Abstract
A sequencing batch reactor (SBR) is a suitable system for evaluating an anaerobic ammonium oxidation (ANAMMOX) process. Response surface analysis was employed to optimize the specific ANAMMOX activity (SAA) and evaluate its technical features in a SBR with a range of total nitrogen (TN) concentration as NH4 +–N and NO2 −–N at 1:1 ratio and an appropriate pH. A partial cubic model adequately fit the response surface with high statistical significance. SAA was optimized at 1.77 mg-N/mg-VSS day with 155.9 mg-N/L TN concentration and pH at 7.34. The inhibition factors of free ammonia, free nitrous acid (FNA), and nitrite gave specific rise to the SAA responses of over 1.4 mg-N/mg-VSS day in square-like boundaries. However, the toxic effect of 2.4 mg-N/L of FNA was identified to induce a drastic decrease at the lowest pH of 5.6. For the most optimized fed-batch mode for ANAMMOX-SBRs, this study found that TN was 100–200 mg/L and pH was 6.5–8, which can become controlling factors for physicochemical reactions at the time of substrate feeding and microbial reacting stages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The operation and maintenance (O&M) costs of a biological nutrient removal (BNR) process are very dependent upon the amount of aeration for nitrification as well as the dosage of external organic carbon sources for denitrification. It was well known that the application of the anaerobic ammonium oxidation (ANAMMOX) reaction to BNR has significantly reduced its operational costs. It is because that ANAMMOX bacteria have a unique metabolic pathway in oxidizing ammonia (NH4 +) with nitrite (NO2 −) as an electron acceptor to produce nitrogen gas (N2) and relatively small amount of nitrate (NO3 −) under autotrophic and anoxic conditions (Eq. 1) (Strous et al. 1998). Because of such unique metabolism, the partial nitritation (PN) process, as a pretreatment upstream of the ANAMMOX process, can oxidize approximately a half of NH4 +–NO2 − (Eq. 2). This will reduce the oxygen requirement by 58%, when compared to a complete nitrification process. Taking advantage of this feature will result in saving the costs of the reduced air requirement along with lower electric consumption, and carbon source can be saved with the use of ANAMMOX bacteria that are completely autotrophic. Even more, a study found that a combined process of PN and ANAMMOX saved up to 90% of O&M costs (Jetten et al. 2001).
ANAMMOX reaction
PN reaction
Implementation of the ANAMMOX process requires a highly skilled cultivation strategy and method during the start-up operation because of the long doubling time of ANAMMOX bacteria such as 15–30 days (Fux et al. 2004; Strous et al. 1998). If this aspect being considered, a process of SBR was proven to be a suitable process with the ANAMMOX process by maintaining a high biomass concentration in a reactor (Scaglione et al. 2015; Pereira et al. 2014). The immobilization technologies using granular sludge or artificial entrapment matrices have been successfully hybridized to improve the biomass retention efficiency of SBRs. For example, an appropriate hydrodynamic shear force facilitated the formation of granular sludge in a SBR, which resulted in highly efficient solid–water separation in a settling stage (Arrojo et al. 2006; Liu and Tay 2002). However, a three-dimensional structure of the granular sludge limits a mass transport of substrates (Ni et al. 2009). To overcome such a drawback, an innovative membrane separation was introduced to cultivate a suspended growing biomass in a membrane SBR (MSBR) and successfully led to the efficient mass transport and the homogeneity of a microbial mixture (van der Star et al. 2008). Suspended biomass with minimal floc size exhibited better biokinetic parameters such as low half saturation concentration and high specific growth rate. The microfiltration (MF) membrane with pore sizes of less than 0.1 μm provided perfect biomass retention in a MSBR and high effluent quality with almost zero suspended solids. MSBR could also skip a settling stage thus simplifying SBR operating procedure. Therefore, the suspended biomass of ANAMMOX bacteria was appropriate for the specific ANAMMOX activity (SAA) in this study.
