Abstract
Groundwater is considered an important water source for agricultural, drinking and domestic uses in many regions of the world including Libya, especially in its coastal area. This study aims to assess the quality of groundwater in north-western area of Libya for drinking and agricultural through examining its physico-chemical characteristics such as pH, electrical conductivity (EC), total dissolved solids (TDS), total hardness, cations and anions. These parameters are used to assess the suitability of groundwater for domestic purposes by comparing their current values with the maximum permissible limits of these parameters that are recommended by World Health Organization (WHO). The EC, sodium adsorption ratio, soluble sodium percentage, Kelly’s ratio and magnesium adsorption ratio of the groundwater are also used to evaluate its suitability for irrigation. Gibbs diagram shows the evaporation–crystallization dominance in controlling the quality of groundwater. The main hydrochemical facies is Ca–Mg–SO4–Cl type of water in both methods of Piper and Chadha’s diagram. The results revealed that most groundwater samples were not suitable for drinking and household uses due to their high levels of most cations and anions, total hardness, EC and TDS. Most of the collected water samples showed the investigated parameter levels exceeded the permissible limits of WHO. Therefore, most of the groundwater samples are considered unsuitable for irrigation due to its high salt content, unless certain measures for salinity control are undertaken.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is an essential component of life. It is mainly obtained from two sources, including surface water and groundwater (Hamzaoui-Azaza et al. 2012). Water resources are harnessed for various purposes like drinking, agricultural, industrial, household, recreation and environmental activities (Trabelsi et al. 2011).
Groundwater is a major water source of drinking all over the world (Prasanth et al. 2012). It is estimated that approximately one-third of the world’s population uses groundwater for drinking (Nickson et al. 2005). Groundwater that is used for domestic and irrigation purposes may vary greatly in its quality depending upon the type and the quantity of dissolved salts. It contains a wide variety of dissolved inorganic chemical constituents at different concentrations that result from chemical and biochemical interactions between water and other geochemical components (Prasanth et al. 2012). Moreover, it becomes the sole source of freshwater for the agricultural production when there is a lack of sufficient surface water sources (Adhikari et al. 2012). In Libya during the last two decades, lack of the surface water resource, limited rainfall rates and escalating water demands led to a severe pumping and an over‐exploitation of the local groundwater aquifers (Nwer 2005).
So, receiving a safe drinking water, free of pathogens, and acceptable with respect to colour, taste and odour, is the priority of consumers and suppliers. The suitability degree of water and its acceptance for drinking and other domestic uses depend upon its physical, chemical and microbiological properties (Al-Tamimi and Alaswd 2014). In the developing countries, 80% of diseases are directly related to the unsanitary conditions of drinking water (UNESCO 2007).
Recently, the competition for water resources has gained an importance in many areas, especially arid and semi-arid regions (Milovanovic 2007; Bagherzadeh and Paymard 2015). Moreover, groundwater quality depends upon the quality of recharged water, atmospheric precipitation, inland surface water and sub-surface geochemical processes (Aghazadeh and Mogaddam 2010). Also, rainfall has an active role in changing the water quality of the underground aquifers (Yidana et al. 2012).
Soil salinization is of a great hazard in arid and semi-arid irrigated lands. Unless this problem is solved, salinity will have a negative effect on land productivity and crop yields and lead to an ecological degradation of land and water resources (Hillel 2000). Use of poor quality groundwater becomes unavoidable for irrigation to compensate the rapid increase in water demands in many arid and semi-arid regions. Low-quality groundwater can convert the soil of such areas to be an unproductive one (Aghazadeh and Mogaddam 2011).
In general, the groundwater quality assessment for domestic, irrigation and industrial activities in the different regions of the world has been studied and reported in recent numerous publications by several researchers, such as Valenzuela et al. (2006), Edmunds and Shand (2008), Hakim et al. (2009), Vasanthavigar et al. (2010), Yidana et al. (2011), Gurunadha et al. (2011), Uhegbu (2012), Venkateswaran and Vediappan (2013), Ewusi et al. (2013), Nag (2014), Mahendra and Patode (2014) and Ravikumar and Somashekar (2015).
This study aims to assess the groundwater quality in the north-western region of Libya for drinking and irrigation purposes through using hydrochemical methods.
Location of study area
The investigated area is located in the north-western region of Libya (Aligeelat). It lies between latitudes 32°30′ and 32°47′N, and longitudes 12°15′ and 12°30′E (Fig. 1). It is characterized by rural setting where most of the people work in agriculture. Groundwater of the area is generally drawn from dug wells which are essential for agricultural purposes.
Study area climate
Libya is located in the Mediterranean climatic zone that is characterized by hot, dry summer with cold rainfall winter. The mean annual temperature is 19.6 °C, with summer temperature rises to a maximum degree of 24.9 °C, whereas winter temperature drops to a minimum one of 14.3 °C. The rainfall is limited in the winter with an average rate of 201.0 mm/year. The average annual relative humidity is 75%, while the average annual evaporation is 1504 mm/year.
Water resources
Libya is an arid country, with an average annual rainfall rate of <100 mm/year (Al-ghraiani 1996). Water resources are divided into surface water, groundwater, and desalinated and treated water. The surface water is very limited and contributes to <3% of the current water resources in use. The groundwater accounts for more than 97% of the water resources in use and it is the main water resource in Libya (Elaalem 2010).
