Abstract
The influence of alkaline aqueous solutions on the properties of bentonite was investigated to evaluate the performance of bentonitic engineered barriers when contacted with alkaline groundwater. Batch and hydraulic conductivity tests were conducted on Na-bentonite using six different alkaline aqueous solutions. For the batch tests, almost no change in the montmorillonite fraction of the bentonite was observed after reacting with alkaline solutions (pH = 8.4–13.1), regardless of the solution type. On the other hand, aluminosilicate minerals (e.g., albite) were dissolved and secondary minerals (e.g., anorthite) were formed in alkaline NaOH solutions (pH > 13). The cation (Ca or Na) concentration primarily affected the swelling properties of bentonite rather than the pH of the solution, which was comparable to the results of the hydraulic conductivity tests. For the Ca solutions, the hydraulic conductivity of the bentonite specimen to the 0.02 mol/L Ca(OH)2 solution (6.5 × 10−9 cm/s) was approximately an order of magnitude lower than that of the bentonite specimen to the 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 solution (5.0 × 10−8 cm/s), whereas the hydraulic conductivity to the 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 solution (pH = 11.3) (5.0 × 10−8 cm/s) was slightly higher than that to the 1 mol/L CaCl2 solution (pHi = 8.4) (4.4 × 10−8 cm/s). For the NaOH solutions with pH > 13, the hydraulic conductivity of the bentonite specimen decreased with increasing Na concentration, suggesting that the effect of Na concentration was more dominant than that of permeant pH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Compacted bentonite has been being considered for use as buffer and backfill materials for deep geological repositories (DGR) of high-level radioactive wastes (HLRWs) in many countries. Cementitious materials, which can be used as construction materials or grouting materials, may dissolve when contacted with groundwater for a long period, resulting in hyper-alkaline leachate with elevated Ca concentration. The hyper-alkaline leachate can interact with the compacted bentonite used as buffer or backfill materials, causing bentonite alteration (e.g., dissolution of minerals, precipitation of secondary minerals, and exchange of Ca for Na). The bentonite alteration may cause changes in the physical properties of compacted bentonite (e.g., porosity, swelling capacity, and hydraulic conductivity) (Gaucher and Blanc 2006; Wilson et al. 2011).
Many laboratory and modeling studies have been conducted on the chemical compatibility of bentonite and alkaline leachate to assess the performance of compacted bentonite (e.g., Savage et al. 2002, 2007, 2010; Pusch et al. 2003; Fernández et al. 2009; Huertas et al. 2009; Gates and Bouazza 2010; Heikola et al. 2013; Calábria et al. 2013; Fernández et al. 2014). These studies showed that bentonite alteration, which involves the dissolution of montmorillonite and the precipitation of secondary minerals, occurs during interactions with hyper-alkaline solutions. The bentonite alteration can be affected by cation type and the concentration as well as the pH of a solution.
For example, Heikola et al. (2013) showed that only small changes in the mineralogy of bentonite for a given test duration (~18 months) occurred at pH < 12, suggesting that bentonite was stable at the pH range (pH < 12). Small amounts of Si and Al were dissolved from the bentonite at pH < 12. Calábria et al. (2013) reported that no alteration of montmorillonite was observed when bentonite was reacted with 1 mol/L NaOH solution (pH ~ 11), whereas small amounts of zeolites (phillipsite and analcime) were found in the bentonite reacted with an alkaline solution containing K and Ca after 28 days.
The dissolution of bentonite (primarily montmorillonite) and the exchange of divalent cations for Na on the exchange complex of bentonite during interactions with hyper-alkaline solutions can increase the hydraulic conductivity of bentonite. On the other hand, the precipitation of secondary minerals (e.g., calcium silicate hydrates, feldspar, carbonates, and polymorphs of silica) in bentonite can decrease the porosity of bentonite, resulting in a decrease in its hydraulic conductivity (Savage et al. 2007).