When it comes to increasing ANAMMOX activity, of importance is the fact that how the major inhibiting factors including free ammonia (FA) and free nitrous acid (FNA) against SAA are manipulated with the correlated equilibrium of NH3/NH4 + and HNO2/NO2 − concentrations, as acidity varies. The relatively significant pH decrease less than 6 in the PN reaction required the addition of alkalinity for optimizing pH to 8 for the following ANAMMOX reaction (Jaroszynski et al. 2011). Technically, the simultaneous optimization of the substrate concentration and pH was highly required. Despite numerous investigations of the inhibition of ANAMMOX activity associated with FA and FNA concentrations (Jin et al. 2012; Strous et al. 1999), little information on the inter-correlated effects of nitrogen concentrations and pH was yet to be found. The objectives of the study therefore were to determine the optimal conditions of nitrogen concentration and pH range for SAA, using a statistical optimization approach (multi-fitting) of RSA (Bae et al. 2015), and evaluate the feasibility of the findings and technical performance to a scale-up system. The results will make a usable statistic database for ANAMMOX activity, particularly, when treating high strength of nitrogenous wastewaters such as piggery wastewater, an anaerobic digester supernatant and side stream from municipal wastewater treatment plants (MWTPs)
Materials and methods
Inoculum
ANAMMOX bacteria were enriched in an upflow anaerobic sludge bed (UASB) with a total reaction volume of 3.35 L, inoculated with granular sludge of anaerobic digestion from a brewery factory and activated sludge from a piggery wastewater treatment plant in Korea (Bae et al. 2010). The enrichment process showed a 90% TN removal, approximately at a TN loading rate of 2.44 kg-N/m3-day. Dominant ANAMMOX bacterium in the granular sludge was “Candidatus Jettenia asiatica”-related species. Before conducting the SAA tests (Trials), the granular biomass was homogenized by a homogenizer (IKA, T10 Basic, Germany) with a high intensity rotor–stator disperser (S10N-19G, ME, USA). The range of concentration of volatile suspended solids (VSS) was from 175.5 to 198.9 mg/L for the SAA tests.
SAA test
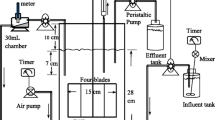
The total reaction volume was 100 mL of a 125 mL serum bottle. For an effective substrate diffusion, a complete mixing was made at 250 rpm in a shaking incubator. The basal medium consisted of 6 mg-P/L of KH2PO4, 12 mg-Mg/L of MgSO4·7H2O, 48 mg-Ca/L of CaCl2·2H2O, and 1 ml/L of trace element solution (I) and 1 ml/L of trace element solution (II). The trace element solution (I) was composed of 5 g/L of ethylenediaminetetraacetic acid (EDTA) and 5 g/L of FeSO4·7H2O, while the trace element solution (II) was composed of 5 g/L of EDTA, 0.43 g/L of ZnSO4·7H2O, 0.24 g/L of CoCl2·6H2O, 0.99 g/L of MnCl2·4H2O, 0.25 g/L of CuSO4·5H2O, 0.22 g/L of Na2MoO4·2H2O, 0.19 g/L of NiCl2·6H2O, 0.21 g/L of Na2SeO4·10H2O, and 0.014 g/L of H3BO3. NH4 +–N and NO2 −–N were added to the basal medium in the forms of (NH4)2SO4 and NaNO2, respectively. The nitrogen concentrations of NH4 + and NO2 − were maintained at a ratio of 1:1. The pH was adjusted with 1 N HCl and 1 N NaOH. The medium was flushed with argon gas before the ANAMMOX inoculum being placed to the serum bottles, which should be kept in anaerobic condition. The ANAMMOX activity was measured by calculating the linear descending slopes of NH4 +–N and NO2 −–N concentrations. The TN removal rates for NH4 + and NO2 − were normalized by VSS concentration to evaluate the SAA [mg-N/mg-VSS day]. The minor controlling factors such as inorganic carbon concentration and temperature were set at 95 mg-C/L and 35 °C.
Analysis
Samples were prepared by filtering through a 0.45-μm syringe filter at 0, 18, 25, and 42 h. The concentration of NH4 +–N was measured by Kjeldahl nitrogen analysis (Kjeltec 1035, Sweden). The concentration of NO2 −–N was analyzed by ion chromatography (Dionex 120, USA), and a colorimetric method was also used to measure the concentration of NO2 −–N. FA and FNA concentrations were calculated with following equations (Eqs. 3–6) (Sinha and Annachhatre 2007):
TNA = total ammonia nitrogen, ionization constant for NH4 + \(\left( {K_{\text{a}} /K_{\text{w}} } \right) = \exp \left( {6334/\left( {273 \, + \, t} \right)} \right)\), K a = ionization constant for ammonia, K w = ionization constant for water, t = temperature in °C, ionization constant for NO2 −, \(K_{\text{n}} = \, \exp \left( { - 2300/\left( {273 \, + \, t} \right)} \right)\).
