Abstract
In the study, the quality of groundwater for irrigation is assessed. The following parameters were analysed using American Public Health Association standard method: pH, turbidity, electrical conductivity, total dissolved solids, Mg2+, SO42−, Cl−, HCO32−, K+, Na2+ and Ca2+. Important constituents that influence the water quality for irrigation are electrical conductivity which ranges from 4 to 1560 µS/cm, soluble sodium percentage which ranges from 4 to 33%, magnesium adsorption ratio (MAR) which ranges from 30 to 81, Kelly’s ratio which ranges from 0 to 0.5, sodium percentage (Na %) which ranges from 4 to 50%, sodium adsorption ratio which ranges from 0 to 0.6, residual sodium carbonate (RSC) which ranges from 1.5 to 3.5, permeability index which ranges from 157 to 675% and total hardness (TH) which ranges from 102 to 614 mg/L in Nkalagu water. The above estimated parameter values satisfy the various permissible standard values for irrigation except MAR, TH, RSC that are slightly above the various permissible standard values. The hydrogeochemical facies analysis plotted in Piper diagram reveals that 53.85% of the samples are predominantly (Cl−–SO42−) facies, 15.38% are (SO42−–Cl− and SO42−–Cl−–HCO3−) facies, while 7.69% are (Mg2+–SO42−–Cl−–HCO3− and Cl−–SO42−–HCO3−) facies. The water type is Ca–Cl2 with much considerable permanent hardness. Although some samples satisfied the various permissible standard values for irrigation, treatment is, however, very strongly recommended to improve the quality and make it more suitable for irrigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Nkalagu area of Ebonyi state, southeastern Nigeria, is known for its large deposit of limestone. The deposit which appears to be one of the largest and most economically viable limestone resource bases of the country was found within the Turonian-Ezeaku Formation and contains over 174 million tonnes of limestone deposit (Odukwe 1980). The presence of this mineral deposit has attracted mining activities within the area. The Nigerian Cement Company (NIGERCEM), the first indigenous cement manufacturing company in Nigeria, is a major company that utilizes this resource for cement manufacture in Nkalagu, Nigeria (Fatoye and Gideon 2013). Exploitation of the mineral is usually by quarrying, and this process has produced many pits that are usually abandoned without acceptable closure and or reclamation. These pits constitute serious threats to the environment (Moses and Ruth 2015), as they are filled by rainwater during the rainy season but become sources of water for whatever purposes to the surrounding community during the dry season. Apart from domestic uses, a more worrisome use of this pit water is recreation (swimming) and for irrigation in large-scale farming since the area is commercially known for agriculture. Irrigation agriculture is dependent on adequate water supply of usable quality (Ketata et al. 2011). Water of poor quality is not suitable for either human or plant uses. Therefore, this type of water doubtfully cannot be satisfactorily used for irrigation (Thakur et al. 2016).
The following water quality characteristics for irrigation and domestic uses will be studied in the present investigations: electrical conductivity (EC), soluble sodium percentage (SSP), magnesium adsorption ratio (MAR), sodium percentage (Na %), sodium adsorption ratio (SAR), Kelly’s ratio (KR). Various studies have been carried out within the Ebonyi state on the assessment of water quality for domestic and irrigation (Eyankware et al. 2016a; Obasi et al. 2015; Ede and Nnabo 2015; Ezeh et al. 2016; Ojobo and Nnabo 2015). In a study on irrigation parameters such as KR, RSBC and SSP in the Ekearu Inymiagu area of Ebonyi state, Eyankware (2016) observed that the values of these parameters were below the set standards for irrigation and affirmed the water fit for irrigation. Eyankware et al. (2016b) also assessed the water of the Mkpuma-Ekwaoku area of Ebonyi state for irrigation and drinking and found out that the water is very hard and the hardness is slightly above the set standard. This, the authors attributed to heavy mining activities within the area. Eyankware (2017) went further to state that the values of irrigation parameters such as PI and SSP were below the set standard, most of the analysed water samples were considered to be very hard and this was attributed to rock water interaction with reference to a Chadha plot and that the water samples are of the (Ca2++ Mg2++ Cl−) water type. Such water is considered to have a permanent hardness and does not deposit residual sodium carbonate during irrigation use. Although various studies have been carried out around Nkalagu and its adjoining areas, these investigations are based on the stratigraphic arrangement and the geology of the area (Reyment 1965; Nwachukwu 1972; Dessauvagie 1974; Fayose and De Klasz 1976; Offodile 1976; Petters 1982; Oko 2002).