Even though many studies regarding bentonite alteration with respect to mineralogical alteration have been conducted, the influence of the bentonite alteration caused by hyper-alkaline solutions with different cations on the hydraulic conductivity of compacted bentonite has been rarely studied. Thus, the objective of this study was to investigate how alkaline solutions alter bentonite and how the bentonite alteration subsequently affects the compacted bentonite properties (i.e., mineral component, swelling capacity, and hydraulic conductivity). Batch tests on Na-bentonite using six different aqueous solutions (i.e., 0.02 mol/L Ca(OH)2, 1 mol/L CaCl2, 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2, 1 mol/L NaOH, 1.5 mol/L NaOH, and 2 mol/L NaOH solutions) were conducted for 40 days. Bentonite samples were analyzed after the completion of batch tests to determine their physical, chemical, and mineralogical properties. The cation concentrations of the filtered leachate obtained from the batch tests were measured to investigate the dissolution of minerals in the bentonite samples. Hydraulic conductivity tests were also conducted on the compacted bentonite specimens using the same aqueous solutions used in the batch tests to evaluate the effect of the solutions on the hydraulic conductivity of compacted bentonite.
Materials and methods
Bentonite
The natural Ca-bentonite was mined in Gyeongsang province, South Korea. The natural Ca-bentonite was ground and treated using Na2CO3 to produce Na-bentonite in a factory. The treated Na-bentonite was used in this study. The Na-bentonite was passed through a U.S. # 100 sieve (<0.15 mm).
Mineral compositions of raw bentonite and reacted bentonite samples obtained from the batch and hydraulic conductivity tests were quantitatively determined using X-ray diffraction (XRD) analysis by Mineralogy Inc., USA.
Chemical solutions
Several short-term experimental studies showed that the montmorillonite in bentonite can only be dissolved in solutions at pH > 13 (Pusch et al. 2003; Meunier 2005). However, long-term modeling studies showed that the montmorillonite in bentonite can be dissolved and secondary minerals can be precipitated in solutions even at pH less than 12.5 (Savage et al. 2002, 2007, 2010). Six different aqueous solutions with different pH and Ca concentrations were used to investigate the effect of pH and secondary mineral precipitation on the bentonite properties (i.e., swelling capacity and hydraulic conductivity).
0.02 mol/L Ca(OH)2, 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2, 1 mol/L CaCl2, 1 mol/L NaOH, 1.5 mol/L NaOH, and 2 mol/L NaOH solutions were used in this study. These solutions were chosen to investigate the effect of pH and type of cations on bentonite alteration. Deionized water (DI) was used as a reference solution. The solutions were prepared by dissolving powered compounds (Ca(OH)2, CaCl2, NaOH: Sigma-Aldrich) in DI water at a designated concentration.
The 0.02 mol/L Ca(OH)2 solution was used to simulate the leachate produced by reacting concrete and groundwater because leachate from concrete generally has a pH and Ca concentration similar to Ca(OH)2 saturated solution (pH = 12.5 and Ca concentration = 0.04 M) (Berner 1992). 1 mol/L CaCl2 solution was chosen to investigate the effect of strong Ca concentration in a slightly alkaline condition (pH = 8.4). The mixed 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 solution was chosen to evaluate the effect of strong Ca concentration in a highly alkaline condition (pH = 11.3). Results with 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 solution were compared to those obtained with 1 mol/L CaCl2 solution to evaluate the effect of pH at approximately similar Ca concentrations. 1–2 mol/L NaOH solutions (pH ~ 13) were used to investigate the effect of pH in highly alkaline conditions.
Batch tests
Batch tests were conducted on the Na-bentonite using the six aqueous solutions for 40 days to investigate how the different aqueous solutions affect bentonite alteration. 20 g of bentonite was placed in a 1000-mL glass bottle with a cap containing 400 mL of each solution (solid-to-liquid ratio: 50 g/L) without purging with nitrogen gas to remove air dissolved in the solution. The mixture of bentonite and each solution was then shaken at 130 rotation/min (rpm) using a water bath shaker machine for 1 h per week.
After 40 days, the mixture was filtered through a 0.45 µm filter. The filtered leachate obtained from the filtration was stored in a 50-mL polyethylene bottle and acidified to pH < 2 with a nitric acid solution for chemical analysis. The solid remaining on the filter was dried in an oven at 105 °C and stored in a vacuum container to characterize its mineralogical and physical properties. The filtered leachates were analyzed to determine the concentrations of Ca, Na, K, Mg, Al, and Si using an inductively coupled plasma atomic emission spectrometer (ICP-AES, OPTIMA 3000XL, PerkinElmer) at the Korea Basic Science Institute (KBSI).