Statistical optimization of the specific ANAMMOX activity
The “One Factor At a Time” (OFAT) design, which independently monitors the response for one factor by setting the other factors at fixed values, was applied as a factor screening tool. The OFAT is quite feasible to simplify a process using approximated responses with a set of independent variables. The simplified analysis of a process by OFAT experiments ignores the statistical interaction between independent variables (Riter et al. 2005). Then, RSA was applied to accurately analyze and optimize the factors that affect the SAA associated with simultaneous changes in TN concentrations including the sum of NH4 +–N and NO2 −–N, and pH, by using an ECHIP Software version 6 (Echip Inc., Delaware, USA). RSA is an iterative statistical technique to approximate multivariate responses and is represented by Eq. 7. A sequential procedure is usable for collecting the data, estimating the polynomials (Eq. 3), and checking the adequacy of the model (Yang et al. 2004).
Here, η a is the experimental value of SAA [mg-N/mg-VSS day], x k is the independent variable k (1 = sum of nitrogen concentrations of NH4 + and NO2 −; 2 = pH), C 0 is the regression constant, and α k is the regression coefficient of the independent variable k. The least squares method was used to estimate the parameters in the appropriate polynomials (Eq. 7). The experimental conditions for RSA were designed based on the central composite cube (CCC) design, minimizing the number of trials required for statistically significant results. The central conditions of the independent variables were set based on a preliminary feasibility test. Because of the significant effects of TN concentration and pH in the specific range of 100–200 mg-N/L, and of 6–8, respectively, a slightly broader range of 65.2–244.8 mg-N/L and of 5.6–8.4 was applied to cover such specific range (Table 1). Nine tests under experimental boundary conditions were conducted to estimate the response rate of SAA. The experiment as well as the measurement was performed once under each experimental condition for RSA, except for the central condition (5 times of replication). The maximum SAA rate calculated from an adequate model was verified through the triplicate batch tests in accordance with identified optimum conditions.
Results and discussion
Preliminary feasibility test
TN concentrations, inorganic carbon concentration, temperature, and pH were independent variables for the SAA optimization. In order to verify the major independent variables exhibiting high sensitivity, OFAT experiments were carried out (Fig. 1). The hypothetical optimum conditions for the OFAT test include 200 mg-N/L of TN, 95 mg-C of HCO3 −/L, 35 °C, and pH = 7. The SAA test for substrate concentration showed the maximum activity at TN concentrations of 50–100 mg/L in Fig. 1a. It was found that the TN concentration higher than 200 mg-N/L eventually came to inhibit the SAA. Inorganic carbon was used as a substrate for the chemolithotrophic ANAMMOX bacteria. As shown in Fig. 1b, an inorganic carbon concentration as low as 55 mg-C/L was insufficient for the high SAA rate, whereas inorganic carbon concentration range of 75–135 mg-C/L showed a relatively insensitive pattern to SAA values of 1.78 ± 0.07 mg-N/L mg-VSS day. Typically, the pH is the major control factor for a biological process because core functional enzymes and proton gradient maintenance require a specific pH. Indeed, pH had a significant impact on the SAA. The optimum pH was 7 in Fig. 1c, while at 5, 9, and 10, SAA was markedly inhibited. The optimum temperature was identified in the range of 30–40 °C in Fig. 1d. The result was consistent with the study that ANAMMOX activity was successfully optimized at 37 °C (Egli et al. 2001). In the study, there was no need to optimize temperature because SAA was stable and insensitive at 30–40 °C. As a result, TN concentration and pH were considered as critical factors for the SAA optimization. So, 160 mg-N/L of TN and neutral pH were selected as optimum conditions for the CCC design (Table 1).
Response surface analysis of SAA
Stepwise changes of 60 mg-N/L for TN and 1 for pH from the center point were initially applied for the first-order model, \(\eta_{i} = C_{0} + \alpha_{1} x_{1} + \alpha_{2} x_{2}\) (Trials 1–5 in Table 1). As a result, the following model was generated for SAA.