Materials and methods
Water sampling
Groundwater samples were collected from 15 wells in different locations in dry, clean, sterilized plastic bottles that previously were well rinsed using the standard sampling procedures (Fig. 1). The depth of tube wells varied from 20 to 70 m. The position of well was recorded using the Global Positioning System (GPS). Before sampling, each tube well was allowed to run for 20 min. The transportation and preservation (4 °C) of the water samples to the laboratory followed the standard methods. The collected water samples were then stored in 500-ml preconditioned clean, high-density polythene bottles for different analysis.
Analytical methods
The water samples were analysed for various physico-chemical parameters. The pH and electrical conductivity (EC) were measured within a few hours by using Elico pH meter and a conductivity meter, respectively, due to the sensitivity of groundwater to the environmental changes. The total dissolved solids (TDS) were calculated using a TDS meter. Calcium, magnesium, sodium, potassium, bicarbonate, sulphate, chloride and nitrate ions were determined using the standard methods suggested by the American Public Health Association (APHA 2005). The turbidity was estimated by using the turbidimeter (Drt-15CE). The total hardness (TH) as CaCO3, calcium and magnesium was titrimetrically determined using a standard EDTA. Sodium and potassium concentrations were measured by using a flame photometer. Chloride concentration was determined by silver nitrate titration. Carbonate and bicarbonate concentrations were estimated by acid–base titration. Sulphate and nitrate concentrations were colorimetrically measured using a spectrophotometer. For the bacteriological analysis, sterile containers were used for collecting water samples. Bacteriological analysis was carried out using the membrane filtration method employing the use of membrane assemblage (vacuum pump, asbestos pad, Buchner flask and membrane funnel) and Leica Quebec dark field colony counter. It was done within 24 h of sample collection (WHO 2004).
Drinking water quality
The suitability of groundwater for domestic purposes was evaluated by comparing the values of different water quality parameters with those of the World Health Organization (WHO 2004) guidelines values for drinking water. The source of the dissolved ions in the water could be determined by adopting the Gibb’s diagram (1970). Also, the suitability of groundwater for drinking purposes has been determined by use of hydrogeochemical facies (Piper trilinear and Chadha’s diagram). The hydrochemical facies (Piper diagram) and the Wilcox plot of the study area were plotted using Aqua Chem (3.7) software, while MS Excel spreadsheet was used to create the Chadha’s diagram.
Irrigation water quality
To evaluate the suitability of the groundwater quality for agricultural purposes, the electrical conductivity (EC) and sodium adsorption ratio (SAR), sodium soluble percentage (SSP), magnesium adsorption ratio (MAR), permeability index (PI) and Kelly’s ratio (KR) parameters were calculated using the standard formulas. The SAR values were plotted over the US salinity diagram against the EC values; SSP values were plotted over the Wilcox diagram against the EC values; the PI values were plotted over Doneen’s chart against the total ionic concentration. The concentrations were interpreted and calculated with irrigation indices using the following formulas of different parameters as follow:
Sodium soluble percentage (SSP)
It was calculated employing the following equation:
Sodium adsorption ratio (SAR)
It was estimated using the following equation:
Permeability index (PI)
It was calculated using the following equation:
Kelly’s ratio (KR)
It was estimated employing the following equation:
Magnesium adsorption ratio (MAR)
It was calculated using the following equation:
All determinations were in meq/l (epm)
Ionic balance
To calculate the ion balance in water, the concentration of each cation and anion in groundwater sample is determined in meq/l. The ionic balance is used to check the correctness of the chemical analyses of these water samples by calculating the differences between the sum total of cations and anions using this equation:
Basic statistical parameters such as the average and standard deviation (SD) were computed along with correlation analysis, and factor analysis was carried out using SPSS (version 10).
Results
In the investigated groundwater samples, the water pH varied between 6.98 and 7.49 with an average value of 7.17 and a standard deviation of 0.16. The turbidity value of groundwater samples ranged from 0.24 to 3.5 NTU with a mean value of 1.00 NTU and a standard deviation of 0.83 NTU. The EC of the groundwater samples varied from 2160 to 9020 µS/cm at 25 °C with an average value of 4691 µS/cm and a standard deviation of 1993.28 µS/cm. The TDS value of the investigated groundwater samples ranged from 1447.7 to 5979.1 mg/l with an average value of 3175.2 mg/l and a standard deviation of 1281.34 mg/l. The total hardness of the groundwater samples varied from 670.0 to 2820.0 mg/l with an average value of 1667.3 mg/l and a standard deviation of 674.53 mg/l (Table 1).
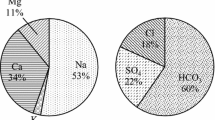
Calcium (Ca2+), magnesium (Mg2+), sodium (Na+) and potassium (K+) were the analysed cations in the groundwater samples. Calcium and sodium were the dominant soluble cations in most locations, followed by magnesium and then potassium. Calcium concentrations varied from 148.2 to 1010.4 mg/l with an average value of 443.1 mg/l and a standard deviation of 217.0 mg/l. Magnesium concentrations varied from 41.4 to 228.9 mg/l with a mean value of 114.6 mg/l and a standard deviation of 53.56 mg/l. Sodium ranged between 222.9 and 923.9 mg/l with an average value of 413.5 mg/l and a standard deviation of 196.0 mg/l. Potassium is the minor cation in groundwater. It ranged between 12.9 and 31.2 mg/l with an average value of 17.4 mg/l and a standard deviation of 5.0 mg/l (Table 1).
