Abstract
Knowing the characteristics and relationships that are important in the accumulation and transfer of heavy metals among native wild plants is beneficial for screening potential accumulator plants and guiding remediation. In this research, nine native wild plants were collected from the Xikuangshan mine in Hunan Province, and the content of heavy metals, bioconcentration factors and translocation factors were determined via analysis. The results showed that the plant rhizosphere was polluted with heavy metals, especially Sb, Hg and Cd. P. aquilinum showed a strong ability to take up and transfer As, Cd, Pb and Zn. I. cylindrica effectively removed Sb from soils, P. vittata removed As and Cd, and D. erythrosora removed Hg and Cd. Native wild plants showed cooperativity to accumulate Cd, Cr, Pb and Zn, similar to As–Cd, As–Cr and As–Pb. There was a significant positive correlation for translocation in Hg–Cd, Hg–Zn, Hg–Pb, Cd–Pb, Cd–Zn, Zn–Cr and Zn–Pb. Knowledge of the ability of native wild plants to take up and transfer heavy metals is useful in screening for potential phytoremediation, and identifying the relationship between heavy metals accumulation and transfer among species will guide the selection of multiple heavy-metal remediation plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many sites around the world are polluted by mining, and heavy metals pollution is a global disaster (Chehregani et al. 2009) and a serious environmental problem to human health and the ecosystem (Brunetti et al. 2009). Heavy metals pollution may result from mining and smelting (Cheng 2003a; Rodríguez et al. 2009), which pose a danger to plants growing in the area (Qian et al. 1996). Contaminated soils pose a serious risk to soil–plant systems. Mining activity discharges wastes into the surrounding environment, and anthropogenic emission of heavy metals has led to significant soil and water pollution (Cheng 2003a). The waste rock and tailings contain high levels of heavy metals and pose a potential risk to the surrounding environment (He 2007; Wang et al. 2010). Tailings spread through air and water, becoming a primary polluted source to the surrounding soil and water (Wiseman et al. 2013). Although plants pose a potential health risk when they accumulate heavy metals (Barthwal et al. 2008), they are suitable for phytoremediation due to the uptake and transferability of these heavy metals.
Phytoremediation is an effective way to remove heavy metals from soils (Chen et al. 2002). Identifying and selecting suitable native wild plant species that tolerate heavy metals is an effective route for phytoremediation. The ability of plants to accumulate heavy metals varies by species (Brunetti et al. 2009). Plants have different physiological responses to heavy metal contamination, while in turn, affected plants accumulate heavy metals (Solís et al. 2005; Guala et al. 2010). Most plants are sensitive to low heavy metal content, while some have high tolerance, and a few plants show hyperaccumulation of some heavy metals (Zacarías et al. 2012). As good candidates for phytoremediation, wild and cultivated plants have been widely used for phytoremediation (Maric et al. 2013). Native wild plants usually have high bioproductivity and easy disposal, which make wild plants most useful in remediating heavy metals onsite (Cheng 2003b).
In the Xikuangshan (XKS) subarea, large amounts of waste rock and tailings have been produced from mining (Fu and Wei 2013). In the study site, the heavy metal content in soils and plants posed different risks to human beings and the surrounding environment (Lei et al. 2005; Zhuang et al. 2009). In the mine subarea, the heavy metal content in wild plants was relatively high and variable (Gonzalez and Gonzalez-Chavez 2006), with different tolerance native wild plants that can be used in phytoremediation. Although the content of heavy metals in that area was high, many wild plant species were still able to grow well (Del Rio et al. 2002). Many recent studies have been published on this subarea, including studies of heavy metals distribution in soils and vegetation that accumulated heavy metals (Brunetti et al. 2009; He 2007; Xue et al. 2014; Zhou et al. 2012), and the ability of native wild plants to accumulate heavy metals was rarely identified. The relationships determining the accumulation and transfer between species are still unclear. Knowledge of the relationships that are important for the heavy metal content as well as the accumulation capacity of different species helps in finding proper methods of phytoremediation. The aim of the study is to analyze the relationships relevant to heavy metal accumulation in wild plant species. This work reports on the phytoremediation ability of native wild plants growing around the Xikuangshan subarea to identify the relationship of heavy metal accumulation and translocation between species and to provide a convincing route for the screening of phytoremediation species in mining areas.