In Eq. 8, η i is the activity of i calculated (mg-N/mg-VSS day, where i = SAA) and x j is the corresponding variable term (j = TN concentration, mg-N/L and pH in order). The first-order model resulted in a significant P value of the lack-of-fit for SAA at 0.5% of α level, and the regression coefficient was not significant with 75% of α level. Thus, four trials were experimented with the stepwise changes of 84.8 mg-N/L (60 mg-N/L × 21/2) and 1.4 (1 × 21/2) for the TN concentration and pH, respectively, from the center point to fit the second and partial cubic order regressions (Trials 6–9 in Table 1). When finding the optimum conditions for the highest level of SAA, a quadratic model was used with Eq. 9.
The regression coefficient for the quadratic model (Eq. 9) evolved to the 10% of α level, compared to the first-order regression, but it still showed a significant P value of the lack-of-fit for SAA at the 0.5% of α level. Therefore, the modified partial cubic model was created by adding the terms x 21 x 2, x 1 x 22 , and x 21 x 22 (Eq. 10). Only the modified partial cubic model adequately fit the response surface with an insignificant P value of the lack of fit and the significant regression coefficient at 0.01% of α level. The R 2 and adjusted R 2 values were 0.992 and 0.975, respectively. Therefore, the modified partial cubic model was selected to adequately describe the response surface of SAA within this region and to find the optimal conditions. Two- and three-dimensional response surfaces of the partial cubic model are shown in Fig. 2, with the corresponding estimated lowest and the highest values of SAA.
The conditions that maximized the SAA rate were calculated based on the modified partial cubic model (Eq. 10). The optimum conditions were identified as 155.9 mg-N/L and 7.34 for TN concentration and pH, respectively. This was quite close to the conditions of Trial 5 in Table 1. The predicted model output under the optimum conditions was identified as SAA of 1.77 ± 0.13 mg-N/mg-VSS day, which was similar to the value of 1.72 ± 0.01 mg/L obtained from the triplicate verification tests. A further observation of the coefficients of the model (Eq. 10) for possible two-way interactions among the independent variables showed that the interaction term of x 1 x 2 was not significant (P values more than 10%). The inter-correlated effects on the response were significant only for the interaction terms of x 21 x 2 and x 21 x 22 ; they were found to be interdependent at 0.5% of α level (P values of 0.0011 and 0.0008, respectively). The square-like red boundary of SAA (η SAA = 1.4) in 100–200 mg-TN/L and pH 6.5–8 discovered the most stable SAA rate between 1.4 and 1.77 mg-N/mg-VSS day in Fig. 2a. However, the peak was transformed to a drastic decrease toward the minimum response of Trial 9 in the steepest ascent of the dashed line in Fig. 2a. This significantly reduced SAA rates from 1.7 to 0.4 mg-N/mg-VSS day (24%), while the other paths toward the edges of the first-order model design such as Trials 6–8, showed less reduction on SAA rates.
Effects of FA and FNA on SAA
The interaction between TN concentration and pH (Eqs. 4, 6) affected the FA and FNA concentrations in accordance to the experimental conditions in Table 1 and in Fig. 3a. The pH was the major factor for determining the FA concentration at the specific range of 0.07 and 21.54 mg-FA/L. Trial 8 at the highest pH, 8.4, had 21.54 mg-FA/L. The NH4 +–N concentrations of 110 and 50 mg/L at the pH of 8 (Trials 1 and 3) had 6.18 and 13.61 mg-FA/L, respectively. However, neutral and mildly acidic conditions exhibited insignificant FA concentrations, less than 1.67 mg/L. In contrast to the variable FA concentrations, FNA concentrations were significant only for the experimental conditions at the pH of 6 and 5.6 (e.g., Trials 2, 4, and 9, 0.29–2.35 mg-FNA/L).