Hydraulic conductivity tests
Six compacted bentonite specimens were prepared for hydraulic conductivity tests. The Na-bentonite was mixed thoroughly with DI water to obtain 50% of the initial water content. After mixing with DI water, the bentonite was placed in a mold with a diameter of 7.1 cm and a length of 14.5 cm. The bentonite was compacted into the mold in 3 layers and each layer was compacted using a 25.0 N rammer dropped at a distance of 30 cm with 3 blows to obtain the same dry density. The height and dry density of the Na-bentonite specimens ranged between 7.3 and 8.9 cm (avg. 8.4 cm) and 0.89 and 0.95 g/cm3 (avg. 0.94 g/cm3), respectively.
After compaction, the specimen was removed from the mold. 0.45 µm filters were placed at the top and bottom of the bentonite specimen and the bentonite specimen encased with flexible membrane was then placed in a permeameter for hydraulic conductivity tests. Hydraulic conductivity tests were conducted on the compacted bentonite specimens in flexible-wall permeameters using a falling headwater–constant tailwater method in accordance with ASTM D 5084 (ASTM 2004a). The flexible-wall permeameter was used to prevent sidewall leakage, which might be caused by shrinkage of the bentonite specimen.
The permeant solutions were prepared using the same six aqueous solutions used in the batch tests. Influent and cell pressures were applied using a gravimetric buret and water tank, respectively. An average effective stress of 28 kPa was applied and the hydraulic gradient was 10. A relatively low effective stress was used to obtain relatively quick equilibrium in terms of hydraulic conductivity, even though the in situ effective stress for engineered barriers is much higher. The hydraulic conductivity of compacted bentonite increased with decreasing effective stress (Ahn and Jo 2009). The permeant solutions were allowed to permeate through the specimens from the bottom to the top to prevent the consolidation of the specimens.
Free swell tests
Free swell tests were conducted on bentonite samples obtained after terminating the batch using DI water in accordance with ASTM D 5890 (ASTM 2004b) to evaluate how bentonite alteration affects the swelling capacity. The bentonite samples were dried and then ground using a mortar and pedestal to pass through a U.S. # 200 sieve (<0.75 mm). A 100-mL graduated cylinder was filled with approximately 90 mL of DI water. 2 g of powdered bentonite obtained after grinding the bentonite sample was then dropped gradually into the graduated cylinder in approximately 0.1 g increments. After the powered bentonite was added, the inside of the cylinder was washed using approximately 10 mL of DI water or an aqueous solution to remove the particles adhering to the inside of the cylinder. The cylinder was filled with DI water to the 100 mL mark after washing. The cylinder was left for 24 h to allow the bentonite to settle to the bottom. After 24 h, the volume of swelled bentonite was measured in ml.
Exchangeable cations and cation exchange capacity
The total exchangeable cations were measured by using the NH4OAc method (Lavkulich 1981). Reacted bentonite samples obtained after terminating the batch tests were dried and then ground to pass through a US # 10 sieve. 40 mL of 1 mol/L NH4OAc solution and 10 g of the reacted bentonite sample were placed in a centrifuge tube. The centrifuge tube was shaken at 115 rpm for 5 min, and the mixture was allowed to stand overnight. The mixture was shaken again for 15 min.
The mixture in the bottle was transferred to a Bucher funnel with a 0.45 µm filter, which was placed above a filtering flask with suction applied. The solid in the Bucher funnel was washed four times with 30 mL of 1 mol/L NH4OAc solution (30 mL for each). The leachates in the filtering flask were transferred to a 250-mL volumetric flask and acidified with a nitric acid solution to pH < 2.
Soluble salts were measured by using a fixed-ratio extract method (Rhoades 1982). 100 mL of DI water and 2 g of reacted bentonite were placed in a centrifuge tube. The mixture in the centrifuge tube was shaken at 115 rpm for 1 h. The mixture in the bottle was transferred to a Bucher funnel with a filter paper, which was placed above a filtering flask with suction applied. The leachate was transferred to a 50-mL volumetric flask and acidified with nitric acid solution to pH < 2.
