Abstract
In this study, the drinking groundwater quality of Lenjanat plain, Iran, is classified based on water quality index (WQI), Takagi–Sugeno–Kang fuzzy water quality index (TSKFWQI) and entropy weighted water quality index (EWQI). Groundwater samples from 79 regional monitoring wells and different resources such as agricultural and potable deep wells, rural dug wells, industrial and recreational facilities and drilled wells in the vicinity of pollution sources of urban and rural sewage discharge points were collected and analyzed during 2009–2010. In this research, physicochemical parameters including As, Pb, Cr, Ni, Cu, NO3, Na, K, F, Cl, Ba, Ca, Mg, Fe, SO4 and TDS were used to calculate the drinking quality rank of water samples using WQI, TSKFWQI and EWQI methods. Calculations showed that ranking the groundwater samples using WQI is very similar to ranks determined by entropy-based calculations of water quality index, while the TSKFWQI clearly indicates that this classification method acts stricter than two other methods (WQI and EWQI). In TSKFWQI, the final rank of any sample is very much affected by toxic parameters. It means that a sample with acceptable range of all parameters, except one toxic parameter, falls in the unacceptable rank. As a result, in areas where water chemistry shows the presence of some toxic elements in the groundwater resources, TSKFWQI classification of water with regard to drinking purposes gives more reliable results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is a dynamic renewable natural resource. Its availability with good quality and adequate quantity is very important for human life and other purposes. Water quality in an aquatic ecosystem is determined by many physical, chemical and biological factors (Sargaonkar and Deshpande 2003). In general, the quality of water is equally important as the quantity.
Various methods are discussed in the literature on drinking water quality criteria and decision making. But most of the reports on the water quality revealed that deterministic approach in decision making by comparing values of parameters of water quality with prescribed limits provided by different regulatory bodies is used without considering uncertainties involved in various steps throughout the entire procedure (Deshpande et al. 1996a, b; Garg et al. 1998; Dahiya and Kaur 1999; Dahiya et al. 2000, 2005; Khaiwal and Garg 2006). However, one of the most popular and commonly used methods during the last few decades was water quality index (WQI).
Decision making using comparison of water quality prescribed limits with various water quality indices has been developed to integrate water quality variables (Mitchell and Stapp 1996; Cude 2001; Liou et al. 2004; Said et al. 2004). This approach has a few drawbacks such as some parameters in the index equations can influence the final score of WQI dramatically without valid scientific justification. There are limitations on which a wrong decision can be taken as it is dependent on the fix weightage assigned to different parameters, whereas the weighting should be varied on the basis of season, rainfall and water intake of individual, ambient temperature, occupational, residential and other environmental factors. These indices are lacking to deal with uncertainties involved at various steps in decision making (Silvert 2000; Chang et al. 2001; Duque et al. 2006). Due to these limitations of the deterministic and WQI approach, an advanced classification method is required, which is capable of accounting for imprecise and vague information in decision making on drinking water quality.
Considering that the water quality assessment for natural water resource is a fuzzy concept with multiple indicators and classes, the fuzzy comprehensive evaluation method, which has been proved effective in solving problems of fuzzy boundaries and controlling the effect of monitoring errors on assessment results (Guleda et al. 2004; Wang 2002), has been studied for the water quality assessment extensively in recent years (Shen et al. 2005; Karmakar and Mujumdar 2006; Icaga 2007; Lermontova et al. 2009; Gharibi et al. 2012; Ocampo-Duquea et al. 2013; Kord and Asghari Moghaddam 2014). These researches find that this model is fit to describe fuzzy character of classified bounds for water quality and can reflect the actual water quality on objectiveness. However, there are still some limits when applying the fuzzy comprehensive evaluation method in water quality assessment, such as when the method emphasizes extreme value action, more information is lost and the scientific character of weight value is not clear. In particular, the weight value which usually contains the information about the individual indicator only but has nothing to do with the relationship between assessment objects.