The specific SAA response in this study was attributable to the inhibitory effects of FA and FNA. In order to verify the effects of FA and FNA on SAA, the 2-D plot of the response was overlaid with the FA and FNA concentrations in Fig. 3b. From the center of the experimental design, the pH effects in vertical paths resulted in the largest difference of FA and FNA concentrations for Trials 8 and 9, respectively. A study (Fernández et al. 2012) reported a critical concentration of FA was found at 35–40 mg-FA/L and IC50 at 38 mg/L. However, a lower inhibitory FA level of 13 mg/L for ANAMMOX activity was also reported (Waki et al. 2007). Consistently, a SAA reduction of 21.2% was shown in Trial 8, which related to the drastic FA increase from 1.09 up to 21.54 mg/L in Trial 5.
On the other hand, ANAMMOX bacteria are more susceptible to FNA inhibition than FA inhibition (Isaka et al. 2007; Dapena-Mora et al. 2007). The toxicity of NO2 −–N was mainly caused by FNA in lower pH conditions (Puyol et al. 2014). The inhibitory effects by FNA were observed in numerous studies with up to 213 μg-FNA/L (Chen et al. 2011; Fernández et al. 2012; Jin et al. 2012). In this sense, following another vertical path from Trial 5 to Trial 9, the FNA increase from 0.05 to 2.4 mg/L showed the most remarkable reduction in SAA. Instead of FNA inhibition, direct NO2 −–N inhibition was found more effective in neutral and moderately alkaline pH conditions of 7–8.4 in this study (Trials 1, 3, 5, 6, 7, and 8). Previous studies reported that the critical NO2 −–N concentration was in the range of 100 to 350 mg/L (Strous et al. 1999; Dapena-Mora et al. 2007; Egli et al. 2001). The highest concentrations of NO2 −–N were 110 and 122.4 mg-N/L of Trials 1, 2, and 6, which was the marginal level for a direct inhibition, and NO2 −–N could partially affect SAA in such condition.
Because of the effects of content of NH4 + and NO2 −, FA and FNA concentrations were more drastically affected by the TN concentration of 220 mg-N/L (Trials 1 and 2) than that of 100 mg-N/L (Trials 3 and 4) as the pH varies. However, FNA and FA exerted insignificant effects on SAA following the vertical paths at both 100 mg-N/L (1.41 and 1.49 mg-N/mg-VSS day for pH 8 and 6, respectively) and 220 mg-N/L (1.30 and 1.29 mg-N/mg-VSS day for pH 8 and 6, respectively). This minor effect on SAA was attributed to the moderate inhibitory effect of FNA and FA in mildly acidic and basic conditions.
ANAMMOX SBR operation
The continuous feeding systems of UASB and expanded granular sludge bed diluted the influent in large working volume. Steady-state ANAMMOX reaction rate also contributed to maintaining constant low NH4 + and NO2 − concentrations. In contrast, the semi-continuous SBR feeding system may have an operation difficulty with high content of NH4 + and NO2 −, especially when treating high strength of nitrogenous wastewater. For instance, high NH4 + and NO2 −concentrations of up to 1000 mg-N/L were reported in the effluent of the PN process (Udert et al. 2003). Also, excessive substrate load (or shock loading) caused by high feeding rate and/or extremely high NH4 + and NO2 − concentrations in the influent induced inhibitory effects on the overall nitrogen removal efficiency (Jin et al. 2008). In particular, suspended biomass in MSBR tends to put inhibitory effects on the biological activity, while immobilized biomass showed better resistance in retarded diffusion (Lotti et al. 2012). The behavior of NH4 + and NO2 − concentrations should be carefully monitored and controlled so as not to inhibit SAA, since the content of NH4 + and NO2 − was known for the microbial toxicity. However, the information on the behavior of substrate concentrations and consequent toxic effects on SAA is little for the process of SBR-type ANAMMOX reactors.
The SBR systems were operated in two modes of feeding and reacting stages. The conventional operational regime clearly separated out the feeding stage from the reacting stages in time series, while the fed-batch strategy combined the two stages. The latter feeding regime reportedly provided a faster growth rate of ANAMMOX bacteria than the conventional regime (Van Hulle et al. 2011). Stable hydraulic and physicochemical conditions caused by slow feeding rate might favor the growth of ANAMMOX bacteria. Therefore, the fed-batch strategy is preferred in most SBR operations (Table 2). The hydraulic residence time (HRT) of the fed-batch strategy was from 1 to 2.5 days, and the appropriate volumetric exchange ratio was 20–25% with 6–12 h cycle duration. The optional polishing stage after the fed-batch stage was applied to remove more nitrogen and to improve operational stability in Nos. 5–7 in Table 2.