Chloride (Cl−), sulphate (\({\text{SO}}_{4}^{2 - }\)) and bicarbonate (\({\text{HCO}}_{3}^{ - }\)) ions are the main anions in groundwater. The chloride content of the investigated groundwater samples ranged between 450.1 and 2000.1 mg/l with an average value of 851.2 mg/l and a standard deviation of 418.7 mg/l. The sulphate concentration in the water samples varied between 360.0 to 2068.8 mg/l with an average value of 1096.4 mg/l and a standard deviation of 487.0 mg/l. Carbonate (CO3) ions were not detected in any water sample. The \({\text{HCO}}_{3}^{ - }\) concentration in these water samples ranged from 109.8 to 439.8 mg/l with an average value of 177.0 mg/l and a standard deviation of 82.6 mg/l. The nitrate concentration ranged from 9.3 to 25.0 mg/l with an average level of 14.7 mg/l and a standard deviation of 3.89 mg/l (Table 1).
The SAR values of the groundwater samples varied from 3.05 to 7.73 with an average value of 4.42 and a standard deviation of 1.27. The SSP of the studied groundwater samples ranged from 29.60 to 43.61% with an average value of 36.48% and a standard deviation of 4.37%. The PI of the investigated groundwater ranged from 31.42 to 49.88% with an average value of 39.72% and a standard deviation of 4.76%. The MAR of groundwater of this study varied from 6.39 to 47.16% with an average value of 33.62% and a standard deviation of 10.15%. The KR of these water samples varied between 0.40 and 0.75 meq/l with an average value of 0.56 meq/l and a standard deviation of 0.11 meq/l (Table 8).
Discussion
Ionic balance
The ionic balance of a water sample is calculated to identify the dominant ionic type, i.e. cationic or anionic of the water sample. According to ionic balance equation, the differences between the sum total of cations and anions in the water samples should not exceed 5%. The obtained results revealed that the differences between the sum total of cations and anions of the investigated water samples ranged between 0.04 and 1.01%. So, it is clear that the ionic balance of these water samples lies in the acceptable limit (Fig. 2).
Water quality for dinking purposes
The physico-chemical characteristics of the investigated groundwater samples are present in Table 1. World Health Organization (WHO 2004) permissible limits are considered in the suitability assessment of these water samples for drinking (Table 2).
pH
The water pH is a measure of the acidity or alkalinity degree of water. It is the most important indicator of water quality in this study. Therefore, the pH of these water samples is within the permissible limit that is prescribed by WHO (2004).
Turbidity
The water turbidity results from the colloidal and extremely fine particles that are dispersed in the water (Sudhakar and Latha 2013). The highly turbid water may cause a human health risk as the excessive turbidity can protect pathogens and also stimulate the bacteria growth during the water storage. High water turbidity can also hamper the smooth movement of aquatic organisms that can cause death. All these water turbidity values are under the maximum permissible limit (<10 NTU) of WHO (2004).
Electrical conductivity (EC)
The electrical conductivity (EC) of water is generally related to the amount of soluble salts in the water samples (ions). The higher EC values of these water samples indicate a salt enrichment in the groundwater. The high groundwater salinity also occurs in shallow-depths wells (20–70 m) that are near the sea coast due to sea water intrusion and encroachment. Moreover, the improper disposal of domestic wastewater could be one of the major salinity sources in the coastal aquifers. According to the maximum permissible limits (Table 2) that is recommended by WHO (2004), the groundwater samples are none suitable for drinking.
Total dissolved solids (TDS)
The total dissolved solids (TDS) are a measure of all dissolved substances in water. It generally reflects the amount of water dissolved minerals that control the water suitability for use. High concentrations of total dissolved solids may cause adverse taste effects. According to WHO (2004), the water TDS value of 1500 mg/l is considered the maximum permissible limit for drinking water (Table 2). All investigated water samples exceeded this maximum permissible limit. Generally, the high TDS values of these groundwaters clearly indicate their unsuitability for drinking.
Total hardness (TH)
Hard water is a potential problem because calcium and magnesium ions can react with bicarbonate ion to form insoluble calcium and magnesium carbonate salts (Robbins 2010). All water samples exceeded the maximum permissible limit of hardness (WHO 2004). They are considered very hard (Table 3) and unsuitable for drinking. The hardness of these waters may be attributed to the limestone aquifer that provides groundwater with calcium and other alkaline earths.
Soluble cations
Calcium and magnesium (Ca and Mg)
The results indicated that calcium was the major cation in the groundwater (Table 1). It contributes to the water hardness. Calcium sources in groundwater are calcite, aragonite, gypsum and anhydrite minerals. The desirable limit of calcium concentration for drinking water is 75 mg/l (WHO 2004). All investigated water samples exceeded this permissible limit of Ca2+ (Table 2). Higher Ca2+ concentrations cause abdominal ailments and undesirable for domestic as well as encrustation and scaling. According to WHO (2004), the maximum permissible limit of Mg2+ concentration in drinking water is 150 mg/l. Few water samples (4) exceeded this limit.
