Abstract
The seasonal and spatial variations of 12 selected antibiotics belonging to four groups, including sulfonamides, quinolones, tetracyclines, and macrolides, were investigated in three main tributaries of Liao River in Jilin Province, northeast China. The concentrations of the antibiotics in the surface water and surface sediments were determined by HPLC–MS/MS. The results indicated that oxytetracycline (OTC), erythromycin (ETM), and ofloxacin (OFL) were dominant antibiotics in the water, with mean concentration of 174.9 ± 266.9, 103.2 ± 95.5, and 67.1 ± 77.3 ng/L, respectively, while OFL, OTC, and norfloxacin were prominent antibiotics in sediments, with mean concentration of 152.2 ± 108.3, 149.5 ± 147.6, and 62.8 ± 83.3 ng/g, respectively. The total antibiotic concentrations in water at each sampling site were 1.2–3 times higher in autumn (November 2015) than those in summer (July 2015), whereas antibiotic concentrations in sediments showed no significant seasonal variation. The highest total antibiotic concentrations in water and sediments were observed around cities with large populations of humans and livestock. Risk assessment based on single antibiotic exposure revealed that OFL, ETM, and OTC were main antibiotics with potential adverse ecological consequences for aquatic organisms. In addition, the mixtures of selected antibiotics posed medium to high risk for aquatic organisms in the Liao River in Jilin Province.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics have been widely applied as humans and veterinary medicines to treat infectious diseases and to promote growth in livestock, agriculture, and aquaculture (Liang et al. 2013). The annual production of antibiotics has been estimated to be 210,000 tons in China. Forty-eight and forty-two percentages of them were applied in agriculture and medicine, respectively, and the remaining 10 % were exported (Luo et al. 2010). Following application to the target organisms, a large fraction (30–90 %) of antibiotics may be released into the environment due to incomplete absorption or metabolism (Sarmah et al. 2006).
Antibiotics in aquatic environments generally originate from municipal wastewater effluents (Miao et al. 2004), agricultural activities, animal waste discharge (Davis et al. 2006), and medical waste (Kümmerer 2009). While some antibiotics may degrade quickly, most are replaced by continual inputs from a variety of pollution sources (Richardson et al. 2005). The ongoing wide use of antibiotics elevates the concentration, so they are pseudopersistent in aquatic environments (Khetan and Collins 2007). Sulfonamides (SAs), quinolones (QNs), tetracyclines (TCs), and macrolides (MLs) are typical antibiotics found in aquatic environments. Once these antibiotics are introduced into an aqueous environment, they may pose a potential risk to the aquatic organisms, increase the spread of antibiotic resistance genes, and eventually pose risks to human health (Gao et al. 2012; Hoa et al. 2011; Kümmerer 2009). In recent years, these antibiotics were frequently detected out in rivers and lakes in China. The concentrations of SAs in water are found even up to mg/L (Li et al. 2012). But most of the measured QNs, TCs, and MLs in water are found at much lower concentration levels (mostly ng/L) (Chen and Zhou 2014; Luo et al. 2011). These antibiotics in sediments are at the concentration levels of ng/g (Chen and Zhou 2014; Li et al. 2012). Moreover, the prominent antibiotics in different rivers vary a lot. For example, MLs are the dominant antibiotics in the surface water from the section of the Liao River in Liaoning Province (Bai et al. 2014), while SAs are prominent in the Huangpu River (Chen and Zhou 2014); TCs and MLs make up the majority of antibiotics in the sediment from the Huangpu River, while QNs have much higher concentrations in Baiyangdian Lake (Li et al. 2012). Thus, complementary regional studies are necessary. In addition, SAs, QNs, TCs, and MLs have different transport and transformation processes in aquatic environments due to the differences in their physicochemical properties. SAs exhibit high solubility and chemical stability in water, whereas MLs tend to be hydrolyzed or sorbed onto sediments (Huang et al. 2011). QNs are susceptible to photodegradation (Jiao et al. 2008) and are also sorbed to sediments (Zhang and Dong 2008), while TCs have a high affinity for sediment organic matter through cation bridging and cation exchange (Figueroa et al. 2004; Rabølle and Spliid 2000; Tolls 2001). Therefore, it is necessary to investigate the concentrations of these antibiotics both in water and in sediment and to estimate the potential risk of these antibiotics to aquatic ecosystems (Chen and Zhou 2014).
Liao River is one of the seven major rivers in China. The river basin is one of the most important heavy industry bases in China. Due to the discharge of wastewater from these industrial sectors, Liao River has become one of the most heavily polluted rivers in China (Dong et al. 2015). Since 2008, some efforts, such as the development of pollution source control technology, have been made for decreasing pollution in the Liao River Basin. The monitoring results for water bodies indicated that the water quality, in terms of indexes COD, BOD, and NH3–N, has been improved year by year. However, there have been concerns regarding the level and ecological risk of emerging contaminants, such as antibiotics, in the river (Hernando et al. 2006; Zhao et al. 2010).