The leachates were analyzed to determine the concentrations of Na, K, Ca, and Mg using ICP-AES at the KBSI. The exchangeable cations were determined by subtracting the soluble salts obtained with the fixed-ratio extract method using DI water from the total exchangeable cations obtained with the NH4OAc method.
Results and Discussion
Bentonite
The physical and chemical properties of natural Ca-bentonite and raw Na-bentonite (i.e., unreacted Na-bentonite) are shown in Table 1. The specific gravity (Gs) of the Ca- and Na-bentonite was 2.7. The average initial water content of the Ca- and Na-bentonite was 5.0%. The paste pHs of the Ca- and Na-bentonite mixed with DI water at a liquid-to-solid ratio of 5 was 9.4 and 9.8, respectively. The free swell index of Ca- and Na-bentonite in DI water was 12 and 33 mL/2g, respectively.
The Ca- and raw Na-bentonite mainly consisted of Na (X Na = 0.10) and Ca (X Ca = 0.82) and Na (X Na = 0.53) and Ca (X Ca = 0.38) as exchangeable cations, respectively. This was determined by the difference between the total exchangeable cations extracted using the ammonium acetate method (Thomas 1982) and the soluble salts extracted with DI water using the fixed-ratio extract method (Rhoades 1982). X Na and X Ca represent the molar ratio of the Na and Ca in the exchangeable complex, respectively. The sum of the exchangeable cation of Ca- and Na-bentonite was 69 meq/100 g and 88 meq/100 g, respectively. The X-ray diffraction (XRD) analysis results show that the raw Na-bentonite contained 83% montmorillonite, 8% feldspar, 7% quartz, and traces of orthoclase and calcite (Table 2). The Ca-bentonite had similar mineral components.
Batch tests
Batch tests were conducted on the raw Na-bentonite using 0.02 mol/L Ca(OH)2, 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2, 1 mol/L CaCl2, 1 mol/L NaOH, 1.5 mol/L NaOH, and 2 mol/L NaOH solutions for 40 days to investigate how the aqueous solutions with different pH and cation concentrations affect bentonite alteration. After terminating the batch tests, the cation concentrations of the filtered leachates were measured to investigate the dissolution characteristics of the bentonite samples. The reacted bentonite samples were also characterized to investigate how the chemical, physical, and mineralogical properties of bentonite were affected by the reaction between the bentonite and the aqueous solution at a given test period (40 days). The results of chemical analysis on the filtered leachates obtained from the batch tests are summarized in Table 3. The exchangeable cations of reacted bentonite, which were determined by the difference between the total exchangeable cations extracted using ammonium acetate and the soluble salts extracted with DI water using the fixed-ratio extract method, are shown in Table 4.
Leachate chemistry
For the 0.02 mol/L Ca(OH)2 and 1 mol/L CaCl2 solutions, the final pH of the filtered leachate remained almost constant, whereas for the mixed solution of 0.02 mol/L Ca(OH)2 and 1 mol/L CaCl2, the final pH (pHf) of the leachate dropped significantly from 11.3 to 8.8. The decrease in the pH of the mixed Ca solution with a high initial pH (pH = 11.3) was probably due to the precipitation of calcite (CaCO3) and dolomite ((Ca, Mg)(CO3)) (Table 2), resulting from reaction with Ca and atmospheric CO2. The pH of aqueous bentonite suspension is affected by calcite precipitation and type and amount of exchangeable cations (Kaufhold et al. 2008). For the NaOH solutions, almost no change in the pH of the filtered leachate occurred due to the very high initial OH concentration, even though aluminosilicate minerals (e.g., albite) were more dissolved in the Ca solutions (Tables 2, 3).
Si concentrations in the filtered leachates were almost 100–2000 times higher in the NaOH solutions at pH > 12.9 than in the Ca solutions at pH < 12.9. Al (0.2–0.3 mmol/L) was only observed in the filtered leachates obtained from tests with the NaOH solutions. These results indicate that the aluminosilicate minerals (e.g., albite) in the bentonite samples were dissolved primarily in the NaOH solutions at pH > 12.9, which is comparable with the results from previous studies (Pusch et al. 2003; Meunier 2005).