Materials and methods
Study area
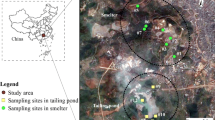
The Xikuangshan antimony (Sb) mine is the largest Sb mine in the world, located in Hunan Province, with geographic coordinates of 27.7°N and 111.4°E. The mine has been exploited since 1897, and large-scale Sb mining/smelting activities have resulted in Sb and other heavy metals pollution of the local soils, water, and plants. Annual rainfall and average temperature is 1354 mm and 16.7 °C.
Sampling and analysis
The dominant native wild plants selected for the sample in the forest subarea (FA), vegetation subarea (VA) and mine subarea (MA) were as follows: Imperata cylindrica—I. cylindrica, Rumex patientia—R. patientia, Fagopyrum dibotrys—F. dibotrys, Erigeron annuus (L) Pers—E. annuus and Oplismenus undulatifolius(A) Bea—O. undulatifolius, collected in MA; Pteriduum aquilinum var. latiusculum—P. aquilinum and Phytolacca americana—P. americana, collected in VA; and Dryopteris erytbrosora—D. erythrosora, R. patientia, F. dibotrys, and Pteris vittata, collected in FA (Fu et al. 2016). A total of nine plant species were sampled for laboratory analysis. Plants were divided into two parts, roots and shoots, and were washed with tap water three times and rinsed with deionized water. The cleaned plants were placed in a convection furnace at 60 °C for 5 days to dry. After drying, the roots and shoots were weighed and a hand mill was used to grind, and the samples were sieved through a 0.6-mm mesh for further analysis. On average, approximately 1 kg plant rhizosphere was collected at each of the geocoded plots, which was sent to bagging and delivered to the laboratory. In the laboratory, plant rhizospheres were air-dried and sieved through a 0.6-mm mesh for further analysis.
An electric hot plate digester was used to digest soil and plant samples; 0.1 g soil sample powder was placed in the tubes, and 6 mL of HNO3 and 2 mL of HCl were added. Then, 0.5 g plant sample powder in the tube was mixed with 10 mL HNO3 and 3 mL of H2O2 for digestion. All tubes were heated at 120 °C for approximately 3 h. A hydride generation atomic fluorescence spectrometer (AFS; Beijing Titan Instruments Co., China) was applied to measure the total concentration of As, Sb and Hg, and inductively coupled plasma optical emission spectroscopy (ICP-OES; PE, USA) was applied for Pb, Cd, Cu and Zn measurement (Fu and Wei 2013; Wei et al. 2011). Blanks, soil (GBW07406) and plant standard reference materials (GBW07604) were used to verify the accuracy of the analysis.
All statistical analysis was performed using SPSS 19. Correlation analysis, cluster analysis and principal component analysis (PCA) were applied in this study to identify the relationships between the heavy metal content in soils and plant rhizospheres, BCFs and TFs.
Results
Heavy metals containment in plant rhizosphere
Table 1 shows the testing of the heavy metal content in plant rhizospheres; the mean content of As, Sb, Hg, Cd, Cr, Pb and Zn in plant rhizospheres were 15.24, 299.00, 1.49, 5.55, 46.58, 72.88 and 253 mg kg−1, respectively. The highest content, with 66.32 mg kg−1 for As, was found in the MA subarea, and the lowest content, with 0.88 mg kg−1 for As, was found in the VA subarea. The Sb content in the MA subarea was very high, ranging from 13.1 to 977 mg kg−1. Hg ranged from 0.09 mg kg−1 in FA to 5.79 mg kg−1 in MA. The content of Cd varied from 0.2 mg kg−1 in VA to 19.17 mg kg−1 in MA. The MA subarea showed the highest Pb content, with 129 mg kg−1, while the VA subarea showed the lowest values, at 5.79 mg kg−1. The highest and lowest content of Zn were 659 mg kg−1 in the MA subarea and 16.31 mg kg−1 in the FA site, respectively. The heavy metal contents in MA and FA were larger than the background of Hunan Province except for Cr, and the contents of As, Cr, Pb and Zn in the VA were less than the background of the province (Fu and Wei 2013).