The section of the Liao River in Jilin Province is located in the southwest of Jilin Province, which is the upper river of the Liao system, with a basin area of 11,283 km2 and a population density of 242 per square kilometer. The per capita share of water of this area is 5 times lower than that of China. The main tributaries of Liao River in Jilin Province are Dongliao River, Tiaozi River, and Zhaosutai River, which flow through Siping City and Liaoyuan City and are transboundary rivers of Jilin and Liaoning Province. The area covers a series of traditional industries, including grain processing, pharmacy, paper-making, and chemical industries. The region’s main commodity is grain-based agriculture and livestock farming. The unique geographical location, large population, outdated produce procedures, and increasing livestock numbers in this basin lead to serious water shortages and high pollution levels in the section of the Liao River in Jilin Province. The pollution in this area should be investigated more fully to understand the occurrence, distribution, and risk for aquatic organisms. However, until now, the pollution from emerging contaminants such as antibiotics in this river remains unknown.
The aims of this study were (1) to investigate the occurrence of four classic antibiotics, including SAs, QNs, TCs, and MLs, in both surface water and surface sediments of the Liao River in Jilin Province; (2) to understand the seasonal and spatial distribution of these antibiotics in water and sediments; and (3) to assess the potential hazards of these antibiotics on aquatic organisms.
Materials and methods
Chemicals and reagents
Twelve target antibiotic standards of SAs including sulfamethoxazole (SMX), sulfamerazine (SMR), sulfamethizole (STL), and sulfadiazine (SDZ), QNs including ofloxacin (OFL), enrofloxacin (ENR), ciprofloxacin (CIP), and norfloxacin (NOR), TCs including oxytetracycline (OTC) and tetracycline (TC), and MLs including erythromycin (ETM) and roxithromycin (RTM) were purchased from Dr. Ehrenstorfer (GmbH, Germany). 13C3-caffeine, used as the surrogate standard, was purchased from Cambridge Isotope Labs (1 mg/mL in methanol, USA). Methanol, acetonitrile, and formic acid of HPLC grade were obtained from Merck (Darmstadt, Germany). Milli-Q water was prepared with a Milli-Q water purification system (Millipore, USA).
The stock solutions (100 mg/L) of individual antibiotics and surrogate standard were prepared in methanol. From these 12 individual standards, a 10 mg/L mixture of working standards containing all selected antibiotics was prepared by diluting the stock solution with methanol. All stock solutions were stored in dark at −20 °C prior to use. Working solutions were freshly prepared daily from the stock solutions by serial dilution to eliminate the effect of their instability.
Sampling sites and sample collection
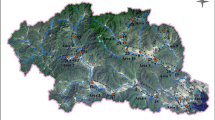
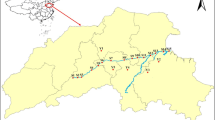
The water and sediments samples were collected from seven sampling sites along the main tributaries of Liao River in Jilin Province, including Dongliao River, Tiaozi River, and Zhaosutai River (Fig. 1). The sampling sites were mainly near the cities or located in the boundary of Jilin Province and Liaoning Province. Four sites are located at the Dongliao River: Lanhezha (S1), Qixiangzhan (S2), Heqing (S3), and Sishaungdaqiao (S4, the boundary section of Dongliao River in Jilin Province). Two sites are located at the Tiaozi River: Huihekou (S5) and Linjia (S6, the boundary section of Tiaozi River in Jilin Province. One site (Lujiazui, S7, the boundary section of Zhaosutai River in Jilin Province) is located in the Zhaosutai River.
Surface water and surface sediment samples were collected in summer (July 2015) and autumn (November 2015). Water samples (2 × 5 L) were collected at each site with a stainless steel bucket which had been pre-cleaned with methanol, Milli-Q water and rinsed by the sample sequentially prior to collection. Sediment samples (about 500 g) were collected from the top 10-cm layer using a grab sampler. Water and sediment samples were stored in 5- and 1-L pre-cleaned amber glass bottles, respectively. The collected samples were immediately placed in a cooler and transported to the laboratory. Once back to the laboratory, the water samples were kept at 4 °C in the dark and target antibiotics were extracted from water samples within 24 h of sample collection. The sediment samples were freeze-dried and passed through a 60-mesh sieve and kept at −20 °C until the analysis. The sediment samples at site 1 in summer and autumn and site 3 in summer were not obtained due to municipal construction and the growth of aquatic plants, respectively.