Hence, this present study determines the chemical composition of water; assesses water quality for irrigation, by evaluating the different irrigation water quality parameters which include soluble sodium percentage (SSP), Magnessium Adsorption Ratio (MAR), sodium percentage (Na %), sodium adsorption ratio (SAR), Kelly’s ratio (KR), total hardness (TH), residual sodium carbonate (RSC), permeability index (PI) and electrical conductivity (EC); check the spread of contaminants on the food chain (plants) and above all monitor the effect of abandoned limestone quarry pit on the environment.
Materials and methods
Study area
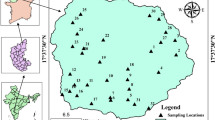
The study area is located in the Nkalagu area of the Ebonyi state, southeastern Nigeria. The area is located along the Enugu–Abakaliki expressway, about 50 km NE of Enugu. It lies between latitudes 6°10′ and 6°40′N and longitudes 7°35′ and 7°50′E. The area is quite accessible through a network of major roads and footpaths as shown in Fig. 1.
Vegetation and climate
The study area belongs to the rainforest region of Nigeria as described by Igbozuruike (1975). The vegetation is characterized by orchard bush of short trees with varying densities of dominant elephant grasses. Two major seasons are experienced in the area—the wet and dry season. The wet season starts from March and ends in October, while the dry season begins in November and ends in February. These two seasons are dependent on the two prevailing winds blowing across the country at different times of the year—the Dry Harmattan Wind, the North East Trade Wind from the Sahara Desert that prevails in the dry season, and the marine wind, the SW Trade Wind, from the Atlantic Ocean which introduces the rainy season. Temperature in the dry season and rainy season ranges from 20 to 38 °C and 16 to 28 °C, respectively. The average monthly rainfall ranges from 3.1 mm in January and 270 mm in July. The average annual rainfall varies from 1750 to 2250 mm. The climate of the area, no doubt, favours the dispersion of the resultant pollutants from the mining activities. This high amount of rainfall results in surface run-off that moves the pollutants and also assists percolation. The drainage pattern of the area is dendritic.
Geology and stratigraphic setting
The Benue Trough is a linear NE–SW trending trough that is divided into three parts, namely the Upper, Middle and Lower parts (Petters 1982, 1991).
Sedimentation in the Abakaliki Basin of the Lower Benue Trough started with deposition from the Asu River Group of Upper Albian age which overlies the Precambrian Basement Complex rocks disconformably. The Asu River Group consists of Abakaliki Shale with volcanoclastics, sandstone and sandy limestone lenses (Benkheil 1989). The Asu River Group is overlain by the Eze-Aku Formation of Turonian age (Table 1). This formation consists of flaggy, grey or black shales with sandstones and subordinate limestone (Reyment 1965; Fig. 2).
An alternating sequence of thick limestone or sandstone units occurs with calcareous shales in places where the Eze-Aku Formation is found (Ikhane et al. 2009). A typical area of the above description is the Nkalagu area in the Nigerian Cement Company (NIGERCEM) quarries (up to 6 m thick) described by Petters (1991) and Umeji (2000) as the Nkalagu Formation.
The hydrogeology of the Nkalagu area is typified by poor aquifer conditions. This is a result of the dominant shale units (Aquiclude) which are neither porous nor permeable and do not transmit water to wells found in the area. Hence, groundwater on a regional scale is impossible to find. However, conditions for the presence of groundwater may occur at weathered/fractured zones or at points of sandstone intercalations.
Sampling and laboratory analysis
Thirteen water samples were collected from various points and analysed for their physicochemical properties as shown in Fig. 2. Precautionary measures were taken by washing the bottles with clean water and cleaning reagents and thoroughly rinsed with distilled, de-ionized water prior to collection of a water sample from the site. Electrical conductivity (EC), pH and total dissolved solids (TDS) were determined at points of collection, and samples were sealed and stored in ice chests and eventually transported to the laboratory within an hour of the collection. Electrical conductivity and total dissolved solids were measured using the (HACH) conductivity and TDS meter (model HQ14D53000000, USA). The pH was measured using a pH meter (Hach sensION + PH1 portable pH meter and Hach sensION + 5050T Portable Combination pH Electrode). Potassium (K), sodium (Na) and calcium (Ca) ion concentrations were obtained with a Jenway Clinical flame photometer (PFP7 model). Magnesium (Mg), bicarbonate (HCO3−) and chloride (Cl) ions were determined using appropriate titrimetric methods described by APHA (2012), and the sulphate concentration was determined by turbidimetry using a UV–Vis spectrometer and spectra manager software. Analysis of all water samples was conducted following standard methods. The accuracy of geochemical analysis was determined by calculating the ion balance, which was within ± 0.01% as applied elsewhere (Aleke et al. 2016). Piper trilinear diagram was plotted using Aquachem 2011.1 software package. Irrigation parameters were determined by calculation using the relations below in (meq/L). The suitability of groundwater for irrigation was evaluated by comparing the water samples with various water quality standards for irrigation.