For the NaOH solutions, the Si concentration in the filtered leachates increased from 54.0 to 67.2 mmol/L as the NaOH concentration increased from 1 to 1.5 mol/L, but decreased from 67.2 to 37.4 mmol/L as the NaOH concentration increased from 1.5 mol/L to 2.0 M. The Al concentration in the filtered leachates increased from 0.20 to 0.28 mmol/L with increasing the NaOH concentration from 1 to 1.5 mol/L. However, almost no changes in the Al concentration were observed when the NaOH concentration increased from 1.5 mol/L to 2.0 M. These results suggest that as the NaOH concentration increased from 1 to 1.5 mol/L, the dissolution of silicate minerals might have predominated, whereas in the 2.0 M NaOH solution, both the dissolution of silicate minerals (e.g., albite) and the precipitation of secondary minerals (e.g., anorthite) might have occurred simultaneously as shown in Table 2 (Knauss and Wolery 1988).
Ca concentrations in the filtered leachates were higher in the Ca solutions due to the higher initial Ca concentration. The Ca concentration was higher in the 1 mol/L CaCl2 solution than in the mixed Ca solution with 0.02 mol/L Ca(OH)2 and 1 mol/L CaCl2 solution, probably because the 1 mol/L CaCl2 solution had a higher initial Ca concentration than the mixed Ca solution. Ca could be precipitated in the mixed Ca solution due to the higher initial pH of the solution (11.3). Almost no Ca was observed in the NaOH solutions.
Among the Ca solutions, the Mg and Na concentrations in the filtered leachates were higher in the 1 mol/L CaCl2 solution, probably due to a greater exchange of Ca for Na or Mg on the exchange complex of the bentonite (Table 2). The K concentration in the filtered leachate was higher in the NaOH solutions than in the Ca solutions except for the 1 mol/L CaCl2 solution. The K concentration in the NaOH solution increased from 1.5 to 2.8 mmol/L with increasing NaOH concentration from 1 to 2 mol/L. More of the K-containing minerals (e.g., orthoclase) dissolved in the NaOH solutions but more K-containing minerals (e.g., mixed-layered illite/smectite) were formed in the solutions with lower NaOH concentration (Table 2).
Exchangeable complex
Na and Ca were primary exchangeable cations in the raw bentonite followed by Mg and K (Table 1). In general, Ca on the exchange complex increased with the initial Ca concentration of the solution, but decreased as the initial Na concentration in the solution increased. Similarly, Na on the exchange complex increased with initial Na concentration in the solution, but decreased with increasing initial Ca concentration (Table 4). The Ca mole fraction (X Ca = 0.93) on the exchangeable complex was higher in the 1 mol/L CaCl2 solution than in the 0.02 mol/L Ca(OH)2 (X Ca = 0.82) and 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 (X Ca = 0.90) solutions due to the higher initial Ca concentration in the solution. The Na mole fraction (X Na) increased from 0.83 to 0.89 and the Ca mole fraction (X Ca) decreased from 0.16 to 0.10 as the NaOH concentration increased from 1 to 2 mol/L. The results suggest that a greater mass of cations in the solution was preferentially exchanged for cations on the exchange complex, regardless of the initial pH of the solution.
No distinct K on the exchange complex was observed in all tests, but the Mg on the exchange complex was higher in the Ca solutions than in the NaOH solutions. The XMg ranged between 0.02 and 0.08 in the Ca solutions and between 0.003 and 0.006 in the NaOH solutions. These results suggest that the exchange of Mg on the exchange complex could occur preferentially in highly alkaline conditions (pH > 12.9).
Mineral component
The mineral components of reacted bentonite obtained after the batch tests are shown in Table 2. Almost no change in the montmorillonite fraction of the reacted bentonite occurred, regardless of the type of solution used, even though Si was dissolved from bentonite in NaOH solutions (Table 3). These results are comparable to those reported by Heikola et al. (2013) and Calábria et al. (2013). They reported that no alternation of montmorillonite occurred when the montmorillonite was reacted with 1 mol/L NaOH and NaOH/KOH/Ca(OH)2 solutions, as well as simulated cement waters for 544 days. On the other hand, albite was dissolved almost completely in all the tests except the test with 1 mol/L CaCl2 solution. These results suggest that no distinct montmorillonite dissolution occurred even in the alkaline solutions (pH > 13) at a given test duration (~40 days), but albite could be dissolved at a lower pH condition (~11) than montmorillonite.