Sample extraction and HPLC–MS/MS analysis
Target antibiotics were extracted from the water and sediment samples according to the method reported in the literature (Cheng et al. 2014; Xu et al. 2007a, 2007b). Water samples were filtered through glass fiber film with a pore opening of 0.45 μm. Before extraction with solid-phase extraction (SPE) using Oasis HLB cartridges (6 mL, 200 mg, Waters), water samples were acidified to pH 3.0 with 0.1 mol/L HCl, followed by the addition of 0.2 g Na2EDTA as the chelating agent and 100 ng 13C3-caffeine as the surrogate standard to 500 mL water samples. The Oasis HLB cartridges were pre-conditioned with 6 mL methanol and 6 mL ultrapure water at a flow rate of 1 mL/min. Then, the water samples were extracted by SPE at a flow rate of 10 mL/min. After loading of samples, cartridges were washed with 10 mL ultrapure water and vacuum-dried for 10 min. Finally, the cartridges were eluted with 6 mL methanol. Two grams of each sediment sample was weighted into a 50-mL glass tube, followed by addition of 0.4 g Na2EDTA and 100 μL 13C3-caffeine solution (1 mg/L) as the surrogate standard. Fifteen milliliters acetonitrile and 15 mL sodium citrate buffer (pH 3) were added into each glass tube followed by mixing on a vortex mixer for 2 min. All the mixed samples were then ultrasonicated for 20 min and centrifuged at 10000r/min for 2 min. The supernatant was removed to a clean tube, and the sediments were extracted for a second time. The supernatants were combined and evaporated at 55 °C to remove the organic solvent and diluted to 200 mL with ultrapure water. Then, the diluted solutions were filtered through 0.45-μm glass fiber filter. The target antibiotics in diluted solutions were extracted with the tandem cartridge (SAX-HLB, SAX (6 mL, 200 mg, CNW) and HLB (6 mL, 200 mg, Waters)). After loading of all the diluted solutions, SAX cartridges were removed. The rest of the SPE procedure was the same as that used for treating water samples. All the eluates were dried with a stream of nitrogen at 45 °C and then re-dissolved with 1 mL methanol. Prior to analysis with high-performance liquid chromatography–electrospray ionization tandem mass spectrometry (HPLC–MS/MS) system, the methanol solutions were filtered through a 0.22-μm nylon filter.
The 12 target antibiotic compounds in the water and sediments were analyzed by HPLC–MS/MS in duplicate. The separation of the target compounds was performed with Agilent 1200 series (Agilent, USA) on a Waters Xbridge C18 column (150 mm × 2.1 mm, 3.5 μm) after 10 μL of sample extract was injected. The mobile phase contained acetonitrile (A) and 0.1 % formic acid (B). The gradient elution program was as follows: 15 % A (4 min), 50 % A (10 min), 60 % A (20 min), 15 % A (25 min), and finally 15 % A (28 min). The column temperature was set at 30 °C and the flow rate was 0.3 mL/min. Mass spectrometric analysis was conducted on an Applied Biosystems API 4000 tandem mass spectrometer (Applied Biosystems/MDS SCIEX, USA) equipped with an electrospray ionization source operated in the positive mode. Quantification of each target antibiotic in the samples was performed in the multiple reactions monitoring mode.
Quality control
Analyses of antibiotics in water and sediment samples were subject to strict quality control procedures. System performance and potential contamination were monitored by running a solvent blank, procedural blank, and the mixed standard solutions at regular intervals. In order to determine the potential losses during the analytical procedure, 13C3-caffeine was used as the surrogate standard. The concentrations of target antibiotics were determined using an external standard method (Grujić et al. 2009; Li et al. 2009; Zhang et al. 2011). When detection is performed with electrospray ionization mass spectrometer, the internal standard method may be more accurate. However, when analyzing mixture of very different analytes, it is difficult to find appropriate internal standard to fit the majority of analytes. It commonly needs multi-internal standards. In this study, the standard curve with seven concentrations of antibiotics (0.5, 1, 10, 20, 50, 100, and 200 ng/L) was established to quantify the antibiotic concentrations. The linearity of calibration curves was confirmed (r 2 > 0.99) in the tested concentration ranges. The limits of quantification (LOQ) for water and sediment were 0.1–0.5 and 0.2–1.3 ng/L, respectively.
Six spiked concentrations (for water samples, 20, 50, and 100 ng/L; for sediment samples, 20, 50, and 100 ng/g) of the target antibiotics were used to determine the relative recoveries in three replicates. The relative recoveries of SAs, QNs, TCs, and MLs concentrations in water were 80.2–93.6 %, 69.3–82.9 %, 78.8–96.7 %, and 75.8–97.9 %, respectively, and those in sediments were 87.8–99.6 %, 60.4–70.8 %, 72.3–81.9 %, and 71.9–89.1 %, respectively. The recoveries of 13C3-caffeine from both water and sediments ranged from 78.5 to 98.7 %. The relative standard deviations (RSDs) for all analytes ranged from 0.1 to 21.7 %.
