in Fig. 6. The PI indicated that the soil of the IN soil category is the most polluted (2.71), whereas soil of the RS, CM, and RE categories are of moderate polluted as they have PI values as 1.09, 1.08, and 1.07, respectively. Additionally, the PI values also pointed out the OS and GS soil categories as of low polluted soil due to the low values of the PI (0.91 and 0.83), respectively. Based on the PI value, all categories of the soil studied have low polluted with each of chromium, cobalt, nickel, and arsenic. In terms of zinc, silver, cadmium and lead, the values of PI showed that copper was moderately polluted for each soil of RE, CM, IN, and RS categories. As for silver, it has moderately polluted each of RE, CM, GS, OS, and RS, while lead is moderately polluted the CM, GS and OS. The important thing is that high pollution level of Ag in the IN soil category and lead in both of the IN and RS as well. The values of PI higher than 3 pointed out high contents of lead and silver in the urban area. The land use categories are affected by heavy metals with respect to IPIs descending as IN > RS > CM > RE > OS > GS. The high level of pollution was diagnosed in area of IN soil category. The areas of the RS, CM, and RE soil types were marked as a soil of middle pollution level, but the low pollution level was restricted in the OS and GS categories. The values of the IPIs indicated the levels of pollution in each category of soil. The IPIs values varied from 0.66 to 2.83 with an average of 1.07 in the RE soil category indicated medium level pollution in places specifically close to the Kirkuk Citadel location (Ctd). The PI value indicated a high contamination with silver and moderate contamination with copper, silver, lead, and zinc. These heavy metals considered an important factor in soil pollution (Martin 2001; Nicholson et al. 2003). In area of RE, atmosphere plays an important role in transferring the pollutants from different source points such as cement manufacture in Laylan Cement Plant, fossil fuel combustion, oil trash burning from Kirkuk Oil refining plant, and municipal waste burning.
Conclusions
This study discussed the concentrations of heavy metals in the different categories of the urban soil. In general, the study usually represented an area of high background of the heavy metals ascribed to both the nature of soils and human beings. The content of heavy metals in these categories is distributed in a heterogeneous manner depending on the supplying sources, harmonized with the nature of the land use. Nickel is predominant heavy metal distributed according to the land use category with a pattern of OS > GS > RE > CM > IN > RS. The predominant heavy metals in the OS soil category are nickel, cobalt and arsenic; in the RE types are chromium, cobalt and nickel; in the IN categories are copper, zinc, lead, Cd and silver. The urban OS soil is mainly affected by emission of the oil trash burning, whereas the GS soil is affected by wastewater that is used for the irrigation purpose of the local agricultural area, particularly, areas close to the Khassa River. The pollution of the IN urban soil is primarily derived from automobile and metal workshops and their association waste. The soil reaction is moderately to slightly alkaline, so the leaching process of heavy metals is unlikely to occur. In contrast, the heavy metals tend to be precipitated under the urban conditions. The geochemical background values as a baseline of the Kirkuk urban soil types were determined. Based on the PI value, the urban soil categories are heavily polluted by lead, but they are moderately polluted by cadmium. Based on the IPI, the IN soil was categorized as a high-polluted soil, the RS, CM, and RE soil types were defined as soil of middle pollution level and the OS and GS soil type is of low pollution level. The current study displays high geochemical backgrounds of the studied heavy metals that are quite different than other places in some countries. The authors accordingly recommend a comparative study to determine the role of geogenic and the influence of anthropogenic factor.