Heavy metal containment in plants
Plants can potentially accumulate heavy metals from the surrounding environment to levels higher than those of the soil (Yoon et al. 2006). The ability of plants to accumulate heavy metals varies with species. The dominating plants at each study site have been sampled and analyzed. The results, as shown in Table 1, showed that the dominating plant species were I. cylindrica, F. dibotrys and R. patientia, while P. americana was dominant in the VA subarea. The heavy metal content in plants is shown in Table 2. Among the collected plants, the As content in plants ranged from 0.89 mg kg−1 (I. cylindrica shoot) to 119 mg kg−1 (P. vittata root). The content in Sb, ranging from 11.86 (D. erythrosora root) to 363 mg kg−1 (P. aquilinum shoot), was higher than in As and other heavy metals. The lowest Hg content was in P. americana roots, while the highest content was in P. vittata roots. The highest and lowest contents of Cd were measured in P. americana shoots and E. annuus shoots, respectively. The lowest content of Pb and Zn presented in F. dibotrys roots, while the largest were in E. annuus roots and D. erythrosora roots. F. dibotrys shoots contained the most Cr, and R. patientia roots contained the least Cr. Compared with the background in Hunan Province, the contents of Sb, Hg and Cd in each plant part were higher, but the content of Cr was lower. The content of Pb was higher than in the background in D. erythrosora roots, E. annuus roots and I. cylindrica roots, and the content of Zn in O. undulatifolius shoots, R. patientia roots and F. dibotrys roots was lower than the background. Their contents were not as high as in the plant rhizosphere.
Accumulation and translocation of metals in plants
Table 2 shows that only As in P. vittata was above 100 mg kg−1 and reached 119 mg kg−1 as its highest adsorbability to As (Chen et al. 2002). Although the other species did not show such high content, their ability to tolerate and accumulate heavy metals is very high. We can identify the plant’s ability to accumulate heavy metals from soils by BCFs, which is defined as the ratio of metal content in plant parts to that in the soil. The ability of metals translocating from roots to shoots is assessed by TFs, which is defined as the ratio of shoot content to root content. Table 3 shows the results of TFs and BAFs, and bold figures in the table showed whether there is a good ability to accumulate and transform heavy metals. For accumulation ability, P. vittata showed high BCFs for all measured heavy metals, but the other species selectively accumulated heavy metals. P. aquilinum presented high BCFs values with As, Cd, Cr, Pb and Zn, P. americana with Cd, Cr, Pb and Zn, D. erythrosora with Hg, Cd, Cr, Pb and Zn, I. cylindrica with Hg, Pb and Zn, and F. dibotrys with Sb. However, the translocation ability was not consistent with accumulation, and each species was selective for heavy metals. F. dibotrys had high TFs for As, Hg, Cd, Cr, Pb and Zn; I. cylindrica for Sb, Cd and Zn; R. patientia for As, Sb, Hg, Cr and Zn; P. aquilinum for Sb, Hg, Cd and Zn; and E. annuus for Sb. P. vittata did not show high TFs for any measured heavy metals, although it displayed high BCFs.