Sodium and potassium (Na and K)
The results indicated that sodium was the second major cation in the groundwater (Table 1). The maximum permissible limit of sodium in drinking water is 200 mg/l (WHO 2004). All samples exceeded this limit. The intake of high levels of Na+ causes increased blood pressure, arteriosclerosis, oedema and hyperosmolarity. The maximum permissible limit of potassium in drinking water is 12 mg/l (WHO 2004). All the samples showed K levels above this limit. Potassium is high due to the weathering of K-feldspars and clay minerals of aquifer matrix. However, its concentration in groundwater is lower compared to sodium due to the fact that potassium minerals are resistant to weathering.
Soluble anions
Soluble anions are dominated by chloride and sulphate in most cases followed by bicarbonate and then nitrate. Such behaviour of soluble cations and anion provides a fairly good reflection of the present data of the total soluble salts.
Chloride (Cl)
Chloride occurs in groundwater probably due to diverse sources such as weathering and leaching of sedimentary rocks and soils, intrusion of salt water, windblown salts in precipitation, domestic and industrial waste discharges, municipal effluents. It obviously affects the taste of the water. The high concentration of chloride in water does not pose any health hazard. The WHO (2004) permissible limit of chloride in drinking water is between 200 and 600 mg/l (Table 2). The chloride concentration excessed this limit in ten water samples but it was within this limit in other five samples.
Sulphate (SO4)
Sulphate is present in water as inorganic sulphate. Most of the groundwater samples exceeded the maximum permissible limit for drinking water of 400 mg/l (WHO 2004). The high sulphate concentration in these samples may be due to gypsum dissolution.
Bicarbonate (CO3 and HCO3)
The most important ions in water that determine its alkalinity are carbonate (\({\text{CO}}_{3}^{2 - }\)) and bicarbonate (\({\text{HCO}}_{3}^{ - }\)). The maximum allowable limit of total bicarbonates in drinking water is 240 mg/l (WHO 2004). So, the bicarbonate concentration in all water samples is within this limit except that of sample no. 6.
Nitrate (NO3)
Nitrates probably come to groundwater from the fertilizers, food preservatives as well as human and animal wastes. They are extremely soluble in the water that easily transfers through the soil into the drinking water supply. All samples showed a nitrate concentration that is within the maximum allowable concentration of WHO (2004) of 50 mg/l.
Total coliforms
The bacteriological content of drinking water is one of the most important quality aspects. The bacterial contamination of drinking water is considered the most common and widespread health risk that is caused either directly or indirectly by human or animal excreta. Total coliforms including faecal coliform and E. coli are indicators of faecal contamination. They come to groundwater from human and animal faecal wastes. In this study, only three groundwater samples (wells Nos. 6, 7 and 13) were found to be contaminated. The permissible limit of bacterial faecal coliforms in drinking water is 4/100 ml and that of E. coli is 0/100 ml (WHO 2004). The groundwater quality of these studied wells is bacteriologically contaminated. Therefore, it is unsuitable for human consumption. Bacteriologically contaminated groundwater is associated with water borne diseases such as viral hepatitis, schistosomiasis and cholera.
Mechanisms controlling groundwater quality
The source of the dissolved ions in the water could be determined by adopting the Gibb’s diagram. The Gibbs ratio for cations, Na/(Na + Ca) (mg/l), and for anions, Cl/(Cl + HCO3) (mg/l), of these water samples are separately plotted against the respective TDS. Gibbs diagram is used to determine the suitability of water for drinking and irrigation purposes and to estimate the source of the pollutants. Gibbs diagram of these water samples suggests that the chemical interaction of evaporation–crystallization of aquifer is the main mechanism in contributing ions to this water (Table 4; Fig. 3).
Hydrochemical facies
The graphical representation of groundwater major dissolved constituents (major cations and major anions) helps in understanding its hydrochemical evolution, grouping and areal distribution. In this study, Piper trilinear diagram and Chadha’s plot were constructed to evaluate the variation in hydrochemical facies.
Piper trilinear diagram
Piper diagram is used to illustrate the major ionic composition of the groundwater samples and assess the geochemical evaluation of groundwater (Fig. 4). It is particularly useful for detecting changes or trends in groundwater chemistry across an area or through time. The Piper diagram not only shows graphically the nature of a given water sample, but also dictates the relationship to other samples. Piper diagrams are drawn by plotting the proportions of the major cations (Ca2+, Mg2+, Na+ and K+ in epm) on one triangular diagram, the proportions of the major anions (\({\text{CO}}_{3}^{2 - }\) + \({\text{HCO}}_{3}^{ - }\), Cl− and \({\text{SO}}_{4}^{2 - }\) in epm) on another one, and combining the information from both triangles on a quadrilateral (diamond). The diamond plot, then, can be analysed to tell what kind of groundwater is looking at (Fig. 5). Samples in the top quadrant are calcium sulphate waters (which are typical of gypsum groundwater and mine drainage), samples in the left quadrant are calcium bicarbonate waters (which are typical of shallow fresh groundwater), samples in the right quadrant are sodium chloride waters (which are salty taste to water or which are typical of marine and deep ancient groundwater), and samples in the bottom quadrant are sodium bicarbonate waters (which are fresh which are typical of deep groundwater influenced by ion exchange). So, Piper diagram indicates the suitability of drinking water based on the type of the water sample in accordance with the classification standards. It clearly explains the variation or domination of cation and anion concentrations. According to Table 5, the alkaline earth type of water (Ca2+ + Mg2+) exceeded the alkalis (Na+ + K+), whereas anion strong acids (Cl− + \({\text{SO}}_{4}^{2 - }\)) exceeded the weak acids (\({\text{CO}}_{3}^{2 - }\) + \({\text{HCO}}_{3}^{ - }\)) which show that the hardness of all samples and the chemical properties are dominated by alkaline earths and strong acids. The majority of the samples (86.7%) are plotted in the Ca–Mg–Cl–SO4 field. About 13.3% of the samples fall in mixed zone, where types of groundwater cannot be identified as neither anion nor cation dominant. However, those falling under zone 6 (86.7%) belong to the permanent hardness category and exhibit the calcium chloride type where non-carbonate hardness exceeds 50%, giving an indication of groundwater from formations that are composed of limestone and dolomite or from active recharge zones with short residence time. None of the samples fall under zone 5 (magnesium bicarbonate type), 7 and 8 (water types originating from halite dissolution (saline) or alkali carbonate enrichment are absent). So, it is obvious that the water samples fall under the Ca–Mg–Cl–SO4 type (sulphate type).