Analytical check (ionic balancing)
The correctness of the chemical results was assessed using the relationship between the anions and the cations in the analysed samples as expressed in milliequivalent per litre. The equation according to Hounslow (1995) is represented as;
The above equation gave a cation–anion ratio of 1:1 ± 0.01, which confirms that the geochemical analysis was accurate.
Irrigation parameters
Soluble sodium percentage (SSP) is expressed in milliequivalent per litre (meq/L) as proposed by Todd (1980)
Magnesium adsorption ratio (MAR) is expressed in milliequivalent per litre (meq/L) (Raghunath 1987)
Sodium percentage (Na %) is expressed in milliequivalent per litre (meq/L) (Eaton 1950; Doneen 1964)
Sodium adsorption ratio (SAR) is expressed in milliequivalent per litre (meq/L) (Richards 1969)
Kelly’s ratio (KR) is expressed in milliequivalent per litre (meq/L) (Kelly 1963)
Total hardness (TH) is measured in milligram per litre (mg/l) as CaCO3. This is equivalent to the values of calcium and magnesium in milliequivalents per litre (meq/l) multiplied by 50 (Hem 1985; Sawyer and McCarty 1967; Raghunath 1987)
Residual sodium carbonate (RSC) expressed in meq/L (Doneen 1964; www.spectrumanalytic.com).
Permeability index (PI) expressed in meq/L (Doneen 1964).
Results and discussion
Physicochemical parameters (pH, EC, TDS)
The result for the physicochemical parameters presented in Table 2 will be used to assess the water for its usability for domestic and irrigation. The ionic concentrations are in the order: Mg2+ > Ca2+ > Na+ and SO42− > Cl− > HCO3−.
pH
From the table, the measured pH is found to range from 5.4 to 8.2. The majority of the samples fall within the slightly acid to neutral pH, while only two samples NKG/07 and NKG/08 are slightly alkaline. It is important to highlight the mechanism of processes taking place in this area, When water is in equilibrium with both CO2 from the atmosphere and carbonate-containing rock, the resultant solution will be a buffer with a pH of 8.3, this is close to the pKa of the weak acid bicarbonate ion HCO3− (pKa = 8.4). This will resist further changes in pH to any added acid or base. This is a natural mechanism for balancing the considerable dissolution of lime from the underlying formation and precipitation of lime from the water body (Ayers and Westcot 1994). However, this range of values is optimal for most organisms and good for irrigation.
Electrical conductivity (EC) and TDS
Electrical conductivity values for water samples from the area range from 4 to 1560 µS/cm. This parameter is related to the concentration of salts dissolved in water (salinity). Figure 3 shows a relationship between EC and TDS. This implies that areas with high electrical conductivity values correspond to areas that have high concentrations of total dissolved solids. TDS values for samples from the area range from 13 to 1015 mg/L. The higher the values of EC, the smaller the amount of water available for use by plants. The values for EC in water samples from the area are within the acceptable limit except for the sample NK/11 1015 mg/L. Table 3 shows a classification of water with reference to EC. Values for no samples are in the unsuitable class.
Soluble sodium percentage (SSP)
SSP values range from 4 to 33% with a mean value of 15% as shown in Fig. 4 and Table 4. When the value of SSP is lower than 50, it indicates good quality of water and higher values show unacceptable quality of water for irrigation. The water samples are suitable for irrigation because the SSP value is lower than 50, the standard limit as shown in Table 6.
Magnesium adsorption ratio (MAR)
Generally, calcium and magnesium maintain a state of equilibrium in most waters. High magnesium in water will adversely affect crop yields as the soil becomes more alkaline. The value of MAR ranges from 30 to 81 with a mean value of 61. Based on the value of MAR, the water is fit for irrigation purpose at sample locations NKG/01 and 09 while the other samples are considered not fit for irrigation; see Table 4. Value below 50 is considered the acceptable limit for MAR.