Statistical analysis and environmental risk assessment
The significance of differences between the antibiotic concentrations detected in two seasons and seven sampling locations was determined using paired t test. A level of p < 0.05 was considered statistically significant differences for pair comparisons. Statistical analysis was processed with the Origin software (Version 8.0, Origin Lab, USA).
The risk quotient (RQ) approach is recommended to assess the environmental risk of pharmaceuticals, and the potential of such compounds to cause undesired environmental effects has been predicted by utilizing this method (Carlsson et al. 2006). RQ for the aquatic environment is calculated as:
where MEC is the measured environmental concentration, and PNEC is the predicted no-effect concentration in water.
The PNEC can be calculated as:
where NOEC is the no observed effect concentration for the most sensitive species which can be estimated from the LC50 (median lethal concentration) or EC50 (half maximal effective concentration) value obtained in the literature and AF is the assessment factor that shows whether the toxicity is acute (1000) or chronic (100) (Leung et al. 2012). In this study, the value of AF was chosen to be 1000. Combined risks of antibiotic mixtures were assessed based on the concentration addition model, which is the sum of all individual antibiotic RQs (Zhao et al. 2010). The aquatic organisms, including algae, plant, and invertebrate, were chosen to assess the environmental risk.
Results and discussion
Occurrence of antibiotics in water and sediments
A summary of target antibiotics in the water and sediments from Liao River in Jilin Province is presented in Table 1. Of the 12 antibiotic compounds, only STL was not detected in any water and sediment samples. Eleven of the 12 target antibiotics were detected in the water. The total concentration of all target antibiotics in each sampling sites ranged from 105.9 ng/L at site 1 in autumn to 1345.6 ng/L at site 5 in autumn. The detection frequencies of OFL, ETM, and RTM in the water were 100 %, followed by OTC (85.7 %). OTC was the most abundant with the highest mean concentration of 174.9 ± 266.9 ng/L, followed by ETM (103.2 ± 95.5 ng/L) and OFL (67.1 ± 77.3 ng/L). The detection frequencies and concentrations of target antibiotics indicated that OTC, ETM, and OFL were the dominant antibiotics in the water. For sediments, 9 of the 12 target antibiotics were detected with range of total concentration from 1.8 ng/g at site 4 in summer to 1190.4 ng/g at site 5 in autumn. Among the detected antibiotics in sediments, OFL, OTC, and ETM showed the highest detection frequency (90.9 %), followed by NOR and TC (both 81.8 %). The highest mean concentration of these detected antibiotics was 152.2 ± 108.3 ng/g for OFL, followed by 149.5 ± 147.6 ng/g for OTC and 62.8 ± 83.3 ng/g for NOR. Although TC and ETM are frequently detected in sediments, their concentrations were lower than that of NOR. These results implied that OFL, OTC, and NOR were dominant antibiotics in the sediments.
In the present study, 12 investigated antibiotics can be categorized into four groups. Their mean concentrations in water and sediments decreased in the following orders: TCs (100.9 ± 215.4 ng/L) > MLs (75.7 ± 80.6 ng/L) > QNs (40.4 ± 52.0 ng/L) > SAs (10.7 ± 12.8 ng/L) and TCs (96.4 ± 122.0 ng/g) > QNs (78.9 ± 99.2 ng/g) > MLs (14.0 ± 18.8 ng/g) > SAs (2.4 ± 1.4 ng/g), respectively. The mean concentrations and standard deviations were calculated with the concentrations of antibiotics in the whole investigation area (seven sites) and different seasons (summer and winter) as shown in Table 1. Because of the great variation range of antibiotic concentrations in different sampling sites and different seasons, the standard deviations were very high, even more than the mean value of concentrations. The different sequence of antibiotics in water and sediments may be due to their different usages and environmental behaviors. In the region of Liao River in Jilin Province, the main commodity is grain-based agriculture and livestock farming. Moreover, the population density (242 per square kilometer) is higher than the average population density of Jilin Province (146 per square kilometer). Thus, antibiotics in aquatic environments may originate primarily from agricultural activities, animal waste discharge, and human activities. The concentration of TCs in water is relatively high, reflecting the high quantity of antibiotic usage and waste discharge into the river. For the concentrations of target antibiotics in sediments, high concentrations of TCs, QNs, and MLs may result from their strong binding capacity to sediments in rivers (Figueroa and MacKay 2005; Huang et al. 2011; MacKay and Canterbury 2005; Robinson et al. 2005; Thuy et al. 2011). Compared with most MLs, QNs are strongly adsorbed onto sediments without biodegradation (Moreno-Bondi et al. 2009), enhancing their concentration in sediments.