Plant strategies
Correlations between heavy metals in plant shoots, roots and the plant rhizosphere are presented in Table 4. There are noticeable interassociations between As and other metals among the three sites. In the plant rhizosphere, positive correlations were seen for Cd, Cr, Pb with Zn, and As with Cd. In roots, Zn with Cd and Zn with Pb showed a significant positive correlation. Similar results were shown in the plant rhizosphere. The heavy metal content differed between roots to shoots, and the correlation between heavy metals in shoots varied between the roots and plant rhizosphere. In shoots, there was a positive correlation for Cd with As and Zn and for Sb with Pb, and Zn was negatively correlated with Pb. Correlation analysis is a good way to identify the source of heavy metals, and cluster analysis helps to show relationships between accumulated heavy metals. Cluster analysis was used to find the relationship between heavy metals uptake and transfer (Fig. 1): Pb, Sb, Hg and Cr in shoots were significant in one group, and Cd, Zn, As, Hg and Pb in roots constituted another group. Nevertheless, Sb in shoots and roots were in one group, and As in the two parts were in another group; similarly, Zn and Cd were in one group. In shoots, Pb and Sb showed a significant correlation (Table 4), and they comprised a group, which indicated a similar origin source, as they were not only transferred from roots (soils) but also accumulated from outside the soil (air dust); Zn and Cd were similar in another group and had a significant correlation in shoots (Table 4; Fig. 1). In roots Zn with Cd and Zn with Pb have good correlations, but only Zn and Cd showed a significant relationship in one group (Fig. 1).
Principal component analysis (PCA) results for BCFs and TFs are shown in Table 5. Five factors explained 72.71% of the total variance. Sb and Pb from shoots are shown in PC1, and Sb and Zn from roots are shown in PC2. Both roots and aerial parts had a potential ability to accumulate Pb. The PC3 and PC4 included As, Cd, Zn from shoots and As, Sb, Cd from roots.
Discussion
In the study, soil showed a relatively high heavy metal content and was heavily polluted, similar to findings from previous research (He 2007). In MA, only Cr (58.51 mg kg−1) was lower in the background of Hunan Province. In addition, Sb (339 mg kg−1), Hg (1.72 mg kg−1) and Cd (0.8 mg kg−1) were higher in VA, similar to FA. According to Chinese maximum permitted content in soils (GB15618-1995), As, Sb, Hg and Cd contents in plant rhizospheres were higher. EF is a good way to identify soil pollution level. EF values between 0.05 and 1.5 indicate that the metal resource is entirely of natural origin (Bhuiyan et al. 2010).
where EF ≥ 1.5 indicated that the source is primarily human being activity, and soils were polluted (Yongming et al. 2006); EF ≥ 2 means the metal was enriched (Wang et al. 2010). Thus, Cr and Pb in the plant rhizosphere of Xikuangshan derive from natural weathering (Table 1). The other test heavy metals mainly originated from mining and smelting activities. In MA, all tested heavy metals showed enrichment, and enrichment of Sb, Hg and Cd was seen in VA; in FA, As, Sb, Hg and Cd were enriched. The contents of As, Cd, Cr, Pb and Zn in plant rhizospheres varied largely between MA VA and FA, which was not the only resource affected; plant transfer and other influencing variables were also altered (surface runoff and strong winds) (Navarro et al. 2008). Sb and Hg content were similar in the three areas, with small changes. Correlation analysis was widely used to analyze sources and geochemical behaviors (Yu et al. 2012), and the correlation between Cd, Cr, Pb and Zn in plant rhizospheres were similar to the bulk soil (Table 4) (Fu and Wei 2013), so the source may be the same as for mine smelting, and the method of transfer from the surface is correlated. In addition, the correlation and cluster analysis results show that the site with high Cd content also tended to have high Cr, Pb and Zn contents, and the four metals have a similar source. Cd, Cr, Pb and Zn in MA were most contaminated (Table 1), while Sb, Hg, Cd and As were all above the background. MA (5.46) had the lower pH than VA (7.12), and the highest organic matter was observed in VA (VA with 3.61% and MA with 2.96%) (He 2007). Waste vegetation will increase organic matter in soils, and low pH may derive from tailing weathering and acid mine drainage. The pH and organic matter affected heavy metal form and activity, as well as plant physiological character, to influence heavy metal uptake (Rosselli et al. 2003). Low pH accelerated heavy metals transfer into soils, while higher organic matter depleted oxygen and resisted soil weathering to prevent heavy metal dissolution. The biochemical properties of soils should be considered to avoid risks, and environmental influence requires further study.