Sodium percentage (Na %)
Sodium percentage is an important criterion for defining the type of irrigation. It helps also in the study of sodium hazard. The value of Na % ranges from 4 to 50 with a mean value of 19 (Table 4).
Figure 5 shows that NKG/02, 04, 05, 06, 10, 12 and 13 are classified as excellent to good, NKG/03, 07 and 11 are classified as good to permissible, while NKG/08 is classified as permissible to doubtful.
Rating of water samples on the basis of electrical conductivity and per cent sodium (after Wilcox 1955)
Sodium adsorption ratio (SAR)
SAR is a property that gives information on the comparative concentrations of Na+, Ca2+ and Mg2+ in the water samples. SAR takes into consideration the fact that the adverse effect of sodium is moderated by the presence of calcium and magnesium ions. When the SAR value rises above 12–15, serious physical soil problems arise and plants have difficulty absorbing water. The value of SAR ranges from 0 to 0.6 with a mean value of 0.2 shown in Fig. 6 and Table 4. Based on this low value of SAR, the water is fit for irrigation.
Figure 6 shows that NKG/04, 05 and 15 are classified as C1 (Excellent), NKG/01 and 10 are classified as C2 (Good), while NKG/03, 06, 07, 08, 09, 11 and 12 are classified as C3 (Doubtful).
Kelly’s ratio (KR)
If the Kelly’s ratio is equal to or below 1, it is indicative of good-quality water for irrigation, whereas a value above 1 is suggestive of unsuitability for agricultural purposes due to alkali hazards. The value of the KR ranges from 0 to 0.5 with a mean value of 0.2, shown Table 4. Based on the result for the KR, the water is considered fit for irrigation.
Total hardness (TH)
The TH value ranges from 102 to 614 mg/L with a mean value of 234 mg/L shown in Table 4. Values for water samples NKG/5, 6, 7, 8, 10, 12 and 13 are classified as moderately hard, NKG/3, 4, 9, 11 are classified as hard while NKG/1 and 2 are classified as very hard, shown in Table 6. Hence, the water can be inferred to be moderately hard to very hard. This is due to the predominance of alkaline earths (Mg and Ca) over alkali earths (Na and K) (Tables 5, 6).
Residual sodium carbonate (RSC)
Effect of bicarbonate and carbonate of water quality for irrigation is inferred by assessing the RSC values of Nkalagu area. From Table 6, the result shows that 38% of the sample water is within the high category, 30.8% of the sample is within the severe category, this class is considered unsuitable for irrigation while the remaining 15.4% of 2 samples each are within the increasing and significant class, respectively. There is usually deposition of lime in the form of white patches on the leaves and roots of plants that are irrigated with water within severe RSC class. This effect is highly undesirable for ornamental plants. However, acid injection into the quarry pit water could help reduce this effect (www.spectrumanalytic.com).
Permeability index (PI)
The permeability index values of abandoned Nkalagu quarry pits range from 157 to 675%. The mean is 391% shown in Table 4. This shows that all the water samples are classified as suitable for irrigation as they are above 75% in the Doneen (1964) chart. Therefore, there will be no apparent permeability and infiltration problems.
Hydrogeochemical facies analysis (Piper trilinear diagram)
The ionic constituents of the water samples from Nkalagu quarry pits are plotted in a Piper trilinear diagram as shown in Fig. 7. The model shows the spatial and temporal variation in the quality, origin and water type of the hydrologic unit (Piper 1944). The major ions (Na+, Ca2+, Mg2+ and HCO3−+CO3, Cl−, SO42−) plotted at the top of the diamond reveals a Ca-Cl2 water type that is high in Ca + Mg and Cl + SO4 ionic constituents. This water type results from area of a permanent hardness. The water type as assessed according to sample locations shows that 53.85% of the samples are (Cl−–SO42−) dominant, 15.38% are (SO42−-Cl−–HCO3−) and (SO42−–Cl−) dominant, while 7.69% are (Mg2+–SO42−–Cl−–HCO3−) and (Cl−–SO42−–HCO3−) dominant type. Ionic contents reveal Mg2+ dominant and SO42−–Cl− dominant water.
Summary and conclusions
Based on SAR, SSP, Na %, KR and PI, it is concluded that the water is considered fit for irrigation but not for MAR due to which sample locations NKG/01 and 09 at 84.6% were considered unsuitable for irrigation. Also, TH and RSC have sample locations NKG/01, 02 at 15.4% and NKG/05, 06, 07, 08 at 30.8% within the very hard and severe classes, respectively, thereby suggesting unsuitable water for irrigation.