In comparison with the antibiotic concentrations in water of Liao River in Liaoning Province (Bai et al. 2014), TCs were dominant in water with a mean concentration of 100.9 ± 215.4 ng/L, which was higher than that in Liaoning Section of the Liao River (mean 24.4 ng/L). As for MLs, QNs and SAs, their mean concentrations (75.7 ± 80.6, 40.4 ± 52.0, and 10.7 ± 12.8 ng/L, respectively) were lower than those in Liaoning Section (mean 201.9, 124.3, and 113.9 ng/L, respectively). For the antibiotic concentrations in sediments, TCs (mean 96.4 ± 122.0 ng/g), QNs (mean 78.9 ± 99.2 ng/g), and SAs (mean 2.4 ± 1.4 ng/g) showed a higher level of contamination in the Jilin Section of the Liao River than in Liaoning Section with the mean concentrations of 32.1, 20.9, and 0.4 ng/g, respectively. In contrast, the mean concentration of MLs (14.0 ± 18.8 ng/g) was lower than that in Liaoning Section of the Liao River (mean 32.8 ng/g). The results suggest a large use of TCs in the region of Liao River in Jilin Province.
Seasonal and spatial variations of antibiotics in Jilin Section of the Liao River
The concentrations of antibiotics in water and sediments at different sampling sites over two seasons are shown in Fig. 2. The total antibiotic concentrations in water at each site were significantly higher (p < 0.05) in autumn than that in summer (about 1.2–3 times higher), with the exception of site 1. In addition, the number of detected antibiotics was higher in autumn than those in summer. Eleven of 12 target antibiotics were detected in autumn, while only 9 of 12 in summer. It was worth noting that the dominating antibiotic group was different between the summer and autumn. Based on the average contribution across different sites, the contributions from different antibiotics in summer and autumn tended to follow the descending order: MLs (47.2 %) > QNs (36.6 %) > TCs (11.0 %) > SAs (5.2 %) and TCs (39.4 %) > MLs (29.1 %) > QNs (28.0 %) > SAs (3.5 %), respectively. The results suggested that MLs and QNs were the dominant antibiotic groups in summer, whereas TCs and MLs were the main antibiotic groups in autumn. The antibiotic concentrations in sediments at each sampling site showed no significant seasonal variations (p > 0.05), in agreement with previous studies on Liaoning Section of the Liao River (Zhou et al. 2011). Seasonal variation of antibiotics in water could be caused by a series of environmental factors and their usages. In summer, water flow-through rate was 8–24 times higher than that in autumn. The high flow rate may dilute antibiotics to lower concentrations (Loftin et al. 2008). Besides, high temperatures in summer could accelerate the decomposition of antibiotics in water due to enhanced microbial activity (Kim and Carlson 2007; Xu et al. 2007b). The results were consistent with previous studies where detected antibiotic concentrations in high flow and warm conditions were lower than those in low flow and cold conditions (Jiang et al. 2011; Yang et al. 2011). As the dominant antibiotics, TCs had higher concentrations in autumn. In autumn, livestock are more likely to be infected with respiratory infections and diarrhea, increasing the use of TCs for prevention of these infectious diseases (Ben et al. 2013). Thus, TCs were dominant in autumn instead of MLs. In comparison with the water column, no significant seasonal variation of antibiotic concentrations in the sediment indicated that antibiotics sorbed onto sediments were not subject to the changes of source input and short-term environmental conditions between seasons.
The sampling strategy was to select four sites representing the Dongliao River (S1, S2, S3, and S4), two sites representing the Tiaozi River (S5 and S6), and one site representing the boundary section of the Zhaosutai River in Jilin Province (S7). The results revealed that the total antibiotic concentrations in the Tiaozi River were at a high level (mean 936.8 ± 401.9 ng/L in water and 789.5 ± 351.5 ng/g in sediments) among the three investigated rivers, while those at the Zhaosutai River were at a low level (mean 187.1 ± 69.2 ng/L in water and 125.9 ± 49.6 ng/g in sediments). Although the spatial distribution of antibiotics showed large variations among the sampling sites, antibiotics had an overall trend of higher concentrations near cities in each river, such as S2 (650.9 ng/L in water and 361.0 ng/g in sediments, autumn) in the Dongliao River and S5 (1345.6 ng/L in water and 1190.4 ng/g in sediments, autumn) in the Tiaozi River. The total antibiotic concentrations decreased from the S2 or S5 to the downstream of each river, with the exception of water samples from the Tiaozi River in summer.