To solve the problems, the information entropy has been introduced. Shannon (1948) introduced the entropy concept into information theory by suggesting entropy as a measure of information or uncertainty. Shannon entropy expresses the degree of uncertainty implicated in predicting the output of a probabilistic event. Mathematically, an inverse relationship exists between the amount of information and the probability of occurrence. If the occurrence of an event can be precisely predicted, the probability value will be great, and inversely, the Shannon entropy will be small. Hence, information and uncertainty as dual terms that reveal the information gained are indirectly measured as the amount of reduced uncertainty. As a measure of the disorder degree of a system, information entropy can measure the amount of useful information with the provided data and has been widely used in engineering, economy and finance, etc. (Chang et al. 1994; Shuiabi et al. 2005; Piplani and Wetjens 2007; Yu and Liu 2011; Castellanoa et al. 2013; Lia et al. 2015). When the difference of the evaluating objects on the same indicator is large, the entropy is small, which illustrates that this indicator provides more useful information and this indicator’s weight should be set relatively large. On the other hand, if the difference is small, the entropy is large, and the corresponding weight would be small. (Zou et al. 2006). Studies show that correlations of entropy weight with mean, standard deviation and coefficient of variation are significant. However, the study on entropy weight is still not perfect and it has close relationship with the number of samples and indexes used for the assessment.
Based on the concept mentioned earlier, this paper compares the WQI with two improved WQI based (fuzzy logic of water quality index (FWQI) and EWQI) to determine the efficiency of these methods in groundwater classification.
Materials and methods
Sampling and chemical analysis
In this study, groundwater from 79 regional monitoring wells was sampled and analyzed annually during 2009–2010. The study was conducted for wet and dry seasons separately. In this study, 10 chemical parameters including calcium (Ca), sodium (Na), potassium (K), magnesium (Mg), sulfate (SO4), chloride (Cl), fluoride (F), nitrate (NO3), iron (Fe), barium (Ba), copper (Cu), nickel (Ni), chromium (Cr), lead (Pb), arsenic (As) and electrical conductivity (EC) were used to evaluate the groundwater quality for drinking purpose. Water samples were collected from pumping wells after minimum of 2 h of pumping prior to sampling in order to remove any standing water from the wells. At each sampling point, samples were stored in two polyethylene bottles and were divided into two groups: (1) non-acidified for anion analysis, and (2) acidified (with a few drops of pure nitric acid) for cation analysis. The collected samples were kept in an ice box and then transferred to a fridge where they were stored at 4 °C until delivery to the laboratory. The Na, K, As, Ba, Pb, Cr, Ni, Cu and Fe concentrations were then measured using atomic absorption spectrometry. Values of NO3, SO4, F and Cl were determined using spectrophotometer technique. The concentration of Ca and Mg was determined titrimetrically using standard EDTA. The EC values were measured in the field, because this parameter is likely to change during transport.
The principles of water quality indices
WQI
In the formulation of WQI, the importance of various parameters depends on the intended use of water; here, water quality parameters are studied from the point of view of suitability for human consumption. WQI is used to assess water quality trends for management purpose. The estimation of WQI requires the selection of parameters of great importance since the selection of many numbers of parameters widen the water quality index. For computing the WQI, the subindex SI i was first determined for each chemical parameter, which was then used to determine the WQI:
where SI i is the subindex of the ith parameter, W i is weight associated with ith water quality parameter, q i is the rating based on concentration of ith parameter and n is the number of parameters.
TSKFWQI
There are mainly two approaches in fuzzy model: First one is the Mamdani approach, and the other is Takagi–Sugeno–Kang (TSK) approach. The TSK fuzzy model is a well-known method of fuzzy reasoning with numerous applications in fuzzy systems, fuzzy control and in general in fuzzy engineering. The main advantage of the TSK model is that is a simple model and can be used in many practical applications for modeling and control of complex systems. Due to its importance, the TSK model has attracted much attention among theoretical academic scientists as well as engineers that work in industry and applied research. The TSK fuzzy model consists of four major elements of member functions, internal functions, rules and outputs. The general algorithm of this fuzzy inference system is calculated as follows.
It is assumed that there are R r (r = 1, 2, …, n) rules.