Anorthite of the reacted bentonite occurred (2%) for the strong alkaline solutions (pH > 11.3) but not for the 1 mol/L CaCl2 solution (pH = 8.4). These results indicate that anorthite was precipitated from the tests with strong alkaline solutions (>11.3). On the other hand, the precipitation of CaCO3 occurred in the tests with only solutions with high pH and Ca concentration. The precipitation of CaCO3 in the alkaline Ca solutions was attributed to the atmospheric CO2.
The mixed-layered illite/smectite occurred only in the tests with NaOH solutions, regardless of the Na concentration. The fraction of the mixed-layered illite/smectite decreased from 5 to 1% as the Na concentration increased from 1 to 2 mol/L. The mixed-layered illite/smectite was probably formed by the dissolution of K-containing minerals (i.e., orthoclase) in the NaOH solutions, resulting in pseudo-illitization of smectite (Table 2).
The results of this study are in agreement with previous studies (e.g., Karnland et al. 2007; Kaufhold and Dohrmann 2010). Karnland et al. (2007) reported that there were no major changes in mineralogy or chemical composition in bentonite contacted with 0.1, 1 mol/L NaOH and saturated Ca(OH)2 solutions. Kaufhold and Dohrmann (2010) also reported that no alteration in bentonite was observed after conducting batch tests on different bentonite samples using saturated Ca(OH)2 solutions at different temperatures (60 and 90 °C) for 3–5 months.
Free swell properties
Free swell tests on the reacted bentonite obtained after the batch tests using DI water were conducted to evaluate how the interaction between bentonite and alkaline solutions affects the free swell properties. The free swell indices of the bentonite reacted with 0.02 mol/L Ca(OH)2 and 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 solutions were 36 and 33 mL/2g, respectively, which were comparable to that of the unreacted bentonite (33 mL/2g) (Fig. 1; Table 1). The similar swell indices of the bentonite samples reacted with the different Ca solutions might be due to the similar amount of dissolved Ca concentrations in the solutions, resulting in similar Ca concentrations on the exchange complex. This suggests almost no effect from pH values between 11.3 and 12.4 on the swelling properties of bentonite after reaction. Even though the 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 solution had a higher initial Ca concentration, Ca ions might have precipitated due to its high pH (~11.3), resulting in the decrease of Ca concentration in the solution.
The free swell index of the bentonite reacted with the 1 mol/L CaCl2 solution (pH = 8.4) was 9 mL/2g, whereas that of the bentonite reacted with the 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 solution (pH = 11.3) was 33 mL/2g (Fig. 1 and Table 5), suggesting that the free swell property was primarily affected by the solution concentration rather than the pH. For the NaOH solutions (pH > 12.9), the free swell index was significantly low and decreased from 15 to 6 mL/2g as the Na concentration increased from 1 to 2 mol/L (Fig. 1). The low swell index of bentonite reacted with the NaOH solutions might be due to the bentonite alteration (e.g., montmorillonite) and the high initial Na concentration in the solution. However, no distinct dissolution of montmorillonite was observed in the bentonite reacted with the NaOH solutions (Table 2). Accordingly, the high initial Na concentration might be primarily attributed to the low free swell index because the bentonite powder for the free swell tests was prepared by filtering, drying, and grinding without pre-washing to remove soluble salts, which might cause excess soluble salts in the reacted bentonite, even though DI water was used for the free swell tests.
Hydraulic conductivity tests
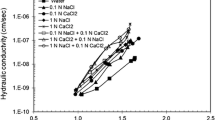
Hydraulic conductivity tests were conducted on bentonite specimens using the same solutions used for the batch tests for 2 years. The results of the hydraulic conductivities are shown in Figs. 2 and 3.