Chadha’s diagram
The hydrochemical diagram proposed by Chadha was applied to identify the different hydrochemical processes. In this scheme, the difference in milliequivalent (epm) between alkaline earths (Ca and Mg) and alkali metals (Na and K) for cations expressed as percentage reacting values was plotted on the x-axis and the difference between weak acidic anions (CO3 and HCO3) and strong acidic anions (Cl and SO4) was plotted on the y-axis (Fig. 6). All samples fall in subfield 6 of Ca–Mg–SO4–Cl type of the hydrochemical facies and such water has permanent hardness (Table 6). This is exactly similar to the results obtained from the piper plot. Finally, the analytical values that were obtained from the groundwater samples when they were plotted on Piper’s and Chadha’s plots reveal that the alkaline earth metals (Ca2+ and Mg2+) are significantly dominant over the alkalis (Na+ and K) and the strong acidic anions (Cl− and \({\text{SO}}_{4}^{2 - }\)) are dominant over the weak acidic anions (\({\text{CO}}_{3}^{2 - }\) and \({\text{HCO}}_{3}^{ - }\)) and the hydrochemical facies is the Ca–Mg–Cl–SO4 type.
Generally, to ascertain the suitability of groundwater for the drinking purpose, the geochemical parameters of the investigated water samples are compared with the guidelines that were recommended by WHO (2004) which indicate that these groundwater samples are not suitable for the drinking purpose.
Water quality for irrigation proposes
The groundwater quality is of paramount importance for irrigation in arid and semi-arid regions. An adequate amount of water is very essential for proper plant growth but the quality of the used water for irrigation purpose should also be well within the permissible limits. Otherwise, it could adversely affect the plant growth. The continuous use of poor quality water without drainage and soil management may lead to a saline or sodic soil, particularly in the clay soils. A good quality of water for irrigation is characterized by its acceptable ranges of electrical conductivity (EC), sodium percentage (Na%), sodium adsorption ratio (SAR), magnesium adsorption ratio (MAR), permeability index (PI), Kelly’s ratio (KR) and chloride concentration (Cl). These parameters determine the suitability of groundwater for agricultural uses. The results of these parameters of the investigated water samples are given in Table 7 with their mean and standard deviation values.
Electrical conductivity (EC)
The electrical conductivity of the groundwater is a good measure of the salinity hazard to crops. It reflects the TDS in the groundwater and affects the available water for plants (Tank and Chandel 2009). Highly saline irrigation water causes an increase in the soil solution osmotic pressure which reduces the absorption of water and nutrients from the soil to plants. According to the US salinity laboratory (1954), 60% of groundwater samples are doubtful for irrigation and 40% of them are unsuitable for irrigation (Table 8). It is attributed to salt leaching and dissolution to the surface aquifers of the studied area.
Sodium adsorption ratio (SAR)
The SAR is a measure of the sodium hazard of water. It estimates the exchangeable sodium percentage of the soil. High concentrations of sodium in water produce harmful effects on soil properties and reduce the soil permeability. They are <10 that indicate an excellent quality for irrigation (Table 8). Therefore, all these groundwater samples are suitable for irrigation.
USSL diagram
The US salinity lab (USSL 1954) diagram, in which the SAR (sodicity) is plotted against the EC (salinity) of the irrigation water, is used to ascribe irrigation water quality through dividing the irrigation water into categories (Fig. 7; Table 9). The USSL plot showed that 6.7% of the groundwater samples fall in the C3S1 category indicating that they are of high salinity and low sodicity. These groundwater samples can be used for irrigation on all soil types with low dangerous effects of the exchangeable sodium. The diagram also indicates that 46.7% of the water samples fall in the C4S1 category that reveals that these samples have very high salinity and low sodicity. These water samples cannot be used for irrigation on all soil types. They are suitable for plants that are high salt tolerant. Thus, their suitability for irrigation is restricted. About 33.3% of the groundwater samples lie in the C4S2 category of very high salinity and medium sodicity. Only 13.3% of the water samples fall in the very high salinity and high sodicity category (C4S3) which can be used only on soils with a considerable drainage system. They can be also used to irrigate salt tolerant and semi-tolerant crops under favourable drainage conditions.