-
(1)
For each implication R i , y i is calculated by the function f i in the consequence
$$y_{r} = f(x_{1} ,x_{2} , \ldots ,x_{p} ) = b_{r} (0) + b_{r} (1)x_{1} + \cdots + b_{r} (p)x_{p}$$(3) -
(2)
The weights are calculated
$$r_{r} = \left( {m_{1}^{r}\Lambda , m_{2}^{r}\Lambda , \ldots ,m_{k}^{r} } \right) \times R_{y}$$(4)where m r1 , m r2 , …, m r k denote the angle cuts of membership functions according to input values for the rth rule. The occurrence probability is shown by R r and Λ stands for min operation. For the sake of simplicity, R r is equal to 1.
-
(3)
The final output y inferred from n implications is given as the average of all y r with the weights r r
$$y = \frac{{\sum\nolimits_{r = 1}^{n} {r_{r} \times y_{r} } }}{{\sum\nolimits_{{r_{i} }}^{n} {r_{r} } }}$$(5)
EWQI
Entropy is the measure of the disorder degree of the system, and it can measure the amount of useful information with the data provided. Therefore, the entropy can be used to determine the weights. When the evaluated objects have quite a large difference between each other on a particular indicator, the entropy is smaller, which shows that when the indicators provide more effective information, the weight of the indicators should be larger; on the contrary, the smaller the difference, the larger the entropy, which shows that the smaller amount of information provided by indicators, the smaller the indicator weights. Therefore, the entropy coefficient method is an objective empowering method. To determine the weight by calculating the entropy is just the way of determining the weights of each indicator based on the evaluated of the variation degree of every evaluation indicator value. The main steps of using entropy coefficient method to determine the weights are formed in the following manner.
-
(1)
Normalization of the original evaluating matrix
Suppose there are n evaluation indicators and m evaluation objects, then the original indicators value matrix X is formed
The matrix is normalized to get the following equation:
where v ij is the standard value of the ith evaluation object on the jth evaluation indicator, v ij ∊ [0, 1]. Among these indicators, to which the bigger the better, there are
while the smaller the better, there are
-
(2)
Definition of the entropy
During the evaluation where there are n evaluation indicators and m evaluation objects, the definition of the entropy of the jth indicator is:
where
-
(3)
Definition of the entropy weight
The definition of the entropy weight of the jth indicator is
where
Study area
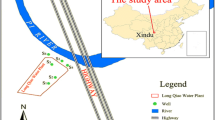
Lenjanat plain located in the southwest of Isfahan province, Iran, is selected for this study. The plain area and average altitude are approximately 1170 km2 and 1800 MSL, respectively. Lenjanat plain is a sub-basin of Zayandehrud River. According to the Emberger climate classification, the area is under the influence of an arid and semiarid desert climate. The geology and geographic location of the area are shown in Fig. 1.
The sediments of the area are included, ranging from Permian to Quaternary geological deposits (Geological Survey of Iran 1976a, b). The most important outcrops in the area are Jurassic shale and widely thick layers of Cretaceous limestone that play the role of bedrock in the most parts of the aquifer. Alluvial deposits are clay (at least 50 %), sand and gravel. Evaporite layers with gypsum and salt minerals have been deposited among the alluvium. The percentage of salt and clay deposits is higher in the bottom layers (Jafarian 1985; Iranian ministry of Energy 1985; Parsabe Sepahan Andish 2008). The Lenjanat alluvial aquifer is mainly recharged from limestone and crushed thick layers of Cretaceous rocks located from west to the south of the mountain line. The general direction of groundwater flow is from west to east, following the overall basin slope. The maximum depth of the water table in the study area is 50 m (east of the area) and its minimum is about 1 m (adjacent to the river) (Nassery and Kayhomayoon 2013).
In the Lenjanat plain, industrial and some part of agricultural (riverbank parts of agriculture) water supplies come from the Zayandehrud River and a major part of agriculture water and domestic supply is obtained from groundwater resources. Agriculture is performed as double planting (spring and autumn), and flooding irrigation is more used in the plain. In recent years, due to groundwater salinization, agricultural activities have been limited to planting of cereals (wheat, alfalfa and clover) in the Lenjanat plain.