Both measured heavy metals in soils were correlated, as seen in plant parts. The heavy metal contents in plants were 0.89–119, 11.86–363, 0.17–1.22, 1.87–26.58, 5.01–100, 11.11–121 and 54.96–896 mg kg−1 for As, Sb, Hg, Cd, Cr, Pb and Zn, respectively, whereas the highest metal plant contents were 157, 456, 1.57, 19.91, 128.98, 144 and 896 mg kg−1 for As, Sb, Hg, Cd, Cr, Pb and Zn, respectively. Most were polluted by heavy metals and had a good correlation. In plant roots, Zn, Cd and Pb showed a positive correlation, and thus there may be some universality in accumulating Zn, Cd and Pb. In shoots, there was a good relationship between Zn and Cd, and As with Cd showed a positive correlation in both shoots and the plant rhizosphere, which indicated the source of Zn and Cd of plants was similar, and the accumulated and transferred amounts showed a positive relationship; As and Cd may result from a similar source and similar transfer.
The heavy metal content in shoots and roots were significantly different between species, and accumulation and translocation also differed between species. Heavy metal phytoremediation ability depends on heavy metals activity and species physiology (Yoon et al. 2006; Zacarías et al. 2012). Phytoextraction generally requires the translocation of heavy metals to easily harvestable plant parts. BCFs and TFs can be used to estimate a plant’s potential for phytoremediation. BCFs can be used to assess the ability of plants to accumulate specific metals or metalloid (Wei et al. 2011), and TFs show the translocation ability for different parts. Coupling BCFs and TFs, we can determine the ability of plants for phytoremediation. Plants exhibiting TFs and particularly BCFs less than one are unsuitable for phytoextraction (Fitz and Wenzel 2002). Accumulators, root compartments and excluders are named to identify the accumulated ability of plants, accumulators have an enrichment coefficient and transfer coefficient larger than 1, and most heavy metals stored in roots have low TFs in root compartments, the last are excluders, with both low BCF and low TF (Lei et al. 2005; Zhou et al. 2012). P. aquilinum absorbs As, Cd, Pb and Zn well, I. cylindrica accumulates Sb well, P. vittata accumulates As and Cd, and D. erythrosora accumulates Hg and Cd (Table 3; Fig. 1). P. vittata was root compartment, F. dibotrys and R. patientia were shoot compartments, E. annuus were excluders with high transfer in shoots for Sb. Plants can remove soil heavy metals by plant absorption and transformation (Lei et al. 2005), which will reduce the heavy metal content in soils and protect deep soils and groundwater (Susarla et al. 2002). Accumulator plant are used for heavy metals stabilization and roots compartments and are relatively stable and good for phytoremediation; some shoot compartments have potential for phytoremediation (Weis and Weis 2004). Although no heavy metal hyper accumulators (except P. vittata) were found in the study, heavy metal-tolerant species with high BCFs and low TFs can be used for potential phytostabilization of contaminated sites (Yoon et al. 2006). In shoots, Pb and Sb had good correlations and formed a group (Fig. 1) due to an origin from a similar resource (soil and air dust) and absorption type; Zn and Cd had a good relationship in another group, because the resource was different from the source of Pb and Sb, and may only be due to root transfer (soils). In roots, Cd and Zn had good correlations and showed significance in one group, and As, Hg, Pb in another group (Fig. 2; Table 6). Principal component analysis (PCA) was applied to identify the ability of plant species to accumulate different heavy metals (Table 5) (Brunetti et al. 2009). Figure 2 shows plant accumulated ability. If the plant was located in sector 1 of Fig. 2a, it accumulated Sb, Pb, Cr and Zn well. When located in sector 1 of Fig. 2b, it had a good ability to accumulate As, Cd, Zn, Sb and Cd. O. undulatifolius was negative in all PC sectors (Fig. 2), meaning it has a weak ability to absorb the test heavy metals. P. aquilinum loading was positive