Irrigation water quality assessment of the groundwater samples using the USSL (1954) diagram
Sodium percentage (Na%)
The soluble sodium percentage (SSP) of the irrigation water is used to evaluate its sodium hazard. The SSP value of water that is less than 50% or equal to 50% indicates a good quality of irrigation water. However, if it is more than 50%, the water will be unsuitable for irrigation. According to SSP results, 73.3% of the groundwater samples are of a good quality and 26.7% of them are permissible for irrigation (Table 8).
Wilcox’s diagram
Wilcox diagram in which the percentage of sodium is plotted against the electrical conductivity indicates that 20% of the groundwater samples are fair, 40% of them are poor, and 40% of them are very poor for irrigation (Fig. 8; Table 10). The high content of Na in groundwater may be making these waters to be not suitable for the agricultural use as it tends to deteriorate the soil.
Permeability index (PI)
The permeability index (PI) also indicates the suitability of groundwater for irrigation. The soil permeability is affected by the long-term use of irrigated water. Evaluation of the suitability of water for irrigation is based on the permeability index. Classes I and II have a PI value that is >75% which their waters are good for irrigation, while the third category (class III) has a PI that is <25% and its waters are unsuitable for irrigation purpose. These PI results reveal that all groundwaters are moderate for irrigation purpose (Table 8).
Doneen diagram
The Doneen’s diagram plots the permeability index, against the total ions content of the water (epm) to evaluate the irrigation water quality and the probable groundwater effects on soil hydraulic properties when it is used for irrigation. A Doneen chart divides the irrigation waters into three major types based on the PI and total ions in solution in meq/l. Class I shows that the waters have low PI values and are the best water types for irrigation. Class II indicates that the waters are acceptable for irrigation but they are lower in the quality compared to Class I. Class III denotes that the waters are unacceptable and may not be used for irrigation. According to the Doneen diagram, all investigated water samples fall in Class I and can be categorized as the best water type for irrigation (Fig. 9; Table 11).
Magnesium adsorption ratio (MAR)
Magnesium adsorption ratio (MAR) indicates the magnesium hazard in water when its concentration is high in groundwater. An excess of magnesium in groundwater affects the quality of soils, which it causes poor crop yields. If the MR in water is <50%, the water will be considered suitable for irrigation, whereas the water with a MAR that is >50% is considered unsuitable for irrigation. The results reveal that all investigated water samples have a MAR that is <50% and are considered suitable for irrigation (Table 8).
Kelly’s ratio (KR)
A Kelley’s ratio (KR) of more than one indicates an excess of sodium in waters. Hence, waters with a Kelley’s ratio of less than one are suitable for irrigation, while those with that ratio of more than one are unsuitable for irrigation. The Kelley’s ratio of the studied groundwaters is <1 indicating good quality waters for irrigation (Table 8).
Chloride classification
Chloride is the most common toxic ion in water used for the irrigation purpose. According to classification of irrigation water based on Cl− ion concentration, 80% of the groundwater samples of the area are of brackish water type and the rest (20%) represent a brackish-salt water type (Table 8).
Statistical analysis (correlation coefficient)
Correlation coefficient (r) is a simple measure to exhibit how well one variable predicts the behaviour of the other (Table 12). The correlation matrix allows distinguishing several relevant hydrochemical relationships. In general, the results show high correlations (>0.7) between some pairs of geochemical parameters, whereas a value between 0.5 and 0.7 shows a moderate correlation (Giridharan et al. 2008). A negative coefficient indicates that the considered variables are evolving in opposite directions. The correlation coefficients of the pH of the groundwater and the EC, TDS, TH, Ca2+, Na+, Cl−, \({\text{SO}}_{4}^{2 - }\) and SAR are negatively highly significant. They are −0.767**, −0.773**, −0.742**, −0.770**, −0.809**, −0.648**, −0.809**and −0.623*, respectively. The negative correlationship indicates the decreasing trend of one parameter (pH) with increasing the others. On other words, pH values are conversely correlated to the concentration of total soluble salts. Also, highly significant positive correlations were found between EC and the respective parameters with r values of 0.986**, 0.911**, 0.944**, 0.961**, 0.937**, 0.936** and 0.789**. The highest r values between EC and other parameters and ions indicate that the EC value of water is affected by these ions and the high mobility of these ions. Calcium, sodium, chloride, and sulphate are the main ions that are responsible for the salinity increase of groundwaters. The TDS shows high positive correlations with TH, Ca2+, Na+, Cl−, \({\text{SO}}_{4}^{2 - }\) and SAR with r values of 0.936**, 0.931**, 0.946**, 0.899**, 0.943** and 0.736**, respectively. The total hardness shows good correlations with Ca2+, Na+, Cl−, \({\text{SO}}_{4}^{2 - }\) and SAR with r values of 0.872**, 0.843**, 0.858**, 0.925** and 0.557*, respectively. High correlations were recorded between calcium and Na+, Cl−, \({\text{SO}}_{4}^{2 - }\) and SAR with r values of 0.872**, 0.886**, 0.835**and 0.663**, respectively. It was also observed that magnesium ion has a high negative correlation with the PI (r = −0.514*). Sodium ions have good correlation with chloride, sulphate and SAR with r values of 0.892**, 0.921**and 0.886**, respectively, indicating that it occurs as NaCl, and Na2SO4 salts. This approves the abundance of calcium- and sodium-rich minerals such as gypsum, halite in the study area. Chloride ions have also high correlations with \({\text{SO}}_{4}^{2 - }\) and SAR with r values of 0.822** and 0.752**, respectively. Sulphate have a good correlation with the SAR (r = 0.718**). In addition, it was observed that the SAR was showed a moderate correlation with the SSP and KR with r value of 0.629* and 0.626*, respectively. Also, a strongly correlation was recorded between the SSP and both PI and KR with r values of 0.889** and 0.999**, respectively. A high correlation was also found between PI and KR (r = 0.895**). However, a poor positive or negative correlation existed between each of K+, NO3 − and MR with other ions.