The untreated and treated wastewater from Liaoyuan and Siping cities were discharged into the upstream of S2 and S5, respectively. Compared with S2, S5 presented a higher total antibiotic concentration. The populations and the breeding scale of livestock and poultry in Siping area were 2.7 and 2.1 times larger than that in Liaoyuan area, respectively (Liaoyuan Statistical Bureau 2014; Siping Statistical Bureau 2014). The larger human and livestock populations may have led to higher contamination level at S5. Previous work indicated that residual antibiotics in the areas close to highly urban locations were higher than those in less populated areas (Zhou et al. 2011). In addition, although some wastewater was treated in sewage treatment plants, antibiotics cannot be removed completely by current technologies and high antibiotic concentrations were still found in the effluents (Xu et al. 2007a). Therefore, the results revealed that sewage discharge from surrounding anthropogenic activities and livestock operations may be the primary reason for the higher antibiotic concentrations at those sampling sites. For S1 in Dongliao River, it is located at the upstream of Liaoyuan. It may be less influenced by the input of wastewater. Besides, the relatively low antibiotic concentration at S1 could be mainly attributed to dilution of relatively less contaminated water from Yangmu Reservoir. For S6 in the Tiaozi River, the relatively high antibiotic concentration in water in summer may be due to the input of highly contaminated water from the tributary originated from Lishu Town, compared with S5. Generally, the spatial distribution of antibiotics in Jilin Section of the Liao River was largely dependent on the distance to major pollution sources in Liaoyuan and Siping cities. Liang et al. (2013) investigated the distribution of antibiotics in the Pearl River Estuary. Similar to our work, they found that total antibiotic concentrations in water were higher along the western coast than the eastern coast, resulting from the distance to major pollution sources on the west coast.
Risk assessment of antibiotics in Jilin Section of the Liao River
Previous studies have reported that antibiotics in the aquatic environment may cause adverse ecological and health impacts on aquatic organisms (Jiang et al. 2011). In the present study, antibiotics were frequently found in water samples from the Jilin Section of the Liao River. Therefore, it is necessary to estimate the environmental risk of these antibiotics to organisms.
Individual RQs of 12 antibiotics in main tributaries of Liao River in Jilin Province were estimated (Fig. 3). When the value of RQ exceeded 1.0, it meant that the risk for the living organisms in water was high. If the value of RQ ranged from 0.01 to 0.1 and 0.1 to 1, it was regarded as low and medium risks, respectively (Hernando et al. 2006). Generally, a large majority of the RQ values were ranged from 0.01 to 1, indicating low to medium risk for the aquatic organisms (algae, plants, and invertebrates) posed by the target compounds in any season. In comparison with plants and invertebrates, algae were relatively susceptible to antibiotics. Previous studies had also reported that exposure to antibiotics tend not to affect invertebrate as much as algae (Lützhøft et al. 1999). The RQ values of OFL and ETM for algae were ranged from 1.2 to 14.1 and 1.0 to 9.8 in three rivers, indicating that these antibiotics were of high risk for algae in the investigated rivers. As for invertebrates, the RQ values of most antibiotics were lower than 0.01, suggesting little risk for them. However, OTC could cause high and medium risk for invertebrates, while ETM could cause medium to low risk. It was worth noting that RTM in the Tiaozi River showed low risk to invertebrates due to the relatively high concentration compared with other two rivers. For the same reason mentioned above, high risk of OFL was found for plants in the Dongliao River and the Tiaozi River. Thus, OFL, ETM, and OTC were the major antibiotics and showed potential adverse ecological consequences for aquatic organisms.
The present study revealed that antibiotics were occurring as mixtures in the three rivers. However, the above risk assessment did not consider effects of mixtures of antibiotics. In view of the precautionary principle, a concentration addition model was used to estimate the mixture effects of 12 antibiotics with similar or dissimilar mechanisms of action by summing the RQ values for the worst-case scenario (Leung et al. 2012; Zhao et al. 2010) and the resultant mixture RQs are shown in Fig. 3. The mixture RQs of 12 antibiotics in water ranged from 0.08 to 24.5, suggesting medium to high risk for the investigated aquatic organisms. Unfortunately, this assessment of the risk for the three rivers only accounted for the toxic effects of 12 measured antibiotics. It did not consider the toxic effects of any other contaminant that might be present in these rivers. The toxicological information of more antibiotics needs to be further investigated to fully assess the risks.
Conclusions
The results showed that OTC, ETM, and OFL were widely distributed in surface water, and OFL, OTC, and NOR were the dominant antibiotics in sediments. The occurrence of the antibiotics in surface water in autumn was significantly higher than that in summer, while no significant seasonal variations were found for antibiotic concentrations in the sediment. As for the spatial variations, antibiotics showed an overall trend of higher concentrations near cities, including Siping and Liaoyuan. The spatial distribution of antibiotics was largely dependent on the distance to major pollution sources leading to relatively low antibiotic concentration in the downstream of each tributary. Based on the worst-case scenario, potential risks were suspected for OFL, ETM, and OTC in the section of the Liao River in Jilin Province. Accounting for the toxicity of mixtures of the antibiotics, it posed medium to high risk for the investigated aquatic organisms. These results revealed the potential source of antibiotics and indicated that the pollution control for Liao River in Jilin Province should be strengthened and more attention should be given to the Siping area.