There was a comparative study by Jin et al. (2008) that a reactor showed consistently high nitrogen removal efficiencies of more than 80% with fixed NH4 + and NO2 − concentrations of 140 mg-N/L, a variable HRT range from 20.2 to 6.9 h, and a nitrogen loading rate of 0.451–1.475 kg-N/m3-day, adjusted by volumetric exchange ratio. However, the same reactor having the shock loading of substrate of the highest NH4 + and NO2 − concentrations up to 515 and 640 mg-N/L, at a fixed HRT of 21.8 h, showed a 50% removal efficiency with a nitrogen loading rate of 1.294 kg-N/m3-day. This indicated that the behavior of the NH4 + and NO2 − concentrations was more critical than a hydraulic condition. However, online monitoring and dynamic control of NH4 + and NO2 − species were somewhat new to the fed-batch strategy, in terms of optimizing the ANAMMOX process. In general, the optimum pH and appropriate substrate concentrations, as well as SAA boundary (η SAA = 1.4–1.77 mg-N/mg-VSS day), discovered from this study can help set a threshold point for controlling constraint or limiting factors during the feeding and reacting stages of ANAMMOX-SBR process, especially when for designing and operating a process.
Alkalinity and temperature
As for alkalinity, with a molar ratio of NH4 +:CaCO3 = 1:0.5, it is only sufficient for PN reaction (Bae et al. 2014). After the PN reaction, the pH of the alkalinity-depleted wastewater drops below 6. Thus, alkalinity was added for the PN reaction to complete, maintaining the suggested pH around 7.34 (in reservoir or by online control). For promptly neutralizing the acidic condition, strong bases such as KOH and NaOH are known effective. Among other acidification buffers, the CaCO3 in limestone has slow solubilization rate, but it will allow sufficient inorganic carbon content last for the growth of ANAMMOX bacteria as suggested in Fig. 1b.
The optimum temperature for SAA was in a range of 30–40 °C. A higher temperature above 40 °C was found to inhibit the ANAMMOX activity because of a biomass cell lysis (Dosta et al. 2008). Most BNR processes such as nitritation, nitratation, and denitrification were feasible with temperature above 40 °C, and mesophilic ANAMMOX bacteria enduring above 55 °C were found (Lopez-Vazquez et al. 2014; Toh et al. 2002). Lotti et al. (2015) reported that SAA rate decreased by 20% depending on the biomass origin when decreasing temperature from 30 to 20 °C, in accordance with different temperature coefficients of the Arrhenius equation. A sharp reduction of SAA occurred by 50% as temperature decreased from 30 to 25 °C in Fig. 1d. However, SAA was insensitive to temperature variation in the range of 30–40 °C in this study. The insensitivity of SAA at relatively higher temperature may be due to the saturated enzyme activity of the maximum reaction rate at 30 °C. Temperature is not a significant controlling factor for process optimization with practical aspects, since most conventional ANAMMOX processes are being operated in the range of 30–35 °C (Van Hulle et al. 2010). Again, any accidental overheating or heterogeneity of temperature distribution in an ANAMMOX reactor should be strictly avoided because ANAMMOX bacteria hardly bear irreversible heat for 1 h at 48 °C (Liu et al. 2015).
Conclusions
In terms of technical performance and operating optimization, an ANAMMOX process was evaluated using the SBR system. TN and pH were a controlling factor for the most optimized ANAMMOX system. The findings from this study confirmed on the optimum operating conditions and technical parameters, which will make a useful data set for a full-scale application. In particular, the optimized ANAMMOX system will be directly employed for treating high content of nitrogen wastewater streams from a number of industries and municipalities. Ultimately, it will relieve conventional biological process of operation and maintenance (O&M) costs, as an alternative to nitrification and denitrification.