Results and discussion
The right prediction of the fuzzy model strongly depends on the number of fuzzy sets. In the case of TSKFWQI approach, these 16 parameters were divided into the four categories on the basis of expert opinion (Saberi Nasr et al. 2013; Kord and Asghari Moghaddam 2014), having their importance with respect to drinking water quality criteria. Toxic heavy metals like As, Pb, Cr and Ni were kept in the first group, Fe, Zn, Cu and Mn were categorized in the second group, K, Ca, Mg and Na in the third group and F, Cl, SO4 and NO3 in the fourth group. The results of physicochemical parameters analysis of these groups are presented in Table 1.
Since each group consists of four input parameters and each parameter consists of three membership functions, the implemented fuzzy rules for this group equal eighty-one (3 × 3 × 3 × 3). Each rule had only one antecedent in order to facilitate the weight assignment. The structure of fuzzy rules was: if indicator i is “Desirable,” then TSKFWQI is “Excellent or Good,” if indicator i is “Acceptable,” then TSKFWQI is “Medium” and if indicator i is “Not-acceptable” then TSKFWQI is “Poor or Extremely Poor” (Table 2).
As listed in Table 3, the concentrations of Fe, Ni, Na, Ba and F are the more affecting parameters in water quality classification based on entropy theory. On the other hand, the major parameters with high impact on WQI calculation include F, NO3, Pb, As, Cd and TDS.
The weights of the five parameters in both of the methods are larger than other parameters. This means that the groundwater assessment results are greatly affected by the concentrations of these parameters. The water quality classification based on mentioned methods is presented in Tables 4 and 5.
In this study, a total of 79 samples were analyzed for classification. Detail on groundwater quality for drinking purposes by using deterministic, TSKFWQI, EWQI and WQI methods is presented in Tables 6 and 7. Distributions of groundwater quality rank in Lenjanat plain are shown in Fig. 2.
The groundwater samples were assessed based on mentioned methods. Table 7 presents the parameters with concentrations greater than the desirable and admissible limits in each sample.
Results show that there are 15 samples that each one of them has the same rank between TSKFWQI, EWQI and WQI. For example, the sample 1 has rank 1 and the sample 3 has rank 3 for three different methods, but the samples 1 and 2 are different in deterministic method. According to Table 6, the quality of samples 23 is worse than other samples of in EWQI and WQI classifications (rank 5 for EWQI and WQI). In sample 32, EWQI shows excellent, WQI good but TSKFWQI poor. Pb is a toxic element and very harmful for human health and in this example Pb concentration is not acceptable (14 μg/l) and TSKFWQI has appropriate assessment of the water quality.
The number of not-acceptable parameters is unique in the water sample 29 and 30, while the decision taken with methods for these two samples is entirely different so that sample 29 in rank of quality parameters is better than sample 30, because of the concentrations of not-acceptable parameters are very high and lie in the range of acceptable, which causes the water sample 29 to be worse than sample 30 (1210 mg/l Ca, 800 mg/l SO4 for sample 29 and 610 mg/l Ca, 208 mg/l SO4 for sample 30).
According to Table 7, seven samples (32, 33, 40, 42, 46, 66 and 79) have the heavy metal contents more than standard values. So, these samples show the worse quality for drinking based on TSKFWQI classification. Results of WQI and EWQI calculations show the two different quality ranks for these samples, because the parameters of the two methods have different weight. For example, based on EWQI and WQI classifications, samples 32 and 42 have the “Excellent” and “Good” quality, respectively. Both EWQI and WQI show the same rank (rank 3 or medium) for sample 33. According to EWQI method, samples 40, 66 and 79 are in rank 2 (good quality), while these samples show the rank 3 (medium quality) based on WQI method calculations.
The maximum coexistence of not-acceptable parameters (six parameters) can be seen in samples 20, 24, 33 and 78. Based on TSK and WQI/EWQI methods, samples 20 and 24 show the “Poor” and “Medium” quality rank, respectively. On the other hand, sample 33 has the “Very poor” and “Medium” quality according to TSKFWQI and WQI/EWQI calculations, respectively. Also, classifications indicate the “Poor,” “Good” and “Medium” quality of sample 78 according to the results of water quality computation based on TSKFWQI, EWQI and WQI methods, respectively.