at the PC1 axis (Fig. 2a) and PC3 axis (Fig. 2b), indicating its shoots were good for accumulating Sb, As and Pb. P. vittata demonstrated weak translocation capacity in shoots and strong accumulated ability on roots, and most of the transformation factors were below 1 (Table 3). I. cylindrica showed higher accumulation of Sb by shoots for the positive PC1 axis. Although I. cylindrica showed the highest TFs for Cd (Table 4), it is not a good choice for phytoremediation in the subarea for low content in plants. D. erythrosora located at PC4 for significantly accumulated Cd and showed positive loading in PC2 for accumulating significant quantities of Pb and Zn in roots (Fig. 2a). F. dibotrys accumulated little heavy metals in roots (Fig. 2b), but their TFs > 1 (Table 3), and thus values were negative for all axes. According to multivariable analysis, we conclude the character of heavy metals accumulated between species and their origin sources (Yu et al. 2012). The heavy metals sources for different parts were different (air, soils and water, or transfer from plant organ) and were more strongly reflected between species. I. cylindrica was good for accumulating Sb from soils and air dust, with a similar ability in P. aquilinum for As, and P. americana for Cd. Relationships of BCFs and TFs between metals were good and were used to identify the similarity of heavy metals accumulation (Yu et al. 2012). There are few studies on the relationship of translocations (Wenzel and Jockwer 1999; Yoon et al. 2006). Accumulated Cd will promote Cd and Cr in wild plant species due to their significant positive correlation (0.678 and 0.89; p < 0.01); plants showed a good relationship in accumulating As, Cd, Cr and Pb for their positive correlation for As with Cd, Cr and Pb (p < 0.01) (Table 6). BCFs relationships were similar to that of the plant rhizosphere (Table 4), but there were differences among TFs of As that were not positively correlated with other heavy metals; TFs were found between Cd and Pb, Pb and Zn had positive correlations (0.776, 0.737; p < 0.01). This indicated a plant was effective in transferring Pb, was also effective in transferring Cd and Zn, and vice versa. For TFs, a negative correlation was shown for Cr–Hg and Cr–Cd and Hg–Cd, which indicated transferring Cr will inhibit Cd and Hg translocation. The wild plants accumulated Cd, Cr, Pb and Zn from soils to roots and showed a positive correlation; accumulated As was positively correlated with Cd, Cr and Pb. From translocation, Zn transfer was positively correlated with Hg, Cd, Cr and Pb, Pb with Hg and Cd, and Cd with Hg. The mechanism of interaction between accumulation and translocation requires further study.
Conclusions
In this study, nine species of native wild plants were sampled and analyzed to identify the phytoremediation ability and relationship of heavy metals accumulation and translocation between species. The accumulated ability of heavy metals varied with land use and plant species. These native wild plants showed relatively high translocation factors for the heavy metals: P. aquilinum for As, Cd, Pb and Zn, I. cylindrica for Sb, P. vittata for As and Cd, D. erythrosora for Hg and Cd. There is potential for phytoremediation for the high TFs. P. vittata and P. aquilinum were root compartments, F. dibotrys and R. patientia were shoot compartments, E. annuus showed excluders but high transfer in shoots for Sb. I. cylindrica accumulated Sb well from soils and air dust, with a similar ability shown in P. aquilinum for As, P. americana for Cd. Native wild plants showed cooperative accumulation of Cd, Cr, Pb and Zn, and similarity in As–Cd, As–Cr and As–Pb. A significant positive correlation of translocation was shown for Hg–Cd, Hg–Zn, Hg–Pb, Cd–Pb, Cd–Zn, Zn–Cr and Zn–Pb. Knowing the character of accumulation and translocation between species is helpful in the search for wild plants for phytoremediation. Identification of the relationships between heavy metals accumulation and transfer among species will guide the selection of multiple heavy-metals remediation plants.