Conclusion
Groundwater is immensely important for water supply in both the urban and rural areas of developing nations. The groundwater quality is assessed for irrigation and domestic suitability purposes. Contamination generally results in a poor drinking water quality, loss of water supply, high cleanup costs, high-cost alternative water supplies and potential health problems. Conclusion and recommendations can be summarized as follows:
- 1.
The chemical analysis reflects that the dominant cations in the investigated groundwater are Ca2+ and Na+ and the dominant anions are \({\text{SO}}_{4}^{2 + }\) and Cl−.
- 2.
The pH of groundwater indicates that it is slightly alkaline (pH around 7).
- 3.
The electrical conductivity, total dissolved solids, total hardness of water samples exceed their maximum permissible limits of WHO.
- 4.
Bacteriologically, most of the water samples are good for human consumption.
- 5.
Using the Gibbs’ diagram, the cations and anions fall within the zone of evaporation–crystallization.
- 6.
All water samples fall in the subfield of Ca–Mg–SO4–Cl type of the hydrochemical facies, and such waters have permanent hardness and salinity problems that are probable not suitable for the drinking purpose according to Piper trilinear diagram and Chadha’s plot. Both plots highlight the contribution from the reverse ion exchange processes besides the dominance of alkaline earth metals (Ca2+ and Mg2+) over the alkalis (Na+ and K+), and strong acidic anions (Cl− and \({\text{SO}}_{4}^{2 - }\)) over the weak acidic anions (CO3 2− and \({\text{HCO}}_{3}^{ - }\)) in the study area.
- 7.
According to the permissible limits prescribed by WHO (2004) for drinking purposes and other parameters, that groundwater samples of the study area are chemically unsuitable for drinking uses. High EC, TH and TDS values in most investigated samples clearly indicate the unsuitability of groundwater for drinking and irrigation purposes.
- 8.
Therefore, the water of these wells must be desalinized before its use for drinking and domestic usages.
- 9.
Based on the water quality parameters such as SAR, SSP, MAR, PI and KR, the suitability of these groundwater samples for irrigation is good to moderate in most cases, indicating low sodic waters. However, other parameters, such as salinity (EC) and chloride (Cl−), suggest that the groundwater of the study area is not suitable for irrigation purpose. Also, according to the salinity hazard diagram, most of the groundwater samples are unsuitable for irrigation due to its high salt content, unless certain measures for the salinity control are undertaken.
- 10.
Finally, recommendations have been made to stringently monitor and control the low groundwater quality in the study area to ensure the sustainable safe use of the groundwater. For this area that has poor groundwater quality for human consumption and irrigation propose due to high salinity, it is recommended that rain water harvesting should be encouraged the excess stored rain water should be directed to recharging wells to reduce the salt concentration in groundwater and deepening the wells to reach the low-salt layer of the aquifer.
- 11.
Regular monitoring of the groundwater level and water quality for compliance as well as the environmental protection of groundwater resource to remove the public apprehensions of groundwater pumping is also recommended.