References
Arrojo B, Mosquera-Corral A, Campos JL, Méndez R (2006) Effects of mechanical stress on Anammox granules in a sequencing batch reactor (SBR). J Biotechnol 123(4):453–463
Bae H, Chung Y-C, Jung J-Y (2010) Microbial community structure and occurrence of diverse autotrophic ammonium oxidizing microorganisms in the anammox process. Water Sci Technol 61(11):2723–2732
Bae H, Yang H, Chung YC, Yoo YJ, Lee S (2014) High-rate partial nitritation using porous poly (vinyl alcohol) sponge. Bioprocess Biosyst Eng 37(6):1115–1125
Bae H, Yang H, Choi M, Chung YC, Lee S, Yoo YJ (2015) Optimization of the mechanical strength of PVA/alginate gel beads and their effects on the ammonia-oxidizing activity. Desalination Water Treat 53(9):2412–2420
Chen T, Zheng P, Shen L, Ding S, Mahmood Q (2011) Kinetic characteristics and microbial community of Anammox-EGSB reactor. J Hazard Mater 190(1):28–35
Dapena-Mora A, Fernandez I, Campos JL, Mosquera-Corral A, Mendez R, Jetten MSM (2007) Evaluation of activity and inhibition effects on Anammox process by batch tests based on the nitrogen gas production. Enzyme Microb Technol 40(4):859–865
Dosta J, Fernandez I, Vazquez-Padin JR, Mosquera-Corral A, Campos JL, Mata-Alvarez J, Mendez R (2008) Short-and long-term effects of temperature on the Anammox process. J Hazard Mater 154(1):688–693
Egli K, Fanger U, Alvarez P, Siegrist H, van der Meer J, Zehnder A (2001) Enrichment and characterization of an anammox bacterium from a rotating biological contactor treating ammonium-rich leachate. Arch Microbiol 175(3):198–207
Fernández I, Vázquez-Padín JR, Mosquera-Corral A, Campos JL, Méndez R (2008) Biofilm and granular systems to improve Anammox biomass retention. Biochem Eng J 42(3):308–313
Fernández I, Dosta J, Fajardo C, Campos JL, Mosquera-Corral A, Méndez R (2012) Short-and long-term effects of ammonium and nitrite on the Anammox process. J Environ Manag 95:S170–S174
Fux C, Marchesi V, Brunner I, Siegrist H (2004) Anaerobic ammonium oxidation of ammonium-rich waste streams in fixed-bed reactors. Water Sci Technol 49(11–12):77–82
Hu Z, Lotti T, de Kreuk M, Kleerebezem R, van Loosdrecht M, Kruit J, Jetten MSM, Kartal B (2013) Nitrogen removal by a nitritation-anammox bioreactor at low temperature. Appl Environ Microbiol 79(8):2807–2812
Isaka K, Sumino T, Tsuneda S (2007) High nitrogen removal performance at moderately low temperature utilizing anaerobic ammonium oxidation reactions. J Biosci Bioeng 103(5):486–490
Jaroszynski LW, Cicek N, Sparling R, Oleszkiewicz JA (2011) Importance of the operating pH in maintaining the stability of anoxic ammonium oxidation (anammox) activity in moving bed biofilm reactors. Bioresour Technol 102(14):7051–7056
Jetten MS, Wagner M, Fuerst J, van Loosdrecht M, Kuenen G, Strous M (2001) Microbiology and application of the anaerobic ammonium oxidation (‘anammox’) process. Curr Opin Biotechnol 12(3):283–288
Jin RC, Zheng P, Hu AH, Mahmood Q, Hu BL, Jilani G (2008) Performance comparison of two anammox reactors: SBR and UBF. Chem Eng J 138(1):224–230
Jin RC, Yang GF, Yu JJ, Zheng P (2012) The inhibition of the Anammox process: a review. Chem Eng J 197:67–79
Liu Y, Tay JH (2002) The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge. Water Res 36(7):1653–1665
Liu S, Zhang Z, Ni J (2015) Behavior detection and activity recovery of damaged anammox bacteria culture after accidental overheating. Chem Eng J 259:70–78
López H, Puig S, Ganigué R, Ruscalleda M, Balaguer MD, Colprim J (2008) Start-up and enrichment of a granular anammox SBR to treat high nitrogen load wastewaters. J Chem Technol Biotechnol 83(3):233–241
Lopez-Vazquez CM, Kubare M, Saroj DP, Chikamba C, Schwarz J, Daims H, Brdjanovic D (2014) Thermophilic biological nitrogen removal in industrial wastewater treatment. Appl Microbiol Biotechnol 98(2):945–956