The samples 24 and 33 have same not-acceptable parameters except one, in sample 24, “Fe” and in 33, “Pb”. These samples show the same rank (rank 3 or medium) based on WQI and EWQI but different in base on TSKFWQI classification. Pb is toxic and the presence of Pb in the sample is more important than Fe, and therefore, the quality of these samples should be different. According to TSKFWQI, sample 24 has the “poor” and sample 33 has “very poor” quality and this method shows good sensitivity to these parameters.
The fuzzy-based classification of groundwater samples (TSKFWQI) in Lenjanat plain indicates that 12 samples have the rank 1 (excellent quality). In six of the 12 samples, the “Excellent” quality despite high concentrations (not acceptable) of some chemical parameters is noteworthy. In fact, the main reason for this contradiction is that the concentration of some parameters is higher than permissible limits but very close to these limits. This phenomenon as a defect in accuracy of a method is seen in the EWQI and WQI methods. As a result, in areas where water chemistry shows the presence of some toxic elements in the groundwater resources, TSKFWQI classification of water for drinking gives more reliable results.
Water quality in many parts of Lenjanat plain shows a large quality change. According to Fig. 3, the majority of sampling points with poor quality are located in urban, rural and industrial areas and in vicinity of Zayandehrud River. Increasing the concentration of toxic heavy metals and NO3, SO4, Cl and F in groundwater is mainly due to infiltration of chemical fertilizers in agricultural areas, gypsum dissolution, cation exchange and leaking of the urban, rural, industrial wastewaters to underground. Under these conditions, concentration of solutes and groundwater pollutants can be changed by climatic factors such as precipitation as the most important agent (by change of dissolution rate and dilution of contaminants) in water quality oscillation.
In order to establish the relationship between two variables, a correlation coefficient is generally used. This is simply a measure to exhibit how well one variable predicts the other. There are two main criteria including Pearson’s correlation and Spearman’s rank correlation to determine the correlation between data. Pearson’s correlation works well if the relationship between variables is linear and if the variables are roughly normal. In the present study, the results of Kolmogorov–Smirnov normality test indicated that the variables have no normal distribution. Therefore, Spearman’s rank correlation coefficients were employed to illustrate and measure associations between variables. If the correlation coefficient (r) is greater than 0.7, two parameters are considered to be strongly correlated, whereas if the r value is between 0.5 and 0.7, it indicates a moderate correlation at a significance level p < 0.05 (Shyu et al. 2011). The statistical software package SPSS 19.0 for Windows was used to calculate Spearman’s correlation coefficients, with the obtained results listed in Table 8.
As seen from Table 8, there is strong positive correlation between TDS with Na, moderate positive correlations between SO4 with NO3 and Ca and F with Na. In addition, there are weak positive correlations between some heavy metals like Ni and Fe with Cr, Cu with Fe, Cu with Pb, Pb and Fe with Cu. No significant correlations are found between the other elements. This shows that the source of these metals is independent from each other.
Groundwater chemistry depends on a number of factors, such as general geology, degree of chemical weathering of the various rock types, quality of recharge water and inputs from sources other than water–rock interaction (Sarath Prasanth et al. 2012). The Gibbs diagram is widely used to establish the relationship of water composition and aquifer lithological characteristics. Three distinct fields such as precipitation dominance, evaporation dominance and rock–water interaction dominance areas are shown in the Gibbs diagram (Gibbs 1970).
According to the Gibbs diagram, the predominant samples fall in the rock–water interaction dominance and evaporation dominance fields (Fig. 4). The rock–water interaction dominance field indicates the interaction between rock chemistry and the chemistry of the percolated waters under the subsurface. In this study, rock-weathered materials derived from the underlying rocks control the groundwater chemistry of water samples. In some samples, evaporation greatly increases concentrations of ions formed by chemical weathering, leading to higher salinity.