A Wilcox diagram shows that the value ranges from excellent/good to good/permissible. The SAR value shows that NKG/04, 05 and 15 are classified as C1 (Excellent), NKG/01 and 10 are classified as C2 (Good), while NKG/03, 06, 07, 08, 09, 11 and 12 are classified as C3 (Doubtful). The values that are within the doubtful ranges are attributed to areas with high concentrations of dissolved salts.
The hydrogeochemical facies analysis plotted in Piper diagram reveals a Ca-Cl2 water type. This type has a permanent hardness. Also, 53.85% of the samples are (Cl−–SO42−), 15.38% are (SO42−–Cl− and SO42−–Cl−–HCO3−) facies, while 7.69% are (Mg2+–SO42−–Cl−–HCO3−) and (Cl−–SO42−–HCO3−) facies.
The ion concentrations are in the order: Mg2+ > Ca2+ > Na+ and SO42− > Cl−. These high concentrations of the Mg2+ and Ca2+ cations show that alkaline earth ions dominate alkali earth ions. There is also evidence of water rock interaction. The high concentration of SO42− is an indication of acid rain (an industrial area). The water of the Nkalagu quarry pits studied is moderately suitable for irrigation. Caution must be applied in the treatment of the water hardness, magnesium hazard and alkalinity. Regular checks, however, are recommended, to monitor the factors that make the water unsuitable for irrigation, and in prolonged use of the water for irrigation.
References
Aleke CG, Okogbue CO, Aghamelu OP, Nnaji NJ (2016) Hydrogeological potential and qualitative assessment of groundwater from the Ajali Sandstone at Ninth Mile area, southeastern Nigeria. Environ Earth Sci 75:290. https://doi.org/10.1007/s12665-015-4843-3
American Public Health Association (APHA) (2012) Standards methods for the examination of water and wastewater, 22nd edn. American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF), New York, p 1360
Ayers RS, Westcot DW (1994) Water quality for agriculture: FAO irrigation and drainage paper 29. Revision. 1, pp 1–130
Benkheil J (1989) The origin and evolution of the Cretaceous Benue Trough, Nigeria. J Afr Earth Sci 8:251–282
Dessauvagie TFJ (1974) Geological Map of Nigeria: Scale 1: 1,000,000. Nigeria Mining Geology Metallurgical Society, Lagos
Doneen LD (1964) Water quality for agriculture. Department of irrigation, University of California, Davis, p 4
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 39:123–133
Ede CO, Nnabo PN (2015) Hydrogeochemical evaluation of groundwater quality of Abakaliki and its environs, south eastern Nigeria. Int J Innov Sci Res 13(2):689–696
Eyankware MO (2016) Hydrochemical appraisal of groundwater for irrigation purpose: a case study of Ekaeru Inyimagu and its Adjoining area, Ebonyi State, Nigeria. Indian J Sci 23(88):924–943
Eyankware MO (2017) Groundwater hydrogeochemical evaluation for irrigation purposes in limestone mining areas of Umuoghara near Abakaliki SE. Nigeria. Sci Technol 3(9):1–19
Eyankware MO, Nnabo PN, Omo-Irabor OO, Selemo OI (2016a) Assessment of the effect of anthropogenic activities on hydrogeochemical quality of water resources of Ekaeru Inyimagu and its environs, southeastern, Nigeria. Sky J Soil Sci Environ Manag Niger 5(5):33–43
Eyankware MO, Obasi PN, Akakuru OC (2016b) Use of hydrochemical approach in evaluation of water quality around the vicinity of Mkpuma Ekwaoku mining district, Ebonyi state, SE. Nigeria for irrigation purpose. Indian J Sci 23(88):881–895
Ezeh VO, Eyankware MO, Irabor OO, Nnabo PN (2016) Hydrochemical evaluation of water resources in Umuoghara and its environs, near Abakaliki, South Eastern Nigeria. Int J Sci Healthc Res. 1(2):23–31
Fatoye FB, Gideon BY (2013) Geology and occurrence of Limestone and marble in Nigeria. J Nat Sci Res 3(11):60–65
Fayose EA, De Klasz I (1976) Microfossils of the Eze Aku formation (Turonian) at Nkalagu quarry eastern Nigeria. Niger J Min Geol 13:51–61
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, 3rd edn. U.S. Geological Survey Water-supply Paper 2254
Hounslow AW (1995) Water quality data: analysis and interpretation. CRS press, Inc. Lewis publishers, Boca Raton, p 397