Lotti T, Van der Star WRL, Kleerebezem R, Lubello C, Van Loosdrecht MCM (2012) The effect of nitrite inhibition on the anammox process. Water Res 46(8):2559–2569
Lotti T, Kleerebezem R, van Loosdrecht MCM (2015) Effect of temperature change on anammox activity. Biotechnol Bioeng 112(1):98–103
Ni BJ, Chen YP, Liu SY, Fang F, Xie WM, Yu HQ (2009) Modeling a granule-based anaerobic ammonium oxidizing (ANAMMOX) process. Biotechnol Bioeng 103(3):490–499
Pereira AD, Leal CD, Dias MF, Etchebehere C, Chernicharo CAL, de Araújo JC (2014) Effect of phenol on the nitrogen removal performance and microbial community structure and composition of an anammox reactor. Bioresour Technol 166:103–111
Puyol D, Carvajal-Arroyo JM, Sierra-Alvarez R, Field JA (2014) Nitrite (not free nitrous acid) is the main inhibitor of the anammox process at common pH conditions. Biotechnol Lett 36(3):547–551
Riter LS, Vitek O, Gooding KM, Hodge BD, Julian RK (2005) Statistical design of experiments as a tool in mass spectrometry. J Mass Spec 40(5):565–579
Scaglione D, Ficara E, Corbellini V, Tornotti G, Teli A, Canziani R, Malpei F (2015) Autotrophic nitrogen removal by a two-step SBR process applied to mixed agro-digestate. Bioresour Technol 176:98–105
Sinha B, Annachhatre AP (2007) Partial nitrification-operational parameters and microorganisms involved. Rev Environ Sci Biotechnol 6(4):285–313
Strous M, Heijnen JJ, Kuenen JG, Jetten M (1998) The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. App Microbiol Biotechnol 50(5):589–596
Strous M, Kuenen JG, Jetten MSM (1999) Key physiology of anaerobic ammonium oxidation. App Environ Microbiol 65(7):3248–3250
Tao Y, Gao DW, Fu Y, Wu WM, Ren NQ (2012) Impact of reactor configuration on anammox process start-up: MBR versus SBR. Bioresour Technol 104:73–80
Toh SK, Webb RI, Ashbolt NJ (2002) Enrichment of autotrophic anaerobic ammonium-oxidizing consortia from various wastewaters. Microbial Ecol 43(1):154–167
Udert K, Fux C, Mnster M, Larsen T, Siegrist H, Gujer W (2003) Nitrification and autotrophic denitrification of source-separated urine. Water Sci Technol 48(1):119–130
van der Star WR, Miclea AI, van Dongen UG, Muyzer G, Picioreanu C, van Loosdrecht M (2008) The membrane bioreactor: a novel tool to grow anammox bacteria as free cells. Biotechnol Bioeng 101(2):286–294
Van Hulle SW, Vandeweyer HJ, Meesschaert BD, Vanrolleghem PA, Dejans P, Dumoulin A (2010) Engineering aspects and practical application of autotrophic nitrogen removal from nitrogen rich streams. Chem Eng J 162(1):1–20
Van Hulle SW, Vandeweyer H, Audenaert W, Monballiu A, Meesschaert B (2011) Influence of the feeding regime on the start-up and operation of the autotrophic nitrogen removal process. Water SA 37(3):289–294
Waki M, Tokutomi T, Yokoyama H, Tanaka Y (2007) Nitrogen removal from animal waste treatment water by anammox enrichment. Bioresour Technol 98(14):2775–2780
Wang T, Zhang H, Gao D, Yang F, Zhang G (2012) Comparison between MBR and SBR on Anammox start-up process from the conventional activated sludge. Bioresour Technol 122:78–82
Yang K, Oh C, Hwang S (2004) Optimizing volatile fatty acid production in partial acidogenesis of swine wastewater. Water Sci Technol 50(8):169–176
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and Future Planning (2014R1A1A2A16054920), and by the Korea Institute of Science and Technology (2E26251).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bae, H., Paul, T., Kim, D. et al. Specific ANAMMOX activity (SAA) in a sequencing batch reactor: optimization test with statistical comparison. Environ Earth Sci 75, 1452 (2016). https://doi.org/10.1007/s12665-016-6252-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6252-7