References
Barthwal J, Smitha N, Kakkar P (2008) Heavy metal accumulation in medicinal plants collected from environmentally different sites. Biomed Environ Sci 21(4):319–324. https://doi.org/10.1016/s0895-3988(08)60049-5
Bhuiyan MA, Parvez L, Islam M, Dampare SB, Suzuki S (2010) Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. J Hazard Mater 173(1):384–392
Brunetti G, Soler-Rovira P, Farrag K, Senesi N (2009) Tolerance and accumulation of heavy metals by wild plant species grown in contaminated soils in Apulia region, Southern Italy. Plant Soil 318(1–2):285–298. https://doi.org/10.1007/s11104-008-9838-3
Chehregani AN, Yazdi M, Lari H (2009) Phytoremediation of heavy-metal-polluted soils: screening for new accumulator plants in Angouran mine (Iran) and evaluation of removal ability. Ecotoxicol Environ Saf 72(5):1349–1353. https://doi.org/10.1016/j.ecoenv.2009.02.012
Chen T, Wei C, Huang Z et al (2002) Arsenic hyperaccumulator Pteris vittata L. and its arsenic accumulation. Chin Sci Bull 47(11):902–905. https://doi.org/10.1360/02tb9202
Cheng S (2003a) Heavy metal pollution in China: origin, pattern and control. Environ Sci Pollut Res 10(3):192–198
Cheng S (2003b) Heavy metals in plants and phytoremediation. Environ Sci Pollut Res 10(5):335–340
Del Rio M, Font R, Almela C, Velez D, Montoro R, Bailon AD (2002) Heavy metals and arsenic uptake by wild vegetation in the Guadiamar river area after the toxic spill of the Aznalcollar mine. J Biotechnol 98(1):125–137. https://doi.org/10.1016/s0168-1656(02)00091-3
Fitz WJ, Wenzel WW (2002) Arsenic transformations in the soil–rhizosphere–plant system: fundamentals and potential application to phytoremediation. J Biotechnol 99(3):259–278
Fu S, Wei C (2013) Multivariate and spatial analysis of heavy metal sources and variations in a large old antimony mine, China. J Soils Sediments 13(1):106–116. https://doi.org/10.1007/s11368-012-0587-9
Fu S, Wei CY, Li LH (2016) Characterizing the accumulation of various heavy metals in native plants growing around an old antimony mine. Hum Ecol Risk Assess 22(4):882–898. https://doi.org/10.1080/10807039.2015.1118676
Gonzalez RC, Gonzalez-Chavez MCA (2006) Metal accumulation in wild plants surrounding mining wastes. Environ Pollut 144(1):84–92. https://doi.org/10.1016/j.envpol.2006.01.006
Guala SD, Vega FA, Covelo EF (2010) Heavy metal concentrations in plants and different harvestable parts: a soil–plant equilibrium model. Environ Pollut 158(8):2659–2663. https://doi.org/10.1016/j.envpol.2010.04.026
He M (2007) Distribution and phytoavailability of antimony at an antimony mining and smelting area, Hunan, China. Environ Geochem Health 29(3):209–219
Lei M, Yue Q, Chen T et al (2005) Heavy metal concentrations in soils and plants around Shizhuyuan mining area of Hunan Province. Acta Ecol Sin 25(05):1146–1151
Maric M, Antonijevic M, Alagic S (2013) The investigation of the possibility for using some wild and cultivated plants as hyperaccumulators of heavy metals from contaminated soil. Environ Sci Pollut Res 20(2):1181–1188. https://doi.org/10.1007/s11356-012-1007-9
Navarro M, Pérez-Sirvent C, Martínez-Sánchez M, Vidal J, Tovar P, Bech J (2008) Abandoned mine sites as a source of contamination by heavy metals: a case study in a semi-arid zone. J Geochem Explor 96(2):183–193
Pan YM, Yang GZ (1988) Hunan soil background value and research. China Environmental Science Press, Beijing
Qian J, Shan X, Wang Z et al (1996) Distribution and plant availability of heavy metals in different particle-size fractions of soil. Sci Total Environ 187:131–141. https://doi.org/10.1016/0048-9697(96)05134-0