Igbozuruike MU (1975) Vegetation Types. In: Oformata GEK (ed) Nigeria in maps, eastern state. Ethiope Publ, Benin, pp 30–31
Ikhane PR, Folorunso AF, Nton ME, Oluwalaanu JA (2009) Evaluations of Turonian limestone formation exposed at NIGERCEM quarry, Nkalagu, southeastern Nigeria: a geochemical approach. Pac J Sci Technol 10(2):763–771
Kelly WP (1963) Use of saline irrigation water. Soil Sci 95(4):355–391
Ketata M, Gueddari M, Bouhlila R (2011) Suitability assessment of shallow and deep groundwaters for drinking and irrigation use in the El Khairat aquifer (Enfidha, Tunisian Sahel). Environ Earth Sci. https://doi.org/10.1007/s12665-011-1091-z
Moses OE, Ruth OE (2015) Environmental degradation on land in Enyigba with reference to Artisan Lead–Zinc miner in south eastern Nigeria. J Multidiscip Sci Res 3(3):32–34
Nwachukwu SO (1972) Tectonic evolution of the southern portion of the Benue trough, Nigeria. Geol Mag 109:411–419
Nwajide CS (1990) Cretaceous sedimentation and paleogeography of the central Benue trough. In: Ofoegbu CO (ed) The Benue trough structure and evolution international monograph series. Vieweg and Sohne Verlag, Braunschweig and Wiesbaden, Germany, pp 19–38
Obasi PN, Eyankware MO, Akudinobi BBE, Nweke MO (2015) Hydrochemical investigation of water resources around Mkpuma Ekwaoku mining district, Ebonyi state southeastern Nigeria. Afr J Geo Sci Res 3(3):01–07
Odukwe GC (1980) Industrial minerals of Nigeria. In: Proceedings 4th Industrial Minerals International Congress Atlanta, London, pp 103–108
Offodile ME (1976) A review of the geology of the cretaceous of the Benue valley. In: Kogbe CA (ed) Geology of Nigeria. Elizabeth Publishing Co., Lagos, pp 319–330
Ojobo JR, Nnabo PN (2015) Levels of heavy metals on groundwater in Abakaliki and its environs, southeastern Nigeria. Int J Innovation Sci Res 12(2):444–452
Oko GO (2002) The Nigeria cement company Plc., Nkalagu Ebonyi State, Re-evaluation of Limestone Reserve in NIGERCEM Leases, 19804, 9806, 9809
Petters SW (1982) Central West African Cretaceous-Tertiary benthic foramnifera and stratigraphy. Paleogeographica 179:1–104
Petters SW (1991) Regional geology of Africa. In: Lecture notes in earth sciences series, vol 40. Springer, Berlin, Heidelberg
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Trans Am Geophys Union 25:914–923
Raghunath IIM (1987) Groundwater, 2nd edn. Wiley, New Delhi, pp 344–369
Reyment RA (1965) Aspects of geology of Nigeria. Ibadan Univ. Press, Ibadan, p 145
Richards LA (ed) (1969) Diagnosis and improvement of saline and alkali soils. In: United States salinity laboratory staff. Agricultural handbook no. 60. The United States Government Printing Office, Washington DC
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers, 2nd edn. McGraw-Hill, New York, p 518
Spectrum Analytic Inc. Guide to interpreting irrigation water analysis, Washington C.H. www.spectrumanalytic.com
Thakur T, Rishi MS, Naik PK, Sharma P (2016) Elucidating hydrochemical properties of groundwater for drinking and agriculture in parts of Punjab, India. Environ Earth Sci 75:467
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley, New York
Umeji AC (2000) Evolution of the Abakaliki and the Anambra sedimentary basins, Southeastern Nigeria. A report submitted to the shell petroluem development company Nigeria, Limited
Wilcox LV (1955) Classification and use of irrigation water. USDA, Circular, Washington, DC, USA, p 96
Acknowledgements
The authors sincerely and graciously acknowledge Prof. Gunter Dörhöfer, Editor-in-Chief, Environmental Earth Science, for his valuable suggestions in improving the manuscript. The anonymous reviewers are also appreciated for their fruitful and constructive suggestions in bringing the manuscript to its present form.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eyankware, M.O., Nnajieze, V.S. & Aleke, C.G. Geochemical assessment of water quality for irrigation in abandoned limestone quarry pit at Nkalagu area, southern Benue Trough, Nigeria. Environ Earth Sci 77, 66 (2018). https://doi.org/10.1007/s12665-018-7232-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-018-7232-x