References
Abbruzzini TF, Silva CA, Andrade DA, Carneiro WJ (2014) Influence of digestion methods on the recovery of iron, zinc, nickel, chromium, cadmium, and lead contents in 11 organic residues. Rev Bras Ciência Solo 38(1):166–176. https://doi.org/10.1590/S0100-06832014000100016
Abdur-Rakib Md, Bhuiyan MAH (2014) Arsenic contamination: food toxicity and local perception. Int J Sci Res Environ Sci 2(1):1–7
Acosta JA, Cano AF, Arocena JM, Debela F, Martínez-Martínez S (2009) Distribution of metals in soil particle size fractions and its implication to risk assessment of playgrounds in Murcia city (Spain). Geoderma 149(1–2):101–109
Albanese S (2014) Determination of the geochemical background and baseline in the soils of Campania region: an overview. Ecoremed, Napes, pp 1–25
Al-Bassam KS, Yousif MA (2014) Geochemical distribution and background values of some minor and trace elements in Iraqi soils and recent sediments. Iraqi Bull Geol Min 10(2):109–156
Al-Dabbas MA, Ali LA, Afaj AH (2012) The effect of Kirkuk oil refinery on air pollution of Kirkuk city-Iraq. In: Proceeding of the 1st Conference on Dust Storms and their environmental effects, pp 8–18
Al-Hamdani JA, Awadh SM, Ibrahim OS (2016) Geochemical partitioning of heavy metals in the urban soil, Kirkuk, Iraq. Iraqi Geol J 39(1):1–24
Al-Jumaily HA (2009) Geochemical evaluation of heavy metals pollution of industrial quarter soils at Kirkuk city. Northern Iraq. J Kirkuk Univ Sci Stud 4(1):1–11
Awadh SM, Al-Kelabi JA (2016) Assessment of groundwater quality using water quality index in Al-Hawija area, Northern Iraq. Iraqi Geol J 39–49(1):67–76
Bander FM, Al-Jumaily RM (1993) Geological Map of Iraq GM 9, Kirkuk Block, Sheet NI-38-2, Library of the Geological Survey and Mineral Investigation, Baghdad, Iraq
Baran A, Wieczorek J (2015) Application of geochemical and ecotoxicity indices for assessment of heavy metals content in soils. Arch Environ Prot 41(2):53–62
Bołzan BD (2017) Effect of pH and soil environment. World News Nat Sci 8:50–60
Boschetti T, Falasca A, Bucci A, De Felice V, Naclerio G, Celico F (2014) Influence of soil on groundwater geochemistry in a carbonate aquifer, Southern Italy. Int J Speleol 43(1):79–94. https://doi.org/10.5038/1827-806X.43.1.8
Chen TB, Zheng Y, Lei M, Huang ZC, Wu HT, Chen H, Fan KK, Yu K, Wu X, Tian QZ (2005) Assessment of heavy metal pollution in surface soils of urban parks in Beijing, China. Chemosphere 60:542–551
Councell TB, Duckenfield KU, Landa ER, Callender E (2004) Tire-wear particles as a source of zinc to the environment. Environ Sci Technol 38:4206–4214
Custodio E (1997) Groundwater quantity and quality changes related to land and water management around urban areas: blessings and misfortunes. In: Chilton J et al (eds) Groundwater in the urban environment: problems, processes and management. Balkema, Rotterdam, pp 11–22
Flem B, Eggen OA, Torgersen E, Kongsvik MK, Ottesen RT (2017) Urban geochemistry in Kristiansand, Norway. J Geochem Explor 187:21–33 (in press)
Grzebisz W, Ciesla L, Komisarek J, Potarzycki J (2002) Geochemical assessment of heavy metals pollution of urban soils. Pol J Environ Stud 11(5):493–499
Hildemann LM, Markowski GR, Jones MC (1991) Submicrometer aerosol mass distributions of emissions from boilers, fireplaces, automobiles, diesel trucks, and meat cooking operations. Aerosol Sci Technol 14:138–152
Houghton JT, Ding Y, Griggs D, Noguer M, Van der Linden P, Xiaosu D (2001) Climate change 2001: the scientific basis—contribution of working group I to the third assessment report of the intergovernmental panel on climate change (IPCC). Cambridge Univ. Press, New York, p 944
Howard JL, Dubay BR, Daniels WL (2013) Artifact weathering, anthropogenic microparticles, and lead contamination in urban soils at former demolition sites, Detroit, Michigan. Environ Pollut 179:1–12
IMOS (2014) Draft: national science and implementation plan 2015–25. imos.org.au/plansreports
Jassim SZ, Goff JC (2006) Geology of Iraq, 1st edn. Czech Republic, Isban, p 341
Jassim SZ, Karim SA, Basi M, Al-Mubarak MA, Munir J (1984) Final report on the regional geological survey of Iraq. Stratigraphy, Vol.3. Library of the Geological Survey and Mineral Investigation, Baghdad, Iraq