Conclusions
In this paper, the fuzzy mathematical method and the information entropy theory are used to improve the conventional water quality index (WQI) and compare the results of drinking water classification based on these three methods. Calculations showed that ranking the groundwater samples using WQI is very similar to ranks determined by entropy-based calculations of water quality index (EWQI). In contrast, the TSKFWQI clearly indicates that this classification method acts stricter than the other two methods (WQI and EWQI). In TSKFWQI, the final rank of any sample is very much affected by toxic parameters. It means that a sample with acceptable range of all parameters (except one toxic parameter) falls the unacceptable rank. Therefore, some samples, which have the good quality based on WQI and EWQI, show the worse quality (higher rank) in TSKFWQI. As a result, in areas where water chemistry shows the presence of some toxic elements in the groundwater resources, TSKFWQI classification of water for drinking gives more reliable results. The results revealed that the hydrochemical properties of the groundwater samples were controlled by rock–water interactions including ion exchange, dissolution of evaporation deposits (halite and gypsum) and precipitation/dissolution of carbonates. In addition to impact of geological formations, various agricultural and anthropogenic activities in industrial areas can change the hydrochemical properties of the water in the Lenjanat aquifer.
References
Castellanoa G, González-Santandera JL, Laraa A, Torrensb F (2013) Classification of flavonoid compounds by using entropy of information theory. Phytochem 93:182–191
Chang CI, Chen K, Wang J, Althouse MLG (1994) A relative entropy based approach to image thresholding. Pattern Recogn 27(9):1275–1289
Chang N, Chen HW, King SK (2001) Identification of river water quality using the fuzzy synthetic evaluation approach. J Environ Manag 63:293–305
Cude CO (2001) Water quality index: a tool for evaluating water quality management effectiveness. J Am Water Resour Assoc 37:125–137
Dahiya S, Kaur A (1999) Assessment of physico-chemical characteristic of underground water in rural areas of Tosham subdivision, Bhiwani-Haryana. J Environ Pollut 6:281–288
Dahiya S, Kaur A, Garg VK, Jain N (2000) Quantification of fluoride in groundwater in rural area of Tosham subdivision, district Bhiwani, Haryana. Environ Sci Pollut Res 19:417–419
Dahiya S, Datta D, Kushwaha HS (2005) A fuzzy synthetic evaluation approach for assessment of physico-chemical quality of groundwater for drinking purposes. Environ Geol 8:158–165
Deshpande AW, Raj DV, Khanna P (1996a) Fuzzy description of river water quality, Paper for International Conference EUFIT
Deshpande AW, Raj DV, Khanna P (1996b) Agreement Index for water consumption. Paper for international conference EUFIT
Duque WO, Huguet NF, Domingo JL, Schuhmacher M (2006) Assessing water quality in rivers with fuzzy inference systems: a case study. Environ Int 32:733–742
Garg VK, Dahiya S, Chaudhary A (1998) Fluoride distribution in underground waters of Jind district, Haryana, India. Ecol Environ Conserv 4:19–23
Geological Survey of Iran (1976a) Geological Map of Iran 1:100000 Series, Sheet 6354 Shahreza. Ministry of Industry and Mines
Geological Survey of Iran (1976b) Geological Map of Iran, 1:100000 Series, Sheet 6254 Riz-e-Lenjan. Ministry of Industry and Mines
Gharibi H, Mahvia AH, Nabizadeha R, Arabalibeikd H, Yunesiana M, Sowlatf MH (2012) A novel approach in water quality assessment based on fuzzy logic. J Environ Manag 112(15):87–95
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 17:1088–1090
Guleda OE, Ibrahim D, Halil H (2004) Assessment of urban air quality in Istanbul using fuzzy synthetic evaluation. Atmos Environ 38(23):3809–3815
Icaga Y (2007) Fuzzy evaluation of water quality classification. Ecol Indic 7:710–718
Iranian Ministry of Energy (1985) The report of geophysical studies in Mobarakeh (Isfahan). Department of Groundwater (in Persian)