For the Ca solutions, the hydraulic conductivity of the bentonite specimen to the 0.02 mol/L Ca(OH)2 solution (6.5 × 10−9 cm/s) was approximately an order of magnitude lower than that of the bentonite specimen to the 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 solution (5.0 × 10−8 cm/s), probably due to the slightly higher Ca concentration in the 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 solution. On the other hand, the hydraulic conductivity to the 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 solution (pH = 11.3) (5.0 × 10−8 cm/s) was slightly higher than that to the 1 mol/L CaCl2 solution (pH = 8.4) (4.4 × 10−8 cm/s), even though the 1 mol/L CaCl2 solution had a higher Ca concentration than the 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 solution. These results suggest that the pH of the permeant slightly affected the hydraulic conductivity. However, even in the higher pH solution (0.02 mol/L Ca(OH)2, pH = 12.4), the hydraulic conductivity of bentonite (6.5 × 10−9 cm/s) was lower than those to the 1 mol/L CaCl2 solution (pH = 8.4) and the 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 solution (pH = 11.3). These results indicate that the effect of Ca concentration on the hydraulic conductivity was more dominant than the pH of the permeant solution.
For the NaOH solutions, the hydraulic conductivity of the bentonite specimen decreased with increasing Na concentration. These results are comparable to the results of the swell tests. The hydraulic conductivity of the bentonite specimen increased more than two orders of magnitude when the Na concentration was increased from 1 to 2 mol/L. The 1, 1.5, and 2 mol/L NaOH solutions had nearly similar pH values (13), but the hydraulic conductivities of the bentonite specimen to the NaOH solutions decreased from 2.4 × 10−8 to 5.5 × 10−6 cm/s (Fig. 3), suggesting that the initial Na concentration affected the hydraulic conductivity more significantly than the pH of the permeant solution.
Conclusions
The effects of alkaline solutions with different concentrations of Ca and Na on the alteration and swelling and hydraulic properties of Na-bentonite were investigated experimentally at 25 °C. Aluminosilicate minerals (i.e., albite) were predominantly dissolved in highly alkaline NaOH solutions (pH > 13), but barely dissolved in alkaline Ca solutions (pH ~ 12). Anorthite appeared (2%) in the bentonite reacted with alkaline solutions (pH > 11), but not with the 1 mol/L CaCl2 solution (pH = 8.4). Mixed-layered illite/smectite appeared only in the bentonite reacted with NaOH solutions, regardless of Na concentration, resulting from the dissolution of K-containing minerals (i.e., orthoclase) in the NaOH solutions.
In this study, the pH of the solution affected bentonite alteration (i.e., dissolution and precipitation of minerals), but no significant pH effect from alkaline solutions was observed on the bentonite properties (i.e., free swell index and hydraulic conductivity). For the Ca solutions with pH between 11.3 and 12.4, the Ca concentration of the solution primarily affected the swelling properties of bentonite rather than the pH of the solution. Similarly, the hydraulic conductivity of the bentonite was more affected by the cation (i.e., Ca or Na) concentrations of permeant solutions than the pHi of the permeant solutions.
However, these results might be attributed to the short test duration (batch tests: 40 days and hydraulic conductivity tests: 2 years). Long-term batch and hydraulic conductivity tests should be required to evaluate the effect of alkaline solutions on bentonite alteration. In addition, the effect of temperature on bentonite alteration should be evaluated. Temperature is one of main factors affecting bentonite alteration because bentonite can be exposed to high temperature in the DGR of HLRWs, which produce heat due to their radioactivity.