Kumar P, Wiltshire A, Mathison C, Asharaf Sh, Ahrens B, Lucas-Picher Ph, Christensen JH, Gobiet A, Saeed F, Hagemann S, Jacob D (2013) Downscaled climate change projections with uncertainty assessment over India using a high resolution multi-model approach. Sci Total Environ 468–469:18–30
Legret M, Pagotto C (1999) Evaluation of pollutant loadings in the runoff water from a major rural highway. Sci Total Environ 235:143–150
Lindsay WL (1979) Chemical equilibria in soils. John Wiley and Sons, New York
Lu SG, Bai SQ (2010) Contamination and potential mobility assessment of heavy metals in urban soils of Hangzhou, China: relationship with different land uses. Environ Earth Sci 60:1481–1490
Martin L (2001) Urban land use influences on heavy metal fluxes and surface sediment concentrations of small lakes. Water Air Soil Pollut 126:363–383
Maslennikova S, Larina N, Larin S (2012) The effect of sediment grain size on heavy metal content. Tyumen State University Tyumen, Russia
McGrath SP, Cunliffe CH (1995) A simplified method for the extraction of the metals in sewage sludge on soils, microorganisms and plants. J Ind Microbiol 14:94–104
Mohammed FA (2009) Pollution caused by vehicle exhausts and oil trash burning in Kirkuk city. Iraqi J Earth Sci 9(2):39–48
Mohrlok U, Schiedek T (2007) Urban impact on soils and groundwater—from infiltration processes to integrated urban water management. JSS J Soils Sediments 7(2):68
Mustafa SS, Mustafa SS, Mutlag AH (2013) Kirkuk municipal waste to electrical energy. Int J Electr Power Energy Syst 44(1):506–513
Nemati K, Bakar NKA, Abas MRB, Sobhanzadeh E, Low KH (2010) Comparative study of open system digestion and microwave assisted digestion methods for metal determination in shrimp sludge compost. J Hazard Mater 182:453–459
Nicholson FA, Smith SR, Alloway BJ, Carlton-Smith C, Chambers BJ (2003) An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci Total Environ 311:205–219
Omar NQ, Ahamad MSS, Hussin WM, Samat N (2014) Modelling land-use and land-cover changes using Markov-CA, and multiple decision making in Kirkuk city. Int J Sci Res Environ Sci 2(1):29–42
Pierson WR, Brachaczek WW (1974) Airborne particulate debris from rubber tires. Rubber Chem Technol 47:1275–1299
Reimann C, De Caritat P (1998) Chemical elements in the environment—factsheets for the geochemist and environmental scientist. Springer-Verlag, Berlin
Reimann C, Filzmoser P, Garrett RG (2005) Background and threshold: critical comparison of methods of determination. Sci Total Environ 346:1–16
Rose AW, Hawkes HE, Webb JS (1979) Geochemistry in mineral exploration, 2nd edn. Academic Press, London, p 658
Smith HIC, Carson BL (1981) Trace metals in the environment. Ann Arbor Science Publishers, Ann Arbor
Soil Survey Division Staff (SSDS) (2017) Soil survey manual, Handbook No. 18. United States Department of Agriculture, USA
Sun R, Chen L (2016) Assessment of heavy metal pollution in Topsoil around Beijing Metropolis. PLoS One 11(5):e0155350. https://doi.org/10.1371/journal.phone.0155350
Thornton I (1991) Metal contamination of soils in urban areas. In: Bullock P, Gregory PJ (eds) Soils in the urban environment. Blackwell, Oxford, pp 47–75
U.S. EPA (2007) Method 3051A (SW-846): microwave assisted acid digestion of sediments, sludges, and oils, Revision 1. Washington, DC
Vidal M, Melgar J, Lopez A, Santoalla MC (2000) Spatial and temporal hydrochemical changes in groundwater under the contaminating effects of fertilizers and wastewater. J Environ Manage 60:215–225
Wong SC, Li X, Thornton I (2006) Urban environmental geochemistry of trace metals. Environ Pollut 142:1–16p
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int Sch Res Netw Ecol. https://doi.org/10.5402/2011/402647 (ID 402647)
Acknowledgements
The authors would like to thank Prof. Dr. Broder J. Merkel and Dr. Nicolai-Alexeji Kummer for their assistance with sample analyses. Ministry of Higher Education and Scientific Research for the scholarship of twining program, General Commission for Groundwater for the financial support. The authors are also thankful to the reviewers for giving their fertile comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Awadh, S.M., Al-Hamdani, J.A.J.M.Z. Urban geochemistry assessment using pollution indices: a case study of urban soil in Kirkuk, Iraq. Environ Earth Sci 78, 587 (2019). https://doi.org/10.1007/s12665-019-8615-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8615-3