References
Bai YW, Meng W, Xu J, Zhang Y, Guo CS (2014) Occurrence, distribution and bioaccumulation of antibiotics in the Liao River Basin in China. Environ Sci Proc Impacts 16:586–593
Ben WW, Pan X, Qiang ZM (2013) Occurrence and partition of antibiotics in the liquid and solid phases of swine wastewater from concentrated animal feeding operations in Shandong Province, China. Environ Sci Proc Impacts 15:870–875
Carlsson C, Johansson AK, Alvan G, Bergman K, Kühler T (2006) Are pharmaceuticals potent environmental pollutants?: part I: environmental risk assessments of selected active pharmaceutical ingredients. Sci Total Environ 364:67–87
Chen K, Zhou JL (2014) Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere 95:604–612
Cheng DM, Liu XH, Wang L, Gong WW, Liu GN, Fu WJ, Cheng M (2014) Seasonal variation and sediment-water exchange of antibiotics in a shallower large lake in North China. Sci Total Environ 476:266–275
Davis JG, Truman CC, Kim SC, Ascough JC, Carlson K (2006) Antibiotic transport via runoff and soil loss. J Environ Qual 35:2250–2260
Dong DM, Liu XX, Guo ZY, Hua XY, Su YL, Liang DP (2015) Seasonal and spatial variations of heavy metal pollution in water and sediments of China’s Tiaozi River. Pol J Environ Stud 24:2371–2379
Figueroa RA, MacKay AA (2005) Sorption of oxytetracycline to iron oxides and iron oxide-rich soils. Environ Sci Technol 39:6664–6671
Figueroa RA, Leonard A, MacKay AA (2004) Modeling tetracycline antibiotic sorption to clays. Environ Sci Technol 38:476–483
Gao PP, Mao DQ, Luo Y, Wang LM, Xu BJ, Xu L (2012) Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res 46:2355–2364
Grujić S, Vasiljević T, Laušević M (2009) Determination of multiple pharmaceutical classes in surface and ground waters by liquid chromatography–ion trap–tandem mass spectrometry. J Chromatogr A 1216:4989–5000
Hernando MD, Mezcua M, Fernández-Alba AR, Barceló D (2006) Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 69:334–342
Hoa PTP, Managaki S, Nakad N, Takada H, Shimizu A, Anh DH, Viet PH, Suzuki S (2011) Antibiotic contamination and occurrence of antibiotic-resistant bacteria in aquatic environments of northern Vietnam. Sci Total Environ 409:2894–2901
Huang CH, Renew JE, Smeby KL, Pinkston K, Sedlak DL (2011) Assessment of potential antibiotic contaminants in water and preliminary occurrence analysis. J Contemp Water Res Educ 120:4
Jiang L, Hu XL, Yin DQ, Zhang HC, Yu ZY (2011) Occurrence, distribution and seasonal variation of antibiotics in the Huangpu River, Shanghai, China. Chemosphere 82:822–828
Jiao SJ, Zheng SR, Yin DQ, Wang LH, Chen LY (2008) Aqueous photolysis of tetracycline and toxicity of photolytic products to luminescent bacteria. Chemosphere 73:377–382
Khetan SK, Collins TJ (2007) Human pharmaceuticals in the aquatic environment: a challenge to green chemistry. Chem Rev 107:2319–2364
Kim SC, Carlson K (2007) Temporal and spatial trends in the occurrence of human and veterinary antibiotics in aqueous and river sediment matrices. Environ Sci Technol 41:50–57
Kümmerer K (2009) Antibiotics in the aquatic environment—a review–part I. Chemosphere 75:417–434
Leung HW, Minh TB, Murphy MB, Lam JC, So MK, Martin M, Lam PKS, Richardson BJ (2012) Distribution, fate and risk assessment of antibiotics in sewage treatment plants in Hong Kong, South China. Environ Int 42:1–9
Li B, Zhang T, Xu ZY, Fang HHP (2009) Rapid analysis of 21 antibiotics of multiple classes in municipal wastewater using ultra performance liquid chromatography-tandem mass spectrometry. Anal Chim Acta 645:64–72
Li WH, Shi YL, Gao LH, Liu JM, Cai YQ (2012) Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China. Chemosphere 89:1307–1315
Liang XM, Chen BW, Nie XP, Shi Z, Huang XP, Li XD (2013) The distribution and partitioning of common antibiotics in water and sediment of the Pearl River Estuary, South China. Chemosphere 92:1410–1416
Liaoyuan Statistical Bureau (2014) Liaoyuan Statistics Year Book 2014. China Statistics Press, Beijing
Loftin KA, Adams CD, Meyer MT, Surampalli R (2008) Effects of ionic strength, temperature, and pH on degradation of selected antibiotics. J Environ Qual 37:378–386