Jafarian MA (1985) The project of soils studies in Mobarakeh Steel Co. Isfahan University, Isfahan (in Persian)
Karmakar S, Mujumdar PP (2006) Grey fuzzy optimization model for water quality management of a river system. Adv Water Resour 29:1088–1105
Khaiwal R, Garg VK (2006) Distribution of fluoride in groundwater and its suitability assessment for drinking purposes. Int J Environ Health Res 16:163–166
Kord M, Asghari Moghaddam A (2014) Spatial analysis of Ardabil plain aquifer potable groundwater using fuzzy logic. J King Saud Univ Sci 26(2):129–140
Lermontova A, Yokoyamab L, Lermontovc M, Augusta Soares Machadod M (2009) River quality analysis using fuzzy water quality index: Ribeira do Iguape river watershed, Brazil. Ecol Indic 9(6):1188–1197
Lia Z, Guc J, Zhuanga H, Kanga L, Zhaoa X, Guoa Q (2015) Adaptive molecular docking method based on information entropy genetic algorithm. Appl Soft Comput 26:299–302
Liou S, Lo S, Wang SA (2004) Generalized water quality index for Taiwan. Environ Monit Assess 96:35–52
Mitchell MK, Stapp WB (1996) Field manual for water quality monitoring: an environmental education program or schools. Thomson-Shore Inc., Dexter, Michigan, p 277
Nassery HR, Kayhomayoon Z (2013) Source of salinity in the groundwater of Lenjanat plain, Isfahan, Iran. Environ Earth Sci 68:413–427
Ocampo-Duquea W, Osorioa C, Piambaa C, Schuhmacherb M, Domingoc JL (2013) Water quality analysis in rivers with non-parametric probability distributions and fuzzy inference systems: application to the Cauca River, Colombia. Environ Int 52:17–28
Parsabe Sepahan Andish (2008) The project of contaminants recognition in Zarinshahr, 13 vols. Zarinshahr Municipality (in Persian)
Piplani R, Wetjens D (2007) Evaluation of entropy-based dispatching in flexible manufacturing systems. Eur J Oper Res 176:317–331
Saberi Nasr A, Rezaie M, Dashti Barmaki M (2013) Groundwater contamination analysis using Fuzzy Water Quality index (FWQI): Yazd province, Iran. Geopersia 3(1):47–55
Said A, Stevens D, Selke G (2004) An innovative index for evaluating water quality in streams. Environ Manag 34:406–414
Sarath Prasanth SV, Magesh NS, Jitheshlal KV, Chandrasekar N, Gangadhar K (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala, India. Appl Water Sci 2:165–175
Sargaonkar A, Deshpande V (2003) Development of an overall index of pollution for surface water based on a general classification scheme in Indian context. Environ Monit Assess 89(1):43–67
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27(379–423):623–656
Shen GQ, Lu YT, Wang MN, Sun YQ (2005) Status and fuzzy comprehensive assessment of combined heavy metal and organo-chlorine pesticide pollution in the Taihu Lake region of China. J Environ Manag 76(4):355–362
Shuiabi E, Thomson V, Bhuiyan N (2005) Entropy as a measure of operational flexibility. Eur J Oper Res 165:696–707
Shyu GS, Cheng BY, Chiang CT, Yao PH, Chang TK (2011) Applying factor analysis combined with kriging and information entropy theory for mapping and evaluating the stability of groundwater quality variation in Taiwan. Int J Environ Res Public Health 8:1084–1109
Silvert W (2000) Fuzzy indices of environmental conditions. Ecol Model 130:111–119
Wang HY (2002) Assessment and prediction of overall environmental quality of Zhuzhou City, Hunan Province, China. J Environ Manag 66(3):329–340
Yu S, Liu Y (2011) Evolution of regional transport structure information entropy and its dynamic analysis. J Transp Syst Eng Inform Technol 11(4):91–96
Zou ZH, Yun Y, Sun JN (2006) Entropy method for determination of weight of evaluating in fuzzy synthetic evaluation for water quality assessment indicators. J Environ Sci 18(5):1020–1023
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamrani, S., Rezaei, M., Amiri, V. et al. Investigating the efficiency of information entropy and fuzzy theories to classification of groundwater samples for drinking purposes: Lenjanat Plain, Central Iran. Environ Earth Sci 75, 1370 (2016). https://doi.org/10.1007/s12665-016-6185-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6185-1