References
Ahn HS, Jo HY (2009) Influence of exchangeable cations on hydraulic conductivity of compacted bentonite. Appl Clay Sci 44(1–2):144–150
ASTM (2004a) Standard test methods for measurement of hydraulic conductivity of saturated porous materials using a flexible wall permeameter. D 5084. American Society for Testing and Materials, Philadelphia
ASTM (2004b) Standard test method for swell index of clay mineral component of geosynthetic clay liners. D 5890. American Society for Testing and Materials, Philadelphia
Berner UR (1992) Evolution of pore water chemistry during degradation of cement in a radioactive waste repository environment. Waste Manag 12:201–219
Calábria JAA, Amaral DND, Ladeira ACQ, Cota DSC, Silva TSS (2013) Determination of the cation exchange capacity of bentonite exposed to alkaline fluid. In: 2013 international nuclear atlantic conference
Fernández RF, Máder UK, Rodíguez M, Villa RV, Cuevas J (2009) Alteration of compacted bentonite by diffusion of highly alkaline solutions. Eur J Mineral 21:725–735
Fernández RF, González Ruiz AI, Cuevas J (2014) Nature of C-(A)-S-H phases formed in the reaction Bentonite/Portlandite. J Geochem 2014:1–8
Gates WP, Bouazza A (2010) Bentonite transformations in strongly alkaline solutions. Geotext Geomembr 28:219–225
Gaucher CE, Blanc P (2006) Cement/Clay interactions—a review: experiments, natural analogues, and modeling. Waste Manag 26:776–788
Heikola T, Kumpulainen S, Vuorinen U, Kiviranta L, Korkeakoski P (2013) Influence of alkaline (pH 8.3–12.0) and saline solutions on chemical, mineralogical and physical properties of two different bentonites. Clay Miner 48:309–329
Huertas FJ, Hidalgo A, Rozalén ML, Pellicione S, Domingo C, García-González CA, Andrade C, Alonso C (2009) Interaction of bentonite with supercritically carbonated concrete. Appl Clay Sci 42:488–496
Karnland O, Olsson S, Nisson U, Sellin P (2007) Experimentally determined swelling pressure and geochemical interactions of compacted Wyoming bentonite with highly alkaline solutions. Phys Chem Earth 32:275–286
Kaufhold S, Dohrmann R (2010) Stability of bentonites in salt solutions III—calcium hydroxide. Appl Clay Sci 51:300–307
Kaufhold S, Dohrmann R, Koch D, Houben G (2008) The pH of aqueous bentonite suspensions. Clay Clay Miner 56:338–343
Knauss KG, Wolery TJ (1988) The dissolution kinetics of quartz as a function of pH and time at 70°C. Geochim Cosmochim Acta 52:43–53
Lavkulich L (1981) Exchangeable cations and total exchange capacity by the ammonium acetate method at pH 7.0. In: Carter MR (ed) Soil sampling and methods of analysis. Canadian Society of Soil Science, Ottawa, pp 173–175
Meunier A (2005) Clays. Springer, Berlin, p 472
Pusch R, Zwahr H, Gerber R, Schomburg J (2003) Interaction of cement and smectitic clay—theory and practice. Appl Clay Sci 23:203–210
Rhoades J (1982) Soluble salts, methods of soil analysis, part 2. In: Page A, Miller R, Keeney D (eds) Chemical and microbiological properties, 2nd edn. Soil Science Society of America, Madison, pp 167–180
Savage D, Noy D, Mihara M (2002) Modelling the interaction of bentonite with alkaline fluids. Appl Geochem 17:207–223
Savage D, Walker C, Arthur R, Rochelle C, Oda C, Takase H (2007) Alteration of bentonite by alkaline fluids: a review of the role of secondary minerals. Phys Chem Earth 32:287–297
Savage D, Benhow S, Watson C, Takase H, Ono K, Oda C, Honda A (2010) Natural systems evidence for the alteration of clay under alkaline conditions: an example from Searles Lake, California. Appl Clay Sci 47:72–81
Thomas GW (1982) Exchangeable cations. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2. Chemical and microbiological properties—agronomy monograph no. 9, 2nd edn. ASA-SSSA, Madison
Wilson J, Savage D, Bond A, Watson S, Pusch R, Bennett D (2011) Bentonite: a review of key properties, processes and issues for consideration in the UK context. QRS-1378ZG-1, Ver. 1.1
Acknowledgements
This research was supported by the Korean Nuclear Energy R&D program (NRF-2015M2A8A5021871) and the National Research Foundation (NRF-2017R1A2B4008238) of the Ministry of Science, ICT & Future Panning, Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anh, H.N., Ahn, H., Jo, H.Y. et al. Effect of alkaline solutions on bentonite properties. Environ Earth Sci 76, 374 (2017). https://doi.org/10.1007/s12665-017-6704-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6704-8