Luo Y, Mao DQ, Rysz M, Zhou QX, Zhang HJ, Xu L, Alvarez PJJ (2010) Trends in antibiotic resistance genes occurrence in the Haihe River, China. Environ Sci Technol 44:7220–7225
Luo Y, Xu L, Rysz M, Wang YQ, Zhang H, Alvarez PJJ (2011) Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin, China. Environ Sci Technol 45:1827–1833
Lützhøft HCH, Halling-Sørensen B, Jørgensen SE (1999) Algal toxicity of antibacterial agents applied in Danish fish farming. Arch Environ Contam Toxicol 36:1–6
MacKay AA, Canterbury B (2005) Oxytetracycline sorption to organic matter by metal-bridging. J Environ Qual 34:1964–1971
Miao XS, Bishay F, Chen M, Metcalfe CD (2004) Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. Environ Sci Technol 38:3533–3541
Moreno-Bondi MC, Marazuela MD, Herranz S, Rodriguez E (2009) An overview of sample preparation procedures for LC–MS multiclass antibiotic determination in environmental and food samples. Anal Bioanal Chem 395:921–946
Rabølle M, Spliid NH (2000) Sorption and mobility of metronidazole, olaquindox, oxytetracycline and tylosin in soil. Chemosphere 40:715–722
Richardson BJ, Lam PKS, Martin M (2005) Emerging chemicals of concern: pharmaceuticals and personal care products (PPCPs) in Asia, with particular reference to Southern China. Mar Pollut Bull 50:913–920
Robinson AA, Belden JB, Lydy MJ (2005) Toxicity of fluoroquinolone antibiotics to aquatic organisms. Environ Toxicol Chem 24:423–430
Sarmah AK, Meyer MT, Boxall ABA (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759
Siping Statistical Bureau (2014) Siping Statistics Yearbook 2014. China Statistics Press, Beijing
Thuy HTT, Nga LP, Loan TTC (2011) Antibiotic contaminants in coastal wetlands from Vietnamese shrimp farming. Environ Sci Pollut R 18:835–841
Tolls J (2001) Sorption of veterinary pharmaceuticals in soils: a review. Environ Sci Technol 35:3397–3406
Xu WH, Zhang G, Li XD, Zou SC, Li P, Hu ZH, Li J (2007a) Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD), South China. Water Res 41:4526–4534
Xu WH, Zhang G, Zou SC, Li XD, Liu YC (2007b) Determination of selected antibiotics in the Victoria Harbour and the Pearl River, South China using high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Environ Pollut 145:672–679
Yang JF, Ying GG, Zhao JL, Tao R, Su HC, Liu YS (2011) Spatial and seasonal distribution of selected antibiotics in surface waters of the Pearl Rivers, China. J Environ Sci Heal B 46:272–280
Zhang JQ, Dong YH (2008) Effect of low-molecular-weight organic acids on the adsorption of norfloxacin in typical variable charge soils of China. J Hazard Mater 151:833–839
Zhang DD, Lin LF, Luo ZX, Yan CZ, Zhang X (2011) Occurrence of selected antibiotics in Jiulongjiang River in various seasons, South China. J Environ Monit 13:1953–1960
Zhao JL, Ying GG, Liu YS, Chen F, Yang JF, Wang L, Yang XB, Stauber JL, Warne MSJ (2010) Occurrence and a screening-level risk assessment of human pharmaceuticals in the Pearl River system, South China. Environ Toxicol Chem 29:1377–1384
Zhou LJ, Ying GG, Zhao JL, Yang JF, Wang L, Yang B, Liu S (2011) Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China. Environ Pollut 159:1877–1885
Acknowledgments
This study was supported by the Major Science and Technology Program for Water Pollution Control and Treatment in China [No. 2012ZX07202009], the National Natural Science Foundation of China [No. 21577047, 21307041 and 21277056], the Science and Technology Development Program of Jilin Province [No. 20150520078JH], and the Science and Technology Program of Department of Education of Jilin Province [No. 2015-494]. The authors would like to thank Dr. Alejandra C. Ortiz for providing valuable comments and revising the English style, thank Dr. Xun Zhang and Dr. Daihui Zhang for the determination of antibiotics, and thank Lufeng Li and Yaojing Xie for the sample collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, D., Zhang, L., Liu, S. et al. Antibiotics in water and sediments from Liao River in Jilin Province, China: occurrence, distribution, and risk assessment. Environ Earth Sci 75, 1202 (2016). https://doi.org/10.1007/s12665-016-6008-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6008-4