References
Adhikari K, Chakraborty B, Gangopadhyay A (2012) Assessment of irrigation potential of groundwater using water quality index tool. Environ Res J 3(6):197–205
Aghazadeh N, Mogaddam AA (2010) Assessment of groundwater quality and its suitability for drinking and agricultural uses in the Oshnavieh area, Northwest of Iran. J Environ Prot 1:30–40
Aghazadeh N, Mogaddam AA (2011) Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer, Northwest of Iran. Environ Monit Assess 176:183–195
Al-ghraiani A (1996) Satisfying future water demand of northern Libya. Water Resources Development, Tripoli
Al-Tamimi RA, Alaswd AM (2014) Suitability assessment of deep wells water for drinking and domestic uses in Al-bewanees region, south of Libya. Int J Curr Res 7(11):9997–10003
APHA. American Public Health Association (2005) Standard method for examination of water and wastewater, 21st edn. APHA, AWWA, WPCF, Washington
Bagherzadeh A, Paymard P (2015) Assessment of land capability for different irrigation systems by parametric and fuzzy approaches in the Mashhad Plain, Northeast Iran. Soil Water Res 10(2):90–98
Edmunds WM, Shand P (2008) Natural groundwater quality. Wiley, New York
Elaalem M (2010) The application of land evaluation techniques in Jeffara Plain in Libya using fuzzy methods. Ph.D. thesis, Department of Geography, University of Leicester
Ewusi A, Obiri-yeboah S, Voigt H, Asabere SB, Bempah CK (2013) Groundwater quality assessment for drinking and irrigation purposes in Obuasi Municipality of Ghana, a preliminary study. Res J Environ Earth Sci 5(1):6–17
Giridharan L, Venugopal T, Jayaprakash M (2008) Evaluation of the seasonal variation on the geochemical parameters and quality assessment of the groundwater in the proximity of River Cooum, Chennai, India. Environ Monit Assess 143:161–178
Gurunadha VVSR, Tamma GR, Surinaidu L, Rajesh R, Mahesh J (2011) Geophysical and geochemical approach for seawater intrusion assessment in the Godavari Delta Basin, A.P., India. Water Air Soil Pollut 217:503–514
Hakim MA, Juraimi AS, Begum M, Hasanuzzaman M, Uddin MK, Islam MM (2009) Suitability evaluation of groundwater for irrigation, drinking and industrial purposes. Am J Environ Sci 5(3):413–419
Hamzaoui-Azaza F, Ameur M, Bouhlila R, Gueddari M (2012) Geochemical characterization of groundwater in a Miocene Aquifer, Southeastern Tunisia. Environ Eng Geosci 18(2):159–174
Hillel D (2000) Salinity management for sustainable agriculture: Integrating science, environment, and economics. The World Bank, Washington, DC
Mahendra GT, Patode HS (2014) Evaluation of groundwater quality and its suitability for drinking and agricultural use in and around Hingoli region, Maharashtra, India. Am Int J Res Humanit Arts Soc Sci 6(3):256–263
Milovanovic M (2007) Water quality assessment and determination of pollution sources along the Axios/Vardar River, Southeast Europe. Desalination 213:159–173
Nag SK (2014) Evaluation of hydrochemical parameters and quality assessment of the groundwater in Gangajalghati Block, Bankura District, West Bengal, India. Arabian J Sci Eng 39:5715–5727
Nickson RT, McArthur JM, Shrestha B, Kyaw-Nyint TO, Lowrt D (2005) Arsenic and other drinking water quality issues, Muzaffargarh District, Pakistan. Appl Geochem 20:55–68
Nwer BAB (2005) The application of land evaluation technique in the north-east of Libya. Ph.D. thesis, Faculty of Environment, Cranfield University, Silsoe
Prasanth SSV, Magesh NS, Jitheshlal KV, Chandrasekar N, Gangadhar K (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala, India. Appl Water Sci. doi:10.1007/s13201-012-0042-5
Ravikumar P, Somashekar RK (2015) Principal component analysis and hydrochemical facies characterization to evaluate groundwater quality in Varahi river basin, Karnataka state, India. Appl Water Sci. doi:10.1007/s13201-015-0287-x
Robbins J (2010) Irrigation water for greenhouses and nurseries. US Department of Agriculture, and County Governments Cooperating no. 6061. Agriculture and Natural Resources, University of Arkansas, Division of Agriculture
Sudhakar G, Latha GS (2013) Seasonal variation of ground-water analysis from Bapatla Mandal, Guntur District, Andhra Pradesh. Int J Sci Res:10–12
Tank DK, Chandel CPS (2009) A hydrochemical elucidation of the groundwater composition under domestic and irrigated land in Jaipur city. Environ Monit Assess. doi:10.1007/s10661-009-0985-7
Trabelsi R, Abid K, Zouari K (2011) Geochemistry processes of the Djeffara palaeogroundwater (Southeastern Tunisia). Quat Int 3:1–13
Uhegbu CA (2012) An investigation to determine the suitability of the Groundwater of Aba Metropolis for Agricultural purposes. Sch Res Libr 4(5):2027–2033
UNESCO (2007) Water portal newsletter no. 161: water related diseases. http://www.unesco.org/water/news/newsletter/161.shtml
USSL (1954) Diagnosis and improvement of saline and alkali soil. USDA Hand Book no. 60, Washington
Valenzuela JC, Quilez D, Lafarga R (2006) Groundwater quality in CR-V irrigation district (Bardenas I, Spain): alternative scenarios to reduce off-site salt and nitrate contamination. Agric Water Manage 84(3):281–289
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Ganthi RR, Chidambaram S, Sarama VS, Anandhan P, Manivannan R, Vasudevan S (2010) Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environ Monit Assess 171(1–4):595–609
Venkateswaran S, Vediappan S (2013) Assessment of groundwater quality for irrigation use and evaluate the feasibility zones through geospatial technology in Lower Bhavani Sub Basin, Cauvery River, Tamil Nadu, India. Int J Innov Technol Explor Eng 3(2):180–187
WHO (2004) Hardness in drinking-water: background document for development of WHO Guidelines for drinking-water quality. World Health Organization
Yidana SM, Sakyi PA, Stamp G (2011) Analysis of the suitability of surface water for irrigation purposes: the Southwestern and Coastal River Systems in Ghana. J Water Resour Prot 3:695–710
Yidana SM, Banoeng-Yakubo B, Aliou A, Akabzaa TM (2012) Groundwater quality in some Voltaian and Birimian aquifers in northern Ghana—application of multivariate statistical methods and geographic information systems. Hydrol Sci J 57(6):1168–1183
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abd El-Aziz, S.H. Evaluation of groundwater quality for drinking and irrigation purposes in the north-western area of Libya (Aligeelat). Environ Earth Sci 76, 147 (2017). https://doi.org/10.1007/s12665-017-6421-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6421-3