Rodríguez L, Ruiz E, Alonso-Azcárate J, Rincón J (2009) Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J Environ Manag 90(2):1106–1116. https://doi.org/10.1016/j.jenvman.2008.04.007
Rosselli W, Keller C, Boschi K (2003) Phytoextraction capacity of trees growing on a metal contaminated soil. Plant Soil 256(2):265–272
Solís C, Andrade E, Mireles A, Reyes-Solís IE, García-Calderón N, Lagunas-Solar MC, Piña CU, Flocchini RG (2005) Distribution of heavy metals in plants cultivated with wastewater irrigated soils during different periods of time. Nucl Instrum Methods Phys Res Sect B 241(1–4):351–355. https://doi.org/10.1016/j.nimb.2005.07.040
Susarla S, Medina VF, McCutcheon SC (2002) Phytoremediation: an ecological solution to organic chemical contamination. Ecol Eng 18(5):647–658
Wang X, He M, Xie J, Xi J, Lu X (2010) Heavy metal pollution of the world largest antimony mine-affected agricultural soils in Hunan province (China). J Soils Sediments 10(5):827–837. https://doi.org/10.1007/s11368-010-0196-4
Wei C, Deng Q, Wu F et al (2011) Arsenic, antimony, and bismuth uptake and accumulation by plants in an old antimony mine, China. Biol Trace Elem Res 144(1–3):1150–1158. https://doi.org/10.1007/s12011-011-9017-x
Weis JS, Weis P (2004) Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environ Int 30(5):685–700
Wenzel W, Jockwer F (1999) Accumulation of heavy metals in plants grown on mineralised soils of the Austrian Alps. Environ Pollut 104(1):145–155
Wiseman CLS, Zereini F, Puttmann W (2013) Traffic-related trace element fate and uptake by plants cultivated in roadside soils in Toronto, Canada. Science Total Environ 442:86–95. https://doi.org/10.1016/j.scitotenv.2012.10.051
Xue L, Liu J, Shi S et al (2014) Uptake of heavy metals by native herbaceous plants in an antimony mine (Hunan, China). Clean Soil Air Water 42(1):81–87. https://doi.org/10.1002/clen.201200490
Yongming H, Peixuan D, Junji C, Posmentier ES (2006) Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci Total Environ 355(1):176–186
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368(2):456–464
Yu R, Ji J, Yuan X et al (2012) Accumulation and translocation of heavy metals in the canola (Brassica napus L.)—soil system in Yangtze River Delta, China. Plant Soil 353:33–45. https://doi.org/10.1007/s11104-011-1006-5
Zacarías M, Beltrán M, Gilberto L, Torres, González A (2012) A feasibility study of perennial/annual plant species to restore soils contaminated with heavy metals. Phys Chem Earth Parts A/B/C 37–39:37–42. https://doi.org/10.1016/j.pce.2010.12.008
Zhou Y, Yang S, Yuan Z et al (2012) Assessment of heavy metal pollution in mine soil and bioaccumulation characteristics of dominant plants in a lead–zinc mineland, West Hunan. Earth Environ 3:361–366
Zhuang P, McBride MB, Xia H, Li N, Li Z (2009) Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci Total Environ 407(5):1551–1561. https://doi.org/10.1016/j.scitotenv.2008.10.061
Acknowledgements
This study was financially supported by the China’s Post-doctoral Science Foundation (2017M612161), Jiangxi postdoctoral research project Foundation (2017KY05), National Natural Science Foundation of China (40971264),and Jiangxi Provincial Department of Education Science and Technology Research Project (60215).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, S., Wei, C., Xiao, Y. et al. Heavy metals uptake and transport by native wild plants: implications for phytoremediation and restoration. Environ Earth Sci 78, 103 (2019). https://doi.org/10.1007/s12665-019-8103-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